1. Introduction
Cryptococcosis is a life-threatening fungal infection caused primarily by
Cryptococcus neoformans, an opportunistic pathogen responsible for severe diseases such as meningitis, particularly in immunocompromised individuals. This infection remains a significant global health burden, with approximately 223,100 cases reported annually among patients with HIV, resulting in an estimated 181,100 deaths, predominantly in sub-Saharan Africa [
1,
2]. While antiretroviral therapy has reduced the incidence of cryptococcosis in developed nations, the emergence of fluconazole-resistant strains and virulent environmental isolates continues to pose challenges for clinical management [
3].
The pathogenicity of
C. neoformans is closely linked to its genetic and phenotypic plasticity, which enables adaptation to diverse ecological niches. Among its key virulence factors is the production of a polysaccharide capsule, composed primarily of glucuronoxylomannan (GXM) and galactoxylomannan (GalXM), which facilitates immune evasion and systemic dissemination [
4,
5]. GXM inhibits phagocytosis and modulates cytokine production to favor an anti-inflammatory response, while GalXM induces macrophage apoptosis, further weakening the host’s immune defenses [
6]. Additionally, melanin synthesis enhances oxidative stress resistance, and thermotolerance allows the pathogen to survive within mammalian hosts [
7,
8]. The capsule’s multifaceted role in immune evasion underscores its importance as a central virulence factor and a potential therapeutic target.
Beyond its clinical adaptations,
C. neoformans thrives in environmental reservoirs such as soil, bird guano, and decaying organic material. Its environmental resilience reflects an evolutionary connection between ecological adaptation and clinical virulence [
9]. Notably, exposure to agricultural triazole fungicides has been implicated in the development of cross-resistance to clinical azoles, further complicating treatment strategies [
10]. Phenetic analyses, including studies on efflux pump proteins, highlight how selective pressures across niches shape the pathogen’s adaptive strategies [
9]. Concurrently, metabolomic studies have shown that clinical isolates exhibit enhanced sulfur metabolism and glutathione pathways to counteract oxidative stress in host environments, while environmental isolates prioritize carbon metabolism to thrive in nutrient-variable conditions [
3,
11].
This study aims to investigate the phenetic and metabolomic adaptations of
C. neoformans strains isolated from clinical and environmental sources. By integrating molecular phenetic analyses and comprehensive metabolomic profiling, we seek to elucidate the distinct adaptive strategies employed by
C. neoformans in varied ecological contexts. These findings provide critical insights into the pathogen’s evolution and may inform the development of novel therapeutic strategies to combat cryptococcosis, particularly in the face of rising antifungal resistance [
1,
9].
2. Materials and Methods
2.1. Study Description and Ethical Aspects
This is an observational in silico study. Open access databases were consulted, and no national or international legislation on scientific research was violated. No human beings or animals were used directly or indirectly, only information of microbial agents and fungi.
2.2. Design and Selection of Strains
Two groups were included in this study: clinical and environmental strains of
C. neoformans, along with a reference strain (
C. neoformans var.
grubii JEC21 was chosen as the reference strain based on its extensive genomic characterization and widespread recognition in the literature as a standard for
C. neoformans var.
grubii [
12,
13]). Six clinical strains (c45, CHC193, MW-RSA1955, Br795, c8, and Th845) and nine environmental strains (VNII, MW-RSA852, A1-35-8, AD1-83a, A5-35-17, D17-1, Ze90-1, Tu259-1, and Gb118) were selected. Including the reference strain JEC21, a total of 16 genomes were analyzed in this study. The reference strain was used as a benchmark but was not included in comparative analyses between the clinical and environmental groups. Variables such as the year of isolation, location (country), and isolation source were recorded to better characterize the collected strains.
We utilized genomes of C. neoformans isolates available in the GenBank/National Center for Biotechnology Information (NCBI) database to ensure consistency in data acquisition. The dataset included strains stratified as clinical or environmental, consistent with the study’s objectives. While other strain types, such as CBS 8710, CBS 7229, and CBS 8273 (C. gattii), are scientifically relevant, their genomes were unavailable in the selected database, and their inclusion could diverge from the study’s focus on C. neoformans adaptations.
Metadata associated with the dbCAN database output, which provides annotations of carbohydrate-active enzymes (CAZymes), as well as descriptions of relative abundance values across strains and virulence data, have been previously deposited in the OSF Home repository, which serves as a publicly accessible platform for storing and sharing metadata and research data, ensuring the transparency and reproducibility of the analyses performed in this study. The complete dataset is available for review at the following link:
https://doi.org/10.17605/OSF.IO/8JF2K (accessed on 15 January 2025).
2.3. Molecular Phenetic Analysis
The molecular phenetic analysis was conducted using MEGA software (v11.0.13) to ensure the precise reproducibility of analytical steps. For this analysis, we specifically selected the multidrug efflux pump protein from environmental and clinical strains of C. neoformans due to its well-documented role in antifungal resistance and environmental adaptation.
Our approach was hypothesis-driven, aiming to determine whether this resistance-associated protein exhibited structured differentiation between clinical and environmental isolates, rather than attempting a whole-genome phylogenetic reconstruction. The rationale for this choice is that whole-genome phylogenetic approaches could introduce confounding variations unrelated to drug resistance, making it more challenging to isolate adaptive signals specific to antifungal resistance mechanisms. Additionally, multi-locus sequence typing (MLST), while valuable for strain classification, does not directly capture protein-level adaptive responses.
