The Heterogenous Presentation of Hepatic Mucormycosis in Adults: A Case Report and Review of the Literature
Abstract
1. Introduction
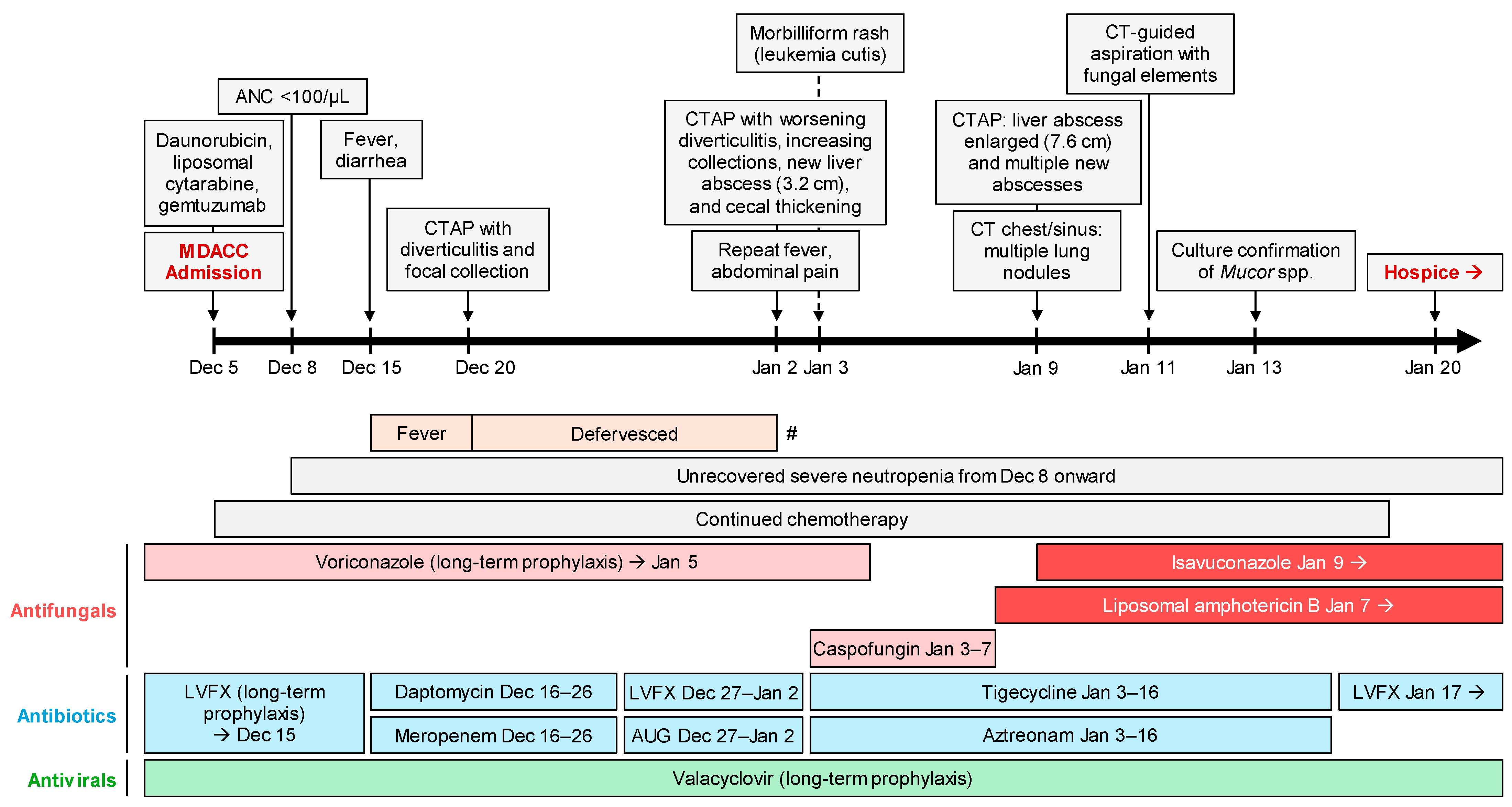
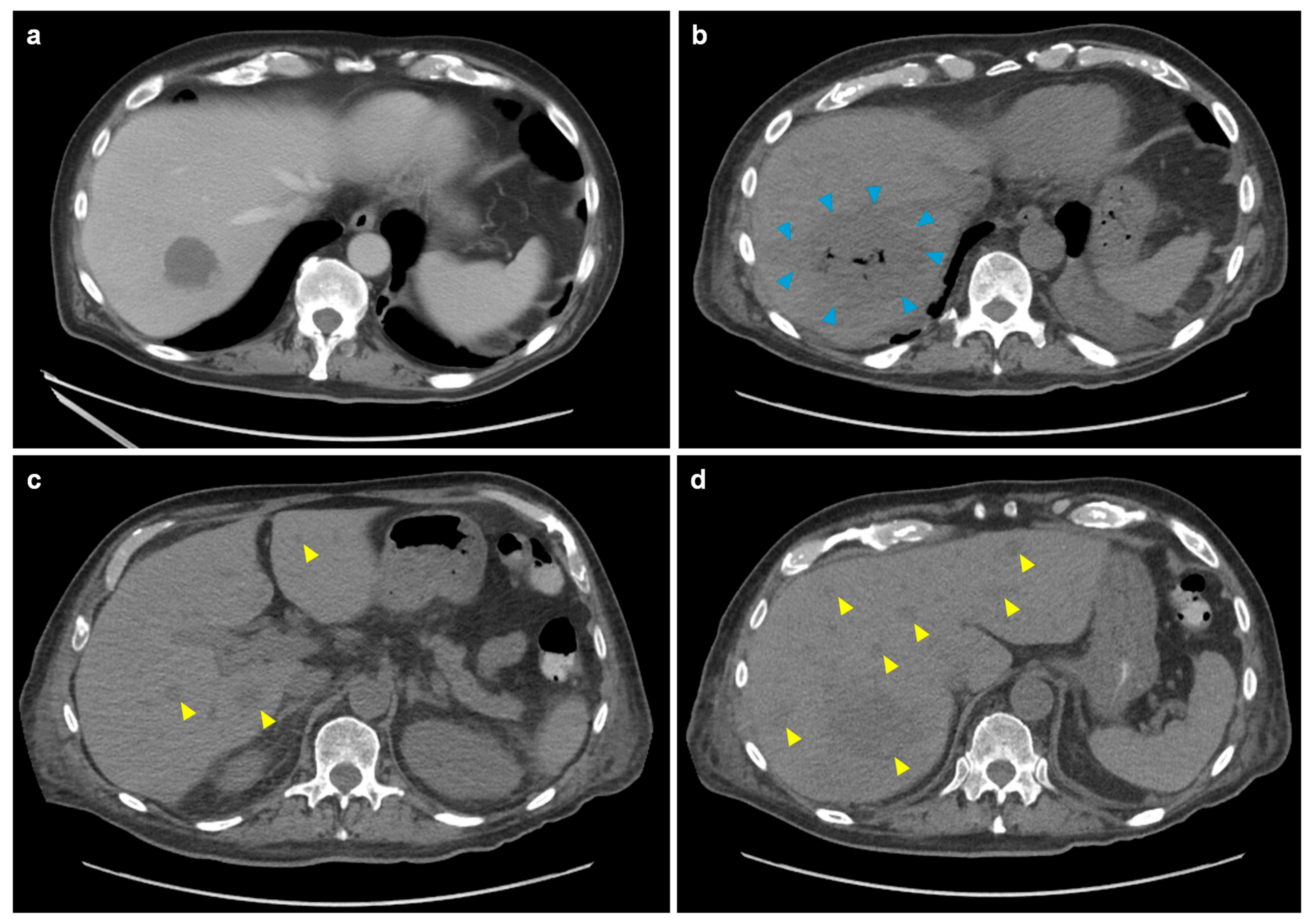
2. Case Report
3. Literature Review
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AST | aspartate aminotransferase |
| cfDNA | Cell-Free Deoxyribonucleic Acid |
| CTAP | computed tomography of the abdomen and pelvis |
| FDG | fludeoxyglucose-18 |
| MCM | mucormycosis |
| NEC | necrotizing enterocolitis |
| PCR | polymerase chain reaction |
| spp. | species |
References
- Spellberg, B.; Kontoyiannis, D.P.; Fredricks, D.; Morris, M.I.; Perfect, J.R.; Chin-Hong, P.V.; Ibrahim, A.S.; Brass, E.P. Risk Factors for Mortality in Patients with Mucormycosis. Med. Mycol. 2012, 50, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Alqarihi, A.; Ibrahim, A.S. Mucormycoses. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 600–612. [Google Scholar] [CrossRef]
- Alqarihi, A.; Kontoyiannis, D.P.; Ibrahim, A.S. Mucormycosis in 2023: An Update on Pathogenesis and Management. Front. Cell Infect. Microbiol. 2023, 13, 1254919. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Lefevre Utile, A.; Jaureguiberry, S.; Angoulvant, A. Gastrointestinal and Intra-Abdominal Mucormycosis in Non-Haematological Patients—A Comprehensive Review. J. Fungi 2025, 11, 298. [Google Scholar] [CrossRef]
