A Barcoded ITS Primer-Based Nanopore Sequencing Protocol for Detection of Alternaria Species and Other Fungal Pathogens in Diverse Plant Hosts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and qPCR
2.3. Primer Design and PCR
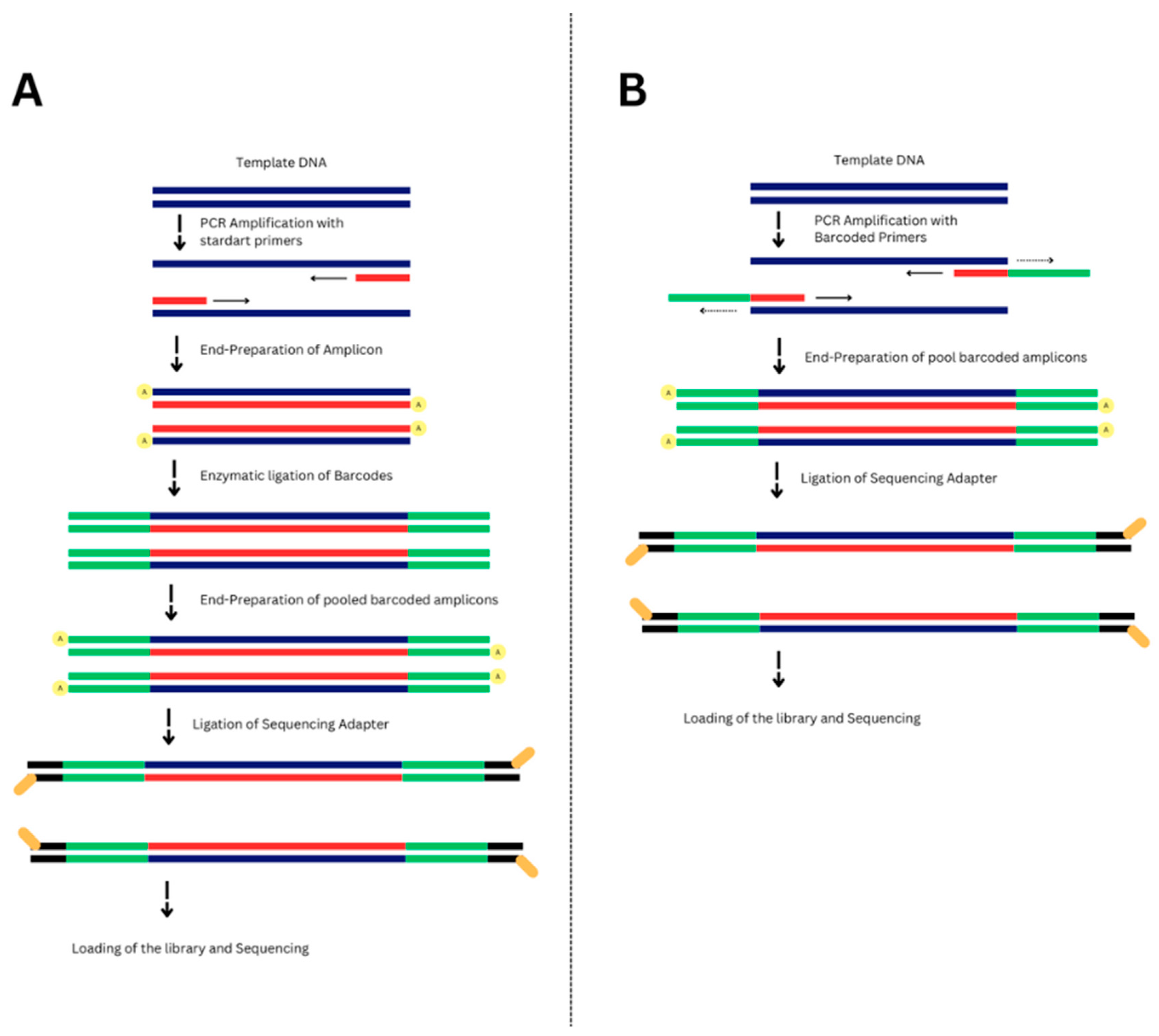
2.4. Library Preparation
2.5. Bioinformatics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meena, M.; Gupta, S.K.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Alternaria toxins: Potential virulence factors and genes related to pathogenesis. Front. Microbiol. 2017, 8, 1451. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, I.; Gilardi, G.; Ortu, G.; Gisi, U.; Gullino, M.L.; Garibaldi, A. Identification and characterization of Alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production. Eur. J. Plant Pathol. 2017, 149, 1190. [Google Scholar] [CrossRef]
- Rovetto, E.I.; La Spada, F.; Aloi, F.; Riolo, M.; Pane, A.; Garbelotto, M.; Cacciola, S.O. Green solutions and new technologies for sustainable management of fungus and oomycete diseases in the citrus fruit supply chain. J. Fungi 2024, 10, 1543. [Google Scholar] [CrossRef]
- Garganese, F.; Schena, L.; Siciliano, I.; Prigigallo, M.I.; Spadaro, D.; De Grassi, A.; Ippolito, A.; Sanzani, S.M. Characterization of citrus-associated Alternaria species in Mediterranean areas. PLoS ONE 2016, 11, e0163255. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, E.; Karpova, D.; Burovinskaya, M.; Vinogradova, S. Leaf spot caused by Alternaria spp. is a new disease of grapevine. Plants 2024, 13, 3335. [Google Scholar] [CrossRef] [PubMed]
- Aloi, F.; Riolo, M.; Sanzani, S.M.; Mincuzzi, A.; Ippolito, A.; Siciliano, I.; Pane, A.; Gullino, M.L.; Cacciola, S.O. Characterization of Alternaria Species Associated with Heart Rot of Pomegranate Fruit. J. Fungi 2021, 7, 172. [Google Scholar] [CrossRef]
- Nizamani, M.M.; Zhang, Q.; Muhae-Ud-Din, G.; Wang, Y. High-throughput sequencing in plant disease management: A comprehensive review of benefits, challenges, and future perspectives. J. Fungi 2023, 9, 210. [Google Scholar] [CrossRef]
- Boža, V.; Brejová, B.; Vinař, T. DeepNano: Deep recurrent neural networks for base calling in MinION nanopore reads. PLoS ONE 2017, 12, e0178751. [Google Scholar] [CrossRef]
- Pereira-Marques, J.; Hout, A.; Ferreira, R.M.; Weber, M.; Pinto-Ribeiro, I.; Van Doorn, L.J.; Knetsch, C.W.; Figueiredo, C. Impact of host DNA and sequencing depth on the taxonomic resolution of whole metagenome sequencing for microbiome analysis. Front. Microbiol. 2019, 10, 1277. [Google Scholar] [CrossRef]
- Gan, M.; Wu, B.; Yan, G.; Li, G.; Sun, L.; Lu, G.; Zhou, W. Combined nanopore adaptive sequencing and enzyme-based host depletion efficiently enriched microbial sequences and identified missing respiratory pathogens. BMC Genom. 2021, 22, 23. [Google Scholar] [CrossRef]