To ensure methodological rigor, protein sequence alignment was performed using ClustalW v2.1, optimizing alignment quality for subsequent analyses. Evolutionary distances among sequences were then estimated using the Dayhoff substitution model, and the robustness of these estimates was assessed with the bootstrap method (1000 replications) to enhance result reliability.
The resulting distance matrix was used to reconstruct the molecular phenetic tree, employing the UPGMA clustering method. The tree was further validated through a bootstrap test (1000 replicates), producing a consensus tree that represents the evolutionary relationships among environmental and clinical strains of C. neoformans. These analyses allowed us to assess whether resistance-related phenetic divergence occurs between clinical and environmental isolates, supporting our initial hypothesis.
2.4. Carbohydrate Pattern Analysis of Enzymes
To investigate potential differences in carbohydrate patterns between environmental and clinical subgroups of C. neoformans, an analysis was conducted using the dbCAN3 program with the HMMER profile (E-value < 1 × 10−15, coverage > 0.35), utilizing the latest HMMdb v13 (14 August 2024). This analysis enabled the identification and characterization of enzymes involved in carbohydrate metabolism for each subgroup. Enzyme frequencies were assessed within each genomic subpopulation of environmental and clinical C. neoformans isolates.
After enzymes were matched across the two subgroups, the frequency relationships were calculated, with differences equal to or greater than 10% serving as criteria for metabolic predictions. Frequencies showing higher abundance in clinical samples were marked for the clinical subgroup, and the reverse was applied to the environmental subgroup.
2.5. Metabolomic Evaluations
After glycolytic enzyme patterns with higher abundance were identified in both clinical and environmental isolates, the corresponding query ID codes were retrieved from the National Center for Biotechnology Information (NCBI) database, and their amino acid residue sequences were extracted in FASTA format. Two datasets were created and subsequently uploaded to the Human Metabolome Database (HMDB) (
https://hmdb.ca/) (accessed on 23 August 2024). Metabolite interactions were analyzed separately for both categories: those with higher abundance in the clinical subgroup and those in the environmental subgroup. The metabolic pathway analysis focused on metabolites exhibiting distinct abundance patterns in each subgroup, allowing for a targeted investigation of potential adaptive metabolic responses.
To conduct metabolic pathway enrichment analysis, we used MetaboAnalyst 6.0 (
https://www.metaboanalyst.ca/) (accessed on 23 August 2024). HMDB codes corresponding to selected enzymes from
C. neoformans isolates were classified based on their association with either clinical or environmental samples. The Hypergeometric test was employed for the enrichment analysis, using Relative Betweenness Centrality as the topological measure. To ensure comprehensive pathway representation, the reference library setting “Use all compounds in the selected pathway library” was applied. The KEGG pathway database was used as the primary reference, integrating data specific to
C. neoformans available as of December 2023. Results were visualized as scatter plots, highlighting significantly enriched pathways and their respective centralities.
A generalized linear model was applied to calculate the Q-stat for each metabolite set, where the Q-stat represents the average of Q values computed for individual metabolites within each pathway. In this context, the Q value quantifies the squared covariance between each metabolite and the observed metabolic adaptation, enabling a refined assessment of pathway-level metabolic differences.
Although this approach provides valuable insights into the metabolic landscape of C. neoformans, it is important to note that our analysis was limited to genomic sequence data. Consequently, we did not assess gene copy number variations (CNVs), post-transcriptional regulatory mechanisms (e.g., alternative splicing, mRNA stability, protein degradation), or single-nucleotide polymorphisms (SNPs) between clinical and environmental strains. While these factors play a crucial role in modulating enzyme activity and metabolic adaptation, their evaluation would require transcriptomic (RNA-seq) or proteomic datasets, which are not consistently available for all isolates in public repositories.
Despite these limitations, our metabolomic enrichment analysis offers a functional prediction of metabolic adaptations, suggesting potential regulatory mechanisms influencing metabolic divergence between clinical and environmental isolates. Future research integrating multiomics approaches (genomics, transcriptomics, and proteomics) will be essential to further validate and expand upon our findings.
2.6. Virulence Profile Analysis of C. neoformans Isolates
Virulence factors in
C. neoformans isolates were identified using the PHI-base database (
http://www.phi-base.org/, accessed on 25 October 2024), a resource cataloging virulence genes across a broad range of pathogens. This database enabled the annotation of potential virulence factors in the genomic sequences of clinical and environmental isolates. Due to the diverse nature of the database, which includes pathogens with differing infection strategies, the identified factors are considered predictive and not definitive for
C. neoformans. Further experimental validation would be required to confirm their relevance in this species.
Data on the occurrence of virulence factors were extracted from PHI-base and organized into a frequency matrix for comparative analysis between clinical and environmental isolates. To ensure data consistency, missing values were substituted with zeroes. The comparative analysis aimed to identify frequency differences in potential virulence factors that could reflect adaptive strategies in distinct ecological niches.
The visualization of virulence profiles was performed using heatmaps generated in R (v4.3.2) with the pheatmap package. The heatmap highlighted the distribution and clustering of virulence factors, organized by “ID_group” (clinical or environmental) and “Virulence Profile”. A gradient color scheme (white to green) was applied to represent frequency values, enhancing the interpretability of the data. To complement this, a stacked bar chart was generated using the ggplot2 package in R, illustrating the proportional distribution of virulence profiles across isolate groups.