- Francis, J.R.; Villanueva, P.; Bryant, P.; Blyth, C.C. Mucormycosis in Children: Review and Recommendations for Management. J. Pediatr. Infect. Dis. Soc. 2018, 7, 159–164. [Google Scholar] [CrossRef]
- Abboud, C.S.; Bergamasco, M.D.; Baía, C.E.S.; Lallée, M.P.; Zan, A.S.C.; Zamorano, M.M.; Pereira, O.I.; Mies, S. Case Report of Hepatic Mucormycosis After Liver Transplantation: Successful Treatment with Liposomal Amphotericin B Followed by Posaconazole Sequential Therapy. Transplant. Proc. 2012, 44, 2501–2502. [Google Scholar] [CrossRef]
- Aceves-Sánchez, B.; Rojas-Castañeda, E.; Ponce-de-León, A.; López-Iñiguez, Á.; Rangel-Cordero, A.; Sánchez, E.; Salgado-Nesme, N.; González-Lara, M.F. Mucormycosis after Liver Transplant: Case Series and Literature Review. Med. Mycol. Case Rep. 2024, 46, 100686. [Google Scholar] [CrossRef]
- Bernardo, R.M.; Gurung, A.; Jain, D.; Malinis, M.F. Therapeutic Challenges of Hepatic Mucormycosis in Hematologic Malignancy: A Case Report and Review of the Literature. Am. J. Case Rep. 2016, 17, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Busca, A.; Marmont, F.; Locatelli, F.; Limerutti, G.; Sorrentino, M.T.; Barbui, A.; Patrono, D.; Salizzoni, M.; David, E.; De Rosa, F. Combined Antifungal Therapy, Iron Chelation and Surgical Resection as Treatment of Hepatic Zygomycosis in a Patient with Haematological Malignancy. Mycoses 2010, 53, 275–278. [Google Scholar] [CrossRef]