- Marotz, C.A.; Sanders, J.G.; Zuniga, C.; Zaramela, L.S.; Knight, R.; Zengler, K. Improving saliva shotgun metagenomics by chemical host DNA depletion. Microbiome 2018, 6, 42. [Google Scholar] [CrossRef]
- Baramidze, V.; Sella, L.; Japaridze, T.; Abashidze, N.; Lamazoshvili, D.; Dzotsenidze, N.; Tomashvili, G. Long amplicon nanopore sequencing of Botrytis cinerea and other fungal species present in infected grapevine leaf samples. Biol. Methods Protoc. 2024, 9, bpad042. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M.; Ziels, R.M.; Kirkegaard, R.H.; Sørensen, E.A.; McDonald, D.; Zhu, Q.; Knight, R.; Albertsen, M. High-accuracy long-read amplicon sequences using unique molecular identifiers with Nanopore or PacBio sequencing. Nat. Methods 2021, 18, 41–45. [Google Scholar] [CrossRef]
- Dommann, J.; Kerbl-Knapp, J.; Albertos Torres, D.; Egli, A.; Keiser, J.; Schneeberger, P.H.H. A novel barcoded nanopore sequencing workflow of high-quality, full-length bacterial 16S amplicons for taxonomic annotation of bacterial isolates and complex microbial communities. mSystems 2024, 9, e00859-24. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, L.; Lai, D.; Xu, W.; Zhang, Y.; Wu, S.; Yang, D.; Zhao, S.; Liu, Z.; Xiao, Y.; et al. Improved targeting of the 16S rDNA nanopore sequencing method enables rapid pathogen identification in bacterial pneumonia in children. Front. Cell. Infect. Microbiol. 2023, 12, 1001607. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.G.; Majumdar, M.; Troman, C.; O’Toole, Á.; Benny, B.; Abraham, D.; Praharaj, I.; Kang, G.; Sharif, S.; Alam, M.M.; et al. Rapid and sensitive direct detection and identification of poliovirus from stool and environmental surveillance samples by use of nanopore sequencing. J. Clin. Microbiol. 2020, 58, e00920-20. [Google Scholar] [CrossRef] [PubMed]
- Leipart, V.; Ludvigsen, J.; Kent, M.; Sandve, S.; To, T.H.; Árnyasi, M.; Kreibich, C.D.; Dahle, B.; Amdam, G.V. Identification of 121 variants of honey bee Vitellogenin protein sequences with structural differences at functional sites. Protein Sci. 2022, 31, 4369. [Google Scholar] [CrossRef]
- Khoo, Y.W.; Khaw, Y.S.; Tan, H.T.; Li, S.F.; Chong, K.P. First report of Fusarium oxysporum causing leaf spot on Basella rubra in Malaysia. Plant Dis. 2023, 107, 850. [Google Scholar] [CrossRef]
- Uchida, J.Y.; Kadooka, C.Y. Cooperative Extension Service plant disease: Colletotrichum leaf spot of red sealing wax palm. Univ. Hawaii Coop. Ext. Serv. 1997, 1, 2–3. [Google Scholar]
- Rhouma, A.; Hajji-Hedfi, L.; Salih, Y.A.; Bousselma, A.; Kouadri, M.E.A.; Khrieba, M.I. Review: Ascochyta leaf spot of wheat: Disease profile and management. Microbiol. Biotechnol. 2024, 11, 1077. [Google Scholar] [CrossRef]