Statistical associations between virulence profiles and isolate origins (clinical vs. environmental) were assessed using a chi-square test. The statistical analysis yielded a chi-square value of 14.40 (p = 0.006), indicating a significant association. These values were incorporated into the bar chart for reference, ensuring clarity and transparency in the presentation of results.
This methodology provided a structured approach to evaluating virulence factors in C. neoformans, offering a foundation for exploring ecological adaptations and their potential impact on pathogenicity and antifungal resistance. While the virulence profiles identified here are valuable for hypothesis generation, they remain predictive and necessitate further validation under laboratory conditions.
2.7. Data Analysis
The statistical analyses in this study were designed to ensure robustness and reliability, given the complexity and volume of comparisons performed. For the molecular phenetic analysis, evolutionary relationships among the strains were inferred using the UPGMA clustering method in MEGA software (v11.0.13) with the Dayhoff substitution model. To validate these inferences, bootstrap tests with 1000 replicates were applied, and a bootstrap consensus tree was generated to represent the relationships among clinical and environmental isolates [
14].
Differences in glycolytic enzyme patterns between clinical and environmental isolates were assessed using dbCAN (HMMER profile; E-value < 1 × 10
−15, coverage > 0.35). Enzymes exhibiting a relative abundance difference of at least 10% between the groups were selected for further analysis [
15]. Enrichment analyses for metabolic pathways were performed in MetaboAnalyst 6.0, applying the Hypergeometric test with Relative Betweenness Centrality as the topological measure. The KEGG pathway database was used as the reference, and results were visualized as scatter plots showing pathway significance and impact [
16]. To control for multiple comparisons, the Benjamini–Hochberg method was used to adjust
p-values, and both raw and FDR-adjusted
p-values (q-values) were reported [
17].
Comparative analyses of virulence profiles were conducted to evaluate associations between ecological niches and the presence of specific virulence factors. Statistical significance was assessed using chi-square tests to determine associations between virulence profiles and isolate origins (clinical vs. environmental) [
18]. Visualizations, including heatmaps and bar charts, were created in R (v4.3.2) using the pheatmap and ggplot2 packages, respectively [
19,
20]. These figures were designed to present statistical insights clearly, with annotations indicating
p-values, FDR-adjusted q-values, and effect sizes.
All statistical analyses adhered to rigorous bioinformatics and statistical standards to maintain methodological consistency [
20]. The significance threshold for all tests was set at
p < 0.05. Adjusted results were cross-verified with the raw data to ensure consistency and reliability.
3. Results
3.1. Overview of Selected C. neoformans Strains for Phenetic and Enzymatic Analysis
Table 1 provides details on the
C. neoformans strains selected as the foundational step for investigating potential adaptation associations between clinical and environmental niches. This dataset includes both clinical and environmental strains, chosen to assess differences in glycolytic enzyme patterns and their involvement in virulence factors and antifungal resistance. The table summarizes key information such as the strain type, specific taxonomic variety, collection date, geographic origin, isolation source, and host. This initial selection of strains was critical for setting up a comparative framework to explore how these organisms may adapt to diverse ecological niches, which could influence their enzymatic profiles and potential for pathogenicity.
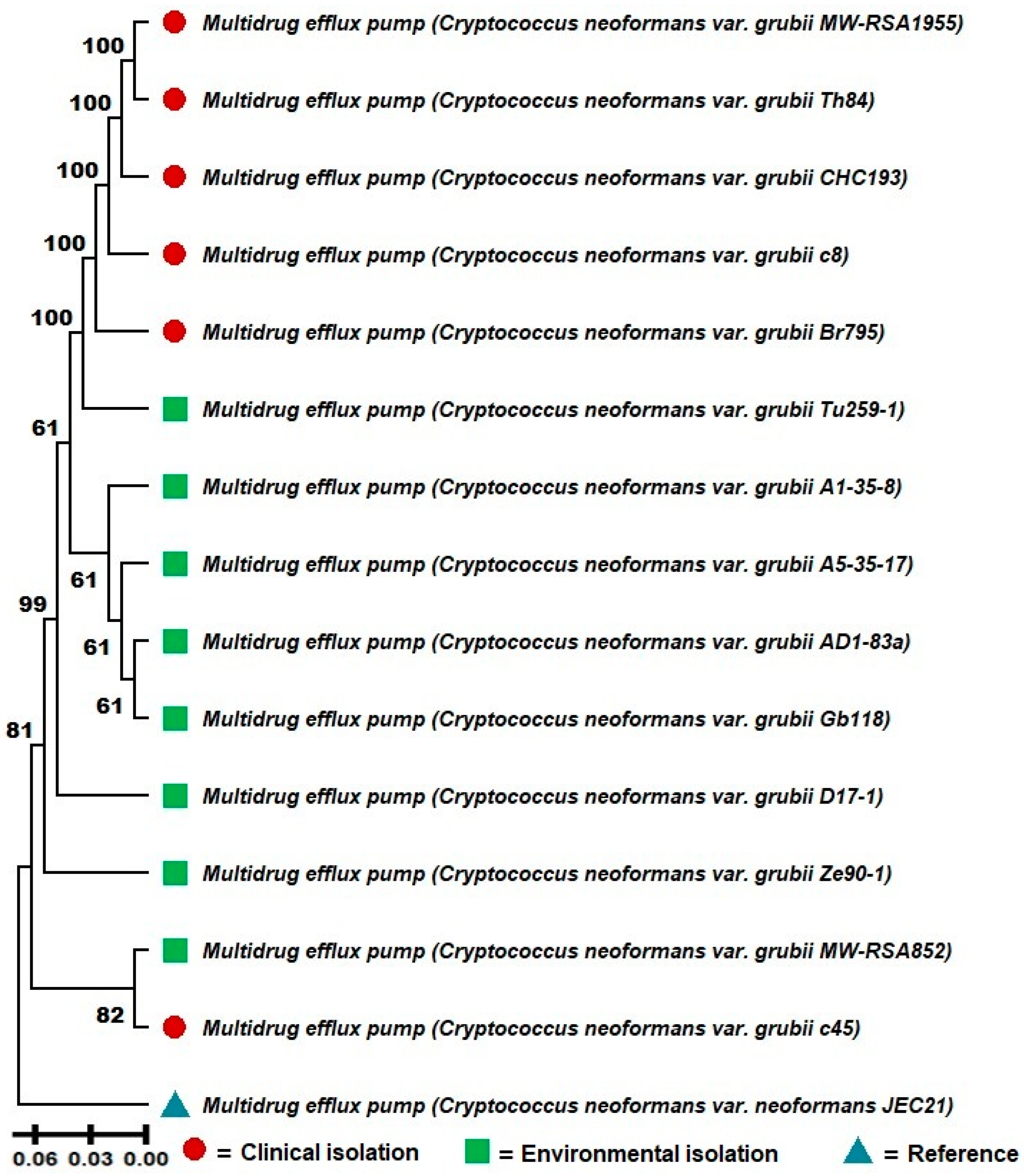
3.2. Significant Phenetic Differentiation Between Clinical and Environmental Isolates of C. neoformans var. grubii and Evidence of Cross-Adaptation
A molecular phenetic analysis was conducted based on the sequences of the multidrug efflux pump protein of
C. neoformans var.