- Chaudhary, R.J.; Choudhary, N.S.; Saraf, N.; Gautam, D.; Piplani, T.; Thiagrajan, S.; Bhangui, P.; Saigal, S.; Rastogi, A.; Soin, A.S. Delayed Graft Dysfunction Due to Invasive Hepatic Mucormycosis After Living Donor Liver Transplantation. J. Clin. Exp. Hepatol. 2020, 10, 629–632. [Google Scholar] [CrossRef]
- Czapka, M.; Santos, C.A.Q.; Proia, L.A. Isolated Hepatic Mucormycosis in the Early Post-Transplant Period: A Case Report and Literature Review. OBM Transplant. 2019, 3, 045. [Google Scholar] [CrossRef]
- Deb, S.; Savio, J.; Padaki, P.A. P181 Mucor in the Land of the Liver: A Case Report. Med. Mycol. 2022, 60, myac072P181. [Google Scholar] [CrossRef]
- Ganesh, K.; Abraham, M.A.; Kumar, J.S.; Simon, S. Invasive Fungal Diseases in Renal Transplantation—Case Series. Indian. J. Transplant. 2021, 15, 169–175. [Google Scholar] [CrossRef]
- Le Gac, G.; Allyn, J.; Coolen-Allou, N.; Lagrange-Xelot, M.; Fernandez, C.; Allou, N.; Hoarau, G. Mucormycose Hépatique à Rhizopus Microsporus: Description d’un Cas. Med. Mal. Infect. 2017, 47, 504–507. [Google Scholar] [CrossRef]
- Gali, S.; Rukmangadha, N.; Prayaga, A.; Kumar, V.S. Hepatic Mucormycosis in a Renal Transplant Recipient: A Rare Presentation. Indian. J. Transplant. 2019, 13, 38. [Google Scholar] [CrossRef]
- Gillrie, M.; Chow, B.; Griener, T.; Johnson, A.; Church, D. Hepatosplenic Mucormycosis Due to Rhizomucor Pusillus Identified by Panfungal PCR/Sequencing of Ribosomal ITS2 and LSU Regions in a Patient with Acute Myelogenous