- Špetík, M.; Eichmeier, A.; Burgová, J.; Groenewald, J.Z.; Crous, P.W. Calophoma clematidina causing leaf spot and wilt on Clematis plants in the Czech Republic. Plant Dis. 2023, 107, 2142. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, J.; Wan, Y.; Han, Y.; Tong, H.; Chen, Y. Characteristics of leaf spot disease caused by Didymella species and the influence of infection on tea quality. Phytopathology 2023, 113, 516–527. [Google Scholar] [CrossRef]
- Viret, O.; Gindro, K. Fungal Diseases of Green Organs. In Science of Fungi in Grapevine; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine powdery mildew (Erysiphe necator): A fascinating system for the study of the biology, ecology, and epidemiology of an obligate biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.B.; Kim, K.D.; Cho, W.D.; Kim, W.G. Didymella acutilobae sp. nov. causing leaf spot and stem rot in Angelica acutiloba. Mycobiology 2023, 51, 313–319. [Google Scholar] [CrossRef]
- Andrew, M.; Peever, T.L.; Pryor, B.M. An expanded multilocus phylogeny does not resolve morphological species within the small-spored Alternaria species complex. Mycologia 2009, 101, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.D.; Barbara, D.J.; Harrison, R.J.; Lane, C.R.; Sreenivasaprasad, S.; Woodhall, J.W.; Clarkson, J.P. Discrete lineages within Alternaria alternata species group: Identification using new highly variable loci and support from morphological characters. Fungal Biol. 2015, 119, 994–1006. [Google Scholar] [CrossRef]
- Woudenberg JH, C.; Seidl, M.F.; Groenewald, J.Z.; De Vries, M.; Stielow, J.B.; Thomma BP, H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- Nicoletti, R.; Zimowska, B. Endophytic fungi of hazelnut (Corylus avellana). Plant Prot. Sci. 2023, 59, 133. [Google Scholar] [CrossRef]
- Nian, L.; Xie, Y.; Zhang, H.; Wang, M.; Yuan, B.; Cheng, S.; Cao, C. Vishniacozyma victoriae: An endophytic antagonist yeast of kiwifruit with biocontrol effect against Botrytis cinerea. Food Chem. 2023, 423, 135442. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Turatsinze, A.N.; Xie, X.; Sha, Y.; Wang, R. First report of Fusarium tricinctum causing root rot on Chinese dwarf cherry (Cerasus humilis) in China. Plant Dis. 2024, 108, 1164. [Google Scholar] [CrossRef]
- Qiu, W.; Feechan, A.; Dry, I. Current understanding of grapevine defense mechanisms against the biotrophic fungus (Erysiphe necator), the causal agent of powdery mildew disease. Hortic. Res. 2015, 2, 15020. [Google Scholar] [CrossRef]