grubii isolates obtained from clinical and environmental samples (
Figure 1). The molecular phenetic tree, constructed using the UPGMA clustering method with bootstrap support of 1000 replications, revealed clear differentiation between clinical and environmental samples, suggesting a possible phenetic divergence between these two groups.
The clinical isolates clustered together in a cohesive clade with high bootstrap support (100), indicating high genetic similarity among these lineages. Notably, environmental isolates TU259-1, A1-35-8, and AD1-83 formed a subgroup with robust statistical support (bootstrap of 61), suggesting a close evolutionary relationship among these sequences. Other environmental isolates, such as GB118 and ZE90-1, also exhibited strong molecular phenetic relationships with each other.
In contrast, the clinical isolates were divided into two main subgroups. The first subgroup, composed of MW-RSA1955, TH84, and CHC193, showed strong bootstrap support (100), indicating high cohesion among these samples. The second subgroup, containing isolates C8 and BR795, also demonstrated high bootstrap support (100). Notably, the clinical isolate C45 clustered with the environmental samples, suggesting a possible environmental origin for this isolate or cross-adaptation. However, other potential explanations, such as sampling errors, misidentification, or contamination, cannot be excluded and warrant further investigation (
Figure 1).
The reference strain JEC21 was included in the analysis to provide a baseline comparison and was positioned outside the clinical and environmental clades. Additionally, it is important to note that the sequence for the multidrug efflux pump protein was not found for the VNII isolate, and therefore, it was excluded from this molecular phenetic reconstruction.
These findings reinforce the hypothesis that although clinical and environmental samples of C. neoformans var. grubii share common genetic characteristics, there is a phenetic divergence that may reflect adaptations to the different selective pressures of the isolation environments. The presence of a clinical isolate within the environmental clade also suggests that transmission events from the environment to the clinical context may have occurred.
3.3. Metabolic Differentiation and Glycolytic Enzyme Patterns in Clinical and Environmental Isolates of C. neoformans
The significant phenetic differentiation observed between clinical and environmental isolates of
C. neoformans var.
grubii (
Figure 1), along with indications of cross-adaptation, prompted further investigation into glycolytic enzyme patterns to explore potential associations with resistance and virulence factors. As such,
Table 2 presents the results of the analysis of glycolytic enzymes related to carbohydrate metabolism in clinical and environmental strains of
C. neoformans, identified using the dbCAN program with the HMMER profile (E-value < 1 × 10
−15, coverage > 0.35) (
Table 2). Only enzyme profiles with a minimum relative abundance difference of 10% between clinical and environmental subgroups were selected. Enzymes with higher abundance in clinical strains are listed in the upper section of the table, while those with higher abundance in environmental strains are listed in the lower section. This table summarizes only the results with significant differences, while the full table containing all analyzed enzyme profiles is available in a public database referenced in the
Section 2 for detailed consultation.
The metabolites associated with glycolytic enzymes in
C. neoformans were identified based on relative abundance in clinical and environmental strains. Following the determination of enzyme patterns for each subgroup, the corresponding amino acid residue sequences were extracted from NCBI and analyzed in the Human Metabolome Database (HMDB). Metabolite–enzyme interactions were categorized according to their higher abundance in each subgroup. Metabolites unique to each niche were utilized to explore potential metabolic pathway products, contributing to the understanding of specific adaptations within each subgroup and their possible role in virulence factors and antifungal resistance (see
Supplementary Table S1).
The metabolic pathway enrichment analysis of
C. neoformans identified significant differences between clinical and environmental isolates concerning the abundance of metabolites across specific pathways, as detailed in
Table 3 and visualized in
Figure 2A,B. To address the high number of comparisons made in this study and reduce the risk of false positives, the Benjamini–Hochberg procedure was applied to adjust
p-values, resulting in false discovery rate (FDR)-corrected q-values. These adjustments provided a robust statistical foundation for interpreting the enriched pathways.
For clinical isolates, sulfur metabolism was the most significantly enriched pathway (p = 0.0053; FDR = 0.39271; impact = 0.0621). This finding suggests that these isolates adapt to oxidative stress, a hallmark of host environments, by employing sulfur metabolism for redox balance and detoxification. Other pathways, such as amino sugar and nucleotide sugar metabolism (p = 0.0237; FDR = 0.87731; impact = 0.1993) and cysteine and methionine metabolism (p = 0.0427; FDR = 0.97982; impact = 0.10096), support amino acid biosynthesis and antioxidant responses essential for survival in the host.
In environmental isolates, methane metabolism emerged as the most significant pathway (p = 0.000070346; FDR = 0.0052056; impact = 0.10417), reflecting adaptations to carbon-rich substrates prevalent in external environments. Glyoxylate and dicarboxylate metabolism (p = 0.0038; FDR = 0.14125) and taurine and hypotaurine metabolism (p = 0.0372; FDR = 0.91726) were also enriched, indicating metabolic strategies to manage diverse environmental stresses, including nutrient variability and oxidative conditions.
These findings, summarized in
Table 3, are visualized in
Figure 2. Panel (a) highlights the pathways with higher abundance in clinical isolates, while panel (b) illustrates pathways enriched in environmental isolates. The scatter plots demonstrate the relationship between the impact score (x-axis) and statistical significance (−log10(
p), y-axis), with color gradients representing adjusted q-values and circle sizes indicating enrichment ratios. This comprehensive visualization reinforces the distinct metabolic adaptations between the two groups.
3.4. Metabolic Pathway Enrichment Analysis of C. neoformans Clinical and Environmental Isolates
The metabolic pathway enrichment analysis revealed distinct pathways enriched in
C. neoformans strains isolated from clinical and environmental samples, as shown in
Table 4 and illustrated in
Figure 3A,B. To ensure statistical robustness and address the multiple comparison issue raised by the large dataset,
p-values were adjusted using the Benjamini–Hochberg procedure to calculate the false discovery rate (FDR). This adjustment ensures that the reported differences are not only statistically significant but also reliable across multiple comparisons.
For clinical isolates, the most enriched pathway was glutathione oxidoreductase (p = 0.259; FDR = 0.0202), a critical pathway involved in the antioxidant response and cellular redox balance, suggesting a key adaptation to the oxidative stress encountered in host environments. Other enriched pathways included iodide oxidoreductase (p = 0.0647; FDR = 0.0635) and thyroid peroxidase (p = 0.0517; FDR = 0.0511), which also contribute to the enhanced antioxidant capacity. Pathways related to sulfur-containing amino acid metabolism, such as cystathionine beta-synthase (p = 0.0776; FDR = 0.0759), highlight a possible role in detoxification and redox regulation within clinical settings.
Environmental isolates showed enrichment in glutathione peroxidase (e) (p = 0.0172; FDR = 0.0172), a pathway linked to oxidative stress defense mechanisms in challenging environments. Additionally, pathways such as oxidized glutathione exchange (p = 0.0172; FDR = 0.0172) and methenyltetrahydrofolate cyclohydrolase, mitochondrial (p = 0.069; FDR = 0.0679) indicate metabolic strategies to counter environmental oxidative conditions. Other pathways, such as glycine hydroxymethyltransferase (p = 0.155; FDR = 0.149) and phosphoserine transaminase (p = 0.198; FDR = 0.189), emphasize the role of serine and folate metabolism in supporting survival under nutrient-limited conditions.
These results, summarized in
Table 4, are visually represented in
Figure 3. Panel (a) shows the enriched pathways in clinical isolates, while panel (b) illustrates pathways enriched in environmental isolates. The scatter plots highlight the relationship between pathway impact scores (x-axis) and −log10(
p) values (y-axis). Circle sizes represent enrichment ratios, and color gradients reflect FDR-adjusted q-values, with darker colors indicating higher statistical significance. This visualization provides a clear distinction between the metabolic adaptations of clinical and environmental isolates of
C. neoformans.
3.5. Comparative Analysis of Enzymatic Patterns and Virulence Profiles in C. neoformans Across Pathogenic Contexts
The results reveal a strong association between the enzymatic pattern of
C. neoformans and various pathogens, as indicated by contingency analysis and the chi-square test. The contingency table used for this analysis, along with the detailed enzymatic frequencies, is available for consultation in the Open Science Framework (OSF) repository (
https://doi.org/10.17605/OSF.IO/8JF2K) (accessed on 15 January 2025). The test yielded a chi-square value of 226.31 with a highly significant
p-value of 6.79 × 10
−11, suggesting a significant correlation between the enzymes present in
C. neoformans and the analyzed pathogens. These findings point to the specificity of
C. neoformans enzymes to certain pathogens, potentially playing an important role in virulence mechanisms and resistance.