- Gao, Y.R.; Han, Y.T.; Zhao, F.L.; Li, Y.J.; Cheng, Y.; Ding, Q.; Wang, Y.J.; Wen, Y.Q. Identification and utilization of a new Erysiphe necator isolate NAFU1 to quickly evaluate powdery mildew resistance in wild Chinese grapevine species using detached leaves. Plant Physiol. Biochem. 2016, 104, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Z.; Wang, Y.B.; Li, S.C.; Wu, F.; Luo, Z.Y.; Chen, J.Y.; Xiang, M.L.; Chen, M. First report of Fusarium tricinctum causing fruit rot on navel orange (Citrus sinensis) in China. Plant Dis. 2023, 107, 891. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Broberg, A.; Andreasson, E.; Stenberg, J.A. Biocontrol potential of beneficial fungus Aureobasidium pullulans against Botrytis cinerea and Colletotrichum acutatum. Phytopathology 2023, 113, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; Romboli, Y.; Barbato, D.; Mari, E.; Venturi, M.; Guerrini, S.; Granchi, L. Indigenous Aureobasidium pullulans strains as biocontrol agents of Botrytis cinerea on grape berries. Sustainability 2021, 13, 9389. [Google Scholar] [CrossRef]
- Morrison, G.A.; Fu, J.; Lee, G.C.; Wiederhold, N.P.; Cañete-Gibas, C.F.; Bunnik, E.M.; Wickes, B.L. Nanopore sequencing of the fungal intergenic spacer sequence as a potential rapid diagnostic assay. J. Clin. Microbiol. 2020, 58, e01972-20. [Google Scholar] [CrossRef]

| Fungal Species | Thuja 01 | Thuja 02 | Maple 01 | Maple 02 | Mandarin 01 | Mandarin 02 | Grape 01 | Grape 02 |
|---|---|---|---|---|---|---|---|---|
| Alternaria spp. 1 | 2.87 | 2.79 | 8.56 | 9.11 | 1.59 | 1.29 | 2.68 | 9.71 |
| Other Fungi 2 | 70.39 | 75.32 | 72.54 | 70.12 | 77.20 | 49.92 | 94.81 | 85.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baramidze, V.; Sella, L.; Japaridze, T.; Dzotsenidze, N.; Lamazoshvili, D.; Abashidze, N.; Basilidze, M.; Tomashvili, G. A Barcoded ITS Primer-Based Nanopore Sequencing Protocol for Detection of Alternaria Species and Other Fungal Pathogens in Diverse Plant Hosts. J. Fungi 2025, 11, 249. https://doi.org/10.3390/jof11040249
Baramidze V, Sella L, Japaridze T, Dzotsenidze N, Lamazoshvili D, Abashidze N, Basilidze M, Tomashvili G. A Barcoded ITS Primer-Based Nanopore Sequencing Protocol for Detection of Alternaria Species and Other Fungal Pathogens in Diverse Plant Hosts. Journal of Fungi. 2025; 11(4):249. https://doi.org/10.3390/jof11040249
Chicago/Turabian StyleBaramidze, Vladimer, Luca Sella, Tamar Japaridze, Nino Dzotsenidze, Daviti Lamazoshvili, Nino Abashidze, Maka Basilidze, and Giorgi Tomashvili. 2025. "A Barcoded ITS Primer-Based Nanopore Sequencing Protocol for Detection of Alternaria Species and Other Fungal Pathogens in Diverse Plant Hosts" Journal of Fungi 11, no. 4: 249. https://doi.org/10.3390/jof11040249
APA StyleBaramidze, V., Sella, L., Japaridze, T., Dzotsenidze, N., Lamazoshvili, D., Abashidze, N., Basilidze, M., & Tomashvili, G. (2025). A Barcoded ITS Primer-Based Nanopore Sequencing Protocol for Detection of Alternaria Species and Other Fungal Pathogens in Diverse Plant Hosts. Journal of Fungi, 11(4), 249. https://doi.org/10.3390/jof11040249