Upon analysis of the contingency table, it was observed that the enzyme alpha-glucosidase was particularly prevalent in Aspergillus fumigatus, with five occurrences, a frequency not observed in other pathogens. However, it was also detected in one occurrence in Fusarium oxysporum and three in Mycobacterium tuberculosis, suggesting that this enzyme may play a role in specific interactions with these organisms. The enzyme N-acetylglucosamine-6-phosphate deacetylase was notably present in two samples of Candida albicans and one of Magnaporthe oryzae, which may be related to the reduced virulence observed in these pathogens. These data indicate that the enzymatic distribution varies considerably among different pathogens, reflecting evolutionary adaptations that may be associated with antimicrobial resistance and pathogenic capability.
The enzyme streptomycin biosynthesis protein StrI, in turn, appeared exclusively in Legionella pneumophila, a pathogen known for its antimicrobial resistance. This finding may suggest that the presence of this protein plays a critical role in resistance mechanisms associated with this specific pathogen. Furthermore, the high prevalence of alpha-glucosidase in M. tuberculosis may be linked to increased virulence in this pathogen, as evidenced by its frequency in the analyzed samples.
Figure 4A presents a heatmap showing the correlation patterns between
C. neoformans enzymatic profiles and various pathogens. The heatmap reflects frequency distributions and association patterns, providing a visual representation of the relationships among the analyzed variables. While the chi-square test quantified the statistical significance of these associations, the heatmap serves as a complementary tool to explore trends and variability in the enzymatic data. The color gradient reflects the intensity of enzymatic presence in each pathogen, with darker colors indicating higher frequency. The visualization also highlights the concentration of specific enzymes in pathogens such as
A. fumigatus and
Pseudomonas aeruginosa, suggesting that enzymatic variability is influenced by pathogen type and possibly correlates with each one’s virulent capacity.
In addition,
Figure 4B presents a comparative analysis of virulence profiles between clinical and environmental isolates of
C. neoformans, revealing significant differentiation between the two groups. Clinical isolates exhibit a higher frequency of profiles associated with both unaffected pathogenicity and hypervirulence, suggesting that these isolates retain or even enhance their virulence potential in clinical settings. This observation does not imply an adaptive reduction in virulence within the human host; instead, it may reflect metabolic and genetic traits that incidentally contribute to increased virulence, rather than a direct selection for lower pathogenicity. In contrast, environmental isolates exhibit a broader range of virulence profiles, with a lower frequency of reduced virulence categories, which may be influenced by different selective pressures acting in their native habitats. These findings highlight distinct evolutionary dynamics between clinical and environmental strains of
C. neoformans.
The hypervirulence category was not widely represented in either group but showed a slight predominance in clinical isolates. This finding may suggest a specific response by some strains to pressures from the hospital or human environment. The plant avirulence determinant profile was observed less frequently across isolates from both groups, indicating that C. neoformans shows a preferential adaptation to animal or human hosts rather than plant hosts.
These observations are supported by the color gradient in
Figure 4B, which illustrates the frequency of each virulence category, with darker shades indicating higher prevalence. Differences between clinical and environmental isolates suggest that
C. neoformans may adjust its virulence and pathogenicity depending on the ecological niche, possibly as a strategy to maximize its survival and infection potential in different contexts. Together,
Figure 4A,B demonstrate that both the enzymatic and virulence profiles of
C. neoformans are shaped by environmental and clinical factors, reflecting adaptations that may have direct implications for virulence and antimicrobial resistance.
4. Discussion
The phenetic and metabolomic adaptations in
C. neoformans, particularly the differentiation between clinical and environmental isolates, provide valuable insights into the pathogen’s resilience. These adaptations underline its ability to navigate diverse ecological niches, offering mechanisms that enhance survival and virulence. Our findings support the hypothesis that
C. neoformans adapts to selective pressures across diverse environments, resulting in distinct genetic and phenotypic traits that enhance its ability to survive in both clinical and environmental settings. This adaptability reinforces the role of niche-specific pressures in shaping
C. neoformans’s evolutionary pathways, adding new insights into its virulence and antimicrobial resistance mechanisms [
1,
9,
21].
The inclusion of other
Cryptococcus species, such as
C. gattii, was not pursued due to their distinct clinical and ecological profiles. By focusing exclusively on
C. neoformans, this study aimed to elucidate specific phenetic and metabolomic adaptations without introducing confounding variables associated with interspecies comparisons. For instance,
C. gattii is more commonly associated with infections in immunocompetent hosts, contrasting with
C. neoformans, which predominantly affects immunosuppressed individuals [
22,
23]. Incorporating additional species would have diverged from the primary objective of exploring phenetic and metabolomic adaptations in
C. neoformans.
A significant finding of this study was the distinct molecular phenetic clustering observed between clinical and environmental strains of
C. neoformans var.
grubii. This clustering highlights evolutionary pressures driving niche-specific adaptations and underscores the pathogen’s genetic and ecological plasticity. The formation of two main clades, with distinct subgroups for clinical and environmental isolates, suggests general trends of phenetic divergence influenced by their isolation source. This analysis includes the reference strain JEC21, which serves as an evolutionary baseline to contextualize the clustering patterns observed [
24,
25].
While the clades predominantly reflect clinical and environmental groupings, the overlap of some isolates, such as the two final strains in the tree belonging to both clinical and environmental groups, underscores the genetic and ecological plasticity of
C. neoformans. These patterns may result from cross-adaptation events or shared evolutionary pressures that allow certain strains to transition between ecological niches [
26,
27].
Using the UPGMA method with 1000 bootstrap replicates, our phylogenetic analysis revealed the distinct clustering of clinical and environmental isolates, underscoring phenetic divergence. However, reliance on a single genetic marker, the multidrug efflux pump gene, may have limited the resolution, and broader genomic analyses are recommended to validate these evolutionary patterns. This phenomenon indicates a genetic and evolutionary divergence shaped by specific environmental factors in clinical and non-clinical settings, as observed in previous studies that examined molecular phenetic relationships in
Cryptococcus species [
9,
28]. The separation of clinical isolates into distinct molecular phenetic groups may be reflective of selective pressures encountered within human hosts, where the pathogen must counter host immune defenses and adapt to intracellular environments rich in oxidative stress, as has been shown in studies emphasizing the metabolic and genetic plasticity of
C. neoformans in response to host immunity [
3,
29].
Clinical strains of
C. neoformans exhibit significant enrichment in sulfur metabolism- and glutathione-associated pathways, emphasizing their adaptation to oxidative stress in host environments [
30,
31]. This metabolic plasticity highlights a key survival strategy under immune pressures. To ensure the robustness of these findings,
p-values were adjusted using the Benjamini–Hochberg procedure, addressing the issue of false discoveries across multiple comparisons and providing reliable insights into metabolic adaptations [
17]. This adaptation aligns with findings from previous studies that identified sulfur-based metabolic pathways as vital for pathogen survival under oxidative stress conditions [
10,
21]. The antioxidant mechanisms in clinical isolates, particularly those involving sulfur metabolism and glutathione pathways, represent key virulence factors. These adaptations enable
C. neoformans to withstand oxidative stress imposed by host immunity, sustaining infection and contributing to its pathogenicity [
32,
33].
Moreover, environmental isolates demonstrated unique adaptations that enable survival in carbon-rich external environments. The significant presence of methane metabolism and glyoxylate pathways in these isolates suggests that
C. neoformans possesses specific metabolic traits to exploit organic compounds in soil and other environmental substrates [
11,
34]. Similarly to the clinical isolates, the statistical analyses for environmental isolates included FDR adjustments to ensure that the observed enrichments are both statistically and biologically meaningful, providing confidence in the distinct metabolic strategies identified. This is consistent with studies indicating that
C. neoformans adapts its metabolism to nutrient availability in different ecological niches, thus enabling survival across a range of habitats [
34,
35,
36]. Furthermore, although our study did not directly evaluate the effects of agricultural fungicides, such as triazoles, previous research has highlighted their role in inducing cross-resistance to clinical azoles. This underscores the need for vigilance in monitoring environmental reservoirs, where fungicide exposure may inadvertently select for resistant strains [
10,
33]. The observed clustering patterns and metabolic adaptations in environmental isolates suggest that metabolic flexibility may confer resilience to environmental pressures, including fungicide exposure. Previous studies have shown that agricultural fungicides, such as triazoles, can inadvertently induce cross-resistance to clinical antifungal agents, a phenomenon that warrants further investigation [
10,
33].
The identification of cross-adapted isolates, such as MW-RSA852, within clinical clades underscores significant public health implications. These findings highlight the potential for environmental strains to acquire virulence traits and transition into human pathogens, complicating clinical management and emphasizing the importance of monitoring environmental reservoirs. Similar cross-environmental adaptations have been documented in other studies, suggesting that
C. neoformans may serve as a model for understanding zoonotic transmission and environmental influences on pathogen emergence [
1,
3,
37]. This finding aligns with global epidemiological trends showing increasing incidences of cryptococcal infections derived from environmental sources, emphasizing the need for vigilant surveillance of environmental reservoirs of
C. neoformans [
11,
21].
This study’s metabolomic profiling further distinguishes the unique metabolic strategies between clinical and environmental isolates. The identification of higher abundances of glycolytic enzymes, particularly those involved in sulfur and nitrogen metabolism in clinical strains, suggests a functional role in supporting intracellular survival by maintaining redox balance and nutrient assimilation under host-imposed stresses. Importantly, the application of the Benjamini–Hochberg correction ensured that the reported enriched pathways and enzymatic profiles are statistically robust, minimizing the likelihood of false positives and strengthening the validity of these observations. These adaptations reflect findings from recent studies that highlight glycolytic enzymes and sulfur-related pathways as targets of metabolic modulation in pathogenic
Cryptococcus strains [
9,
28,
38]. Conversely, environmental strains’ reliance on methanotrophic pathways for survival in organic matter-rich environments emphasizes the metabolic flexibility that enables
C. neoformans to colonize a range of habitats and presents potential areas for targeted intervention strategies in environmental reservoirs [
11,
39].
The analysis of enzymatic patterns and virulence profiles revealed significant associations between the enzymatic profiles of
C. neoformans and its adaptability and pathogenicity in various contexts. The correlation between the enzymes present and the observed pathogenicity highlights the relevance of specific enzymatic factors in virulence mechanisms and antimicrobial resistance. This association reinforces previous studies that identify enzymes as critical factors in fungal pathogenesis, including
C. neoformans [
40].
The prominence of the enzyme alpha-glucosidase, predominantly found in
Aspergillus fumigatus, with additional occurrences in
Fusarium oxysporum and
Mycobacterium tuberculosis, suggests an adaptive role in interactions with specific pathogens. Previous studies have demonstrated that glucose-related enzymes can influence infection and dissemination processes in various microorganisms, indicating that the presence of this enzyme may be linked to enhanced survival in specific environments [
41].
Another significant finding was the prevalence of the StrI protein, associated with streptomycin biosynthesis, exclusively in
Legionella pneumophila. This enzyme may play a central role in antimicrobial resistance, a phenomenon widely documented in pathogens adapted to hospital environments [
42]. The detection of the enzyme N-acetylglucosamine-6-phosphate deacetylase in
Candida albicans and
Magnaporthe oryzae suggests a reduction in virulence in these organisms, supporting the idea that alterations in enzymatic metabolism can directly affect infectious capacity [
21].
Our use of the PHI-base database enabled the identification of putative virulence genes in
C. neoformans isolates. While PHI-base is a robust tool for pathogenicity-related genes, it includes diverse pathogens with varying virulence strategies, some of which may not directly apply to
C. neoformans. For example, genes associated with intracellular growth in macrophages, as seen in
Legionella pneumophila, may not align with
C. neoformans’s mechanisms of evading host defenses [
43,
44]. Despite this limitation, the database provides predictive insights that can guide experimental validation under host-relevant conditions. Future studies integrating genomic data with functional assays will be crucial to confirming the role of these genes in the pathogenesis of
C. neoformans.
The virulence profiles, presented in the heatmap, revealed marked differences between clinical and environmental isolates of
C. neoformans. Clinical isolates exhibited a higher frequency of profiles associated with unaffected pathogenicity, indicating an adaptation to the human environment that preserves infectious capacity even under environmental challenges. Conversely, environmental isolates showed a lower rate of reduced virulence, reflecting distinct selective pressures likely associated with survival in environments that are nutrient-rich but less prone to immune challenges [
45].
The subtle predominance of the hypervirulence category in clinical isolates suggests specific responses to hospital-related pressures, aligning with studies showing that virulence factors can be modulated by environmental conditions such as pH, nutrient availability, and quorum sensing signals [
21,
41]. The role of glutathione in redox regulation, demonstrated by Black et al. (2024) [
45], may also contribute to these adaptations, providing a crucial metabolic advantage for countering host oxidative stress.
Finally, the lower number of virulence profiles associated with plant avirulence determinants highlights the specialization of
C. neoformans in animal and human hosts. Recent studies reinforce that the environment plays a critical role in regulating virulence factors, modulating not only gene expression but also susceptibility to antifungal therapies [
41].
These findings, contextualized with the literature, underscore the need for complementary experimental investigations to validate the mechanisms observed in silico. Future studies could delve deeper into the interactions between enzymatic profiles and virulence determinants, elucidating the molecular bases guiding C. neoformans adaptation to diverse ecological niches and clinical contexts.
This study provides important insights into the phenetic and metabolomic adaptations of
C. neoformans; however, certain limitations should be acknowledged. While this study leveraged an in silico approach to identify genomic and metabolic trends, the absence of direct experimental validation limits the ability to fully capture the complex interplay between environmental and host-specific pressures on
C. neoformans adaptation. Nonetheless, statistical corrections, such as false discovery rate adjustments, ensured the reliability and interpretability of the findings across a large dataset. Experimental validation through in vitro and in vivo models would significantly strengthen these findings, offering a more direct view of phenotypic plasticity and adaptive mechanisms under controlled stress conditions [
28,
29].
Although this study included isolates from diverse geographic regions, the limited sample size within each category may restrict the generalizability of our findings. Expanding the dataset in future research could provide deeper insights into regional adaptations and enhance the robustness of observed trends. Although the trends observed are meaningful and supported by statistical analyses, expanding the dataset to include a broader and more globally representative range of isolates would provide deeper insights into regional adaptations and cross-resistance patterns. This would also mitigate potential biases arising from small sample sizes and enhance the robustness of the findings [
3,
9].
A notable limitation of this study is the temporal aspect of the isolates included. Some isolates were collected over two decades ago; however, it is important to emphasize that their sequences were deposited more recently in the NCBI database, reflecting advancements in sequencing technology and data sharing practices. These deposited sequences remain relevant for studying core mechanisms of C. neoformans pathogenicity and adaptation, as traits such as capsule formation, melanin synthesis, and sulfur metabolism are evolutionarily conserved. Additionally, newer genomic datasets with comprehensive annotations are often not immediately available on open access platforms. It is anticipated that future studies will benefit from more recent isolates as these data are deposited and shared in public repositories, enabling a broader temporal perspective on emerging adaptations and resistance mechanisms.
This study primarily focused on glycolytic and sulfur-related pathways due to their established associations with virulence and oxidative stress resistance. However, other metabolic pathways potentially influencing these traits, such as lipid metabolism, amino acid biosynthesis, and secondary metabolite production, were not fully explored. Future metabolomic analyses encompassing these pathways could provide a more comprehensive understanding of the biochemical adaptations that underlie virulence and antifungal resistance in C. neoformans.
Lastly, the observed cross-adaptation event involving the C45 isolate requires further investigation. This clinical isolate’s unexpected clustering with environmental strains suggests the presence of transitional mechanisms that may enable environmental strains to acquire pathogenic traits. To better elucidate these mechanisms, future studies should integrate genomic, metabolomic, and phenetic analyses, complemented by functional experiments. Such an approach would offer critical insights into the genetic and phenotypic factors driving environmental-to-clinical transitions and highlight the evolutionary pressures shaping
C. neoformans adaptability, with implications for public health [
10,
33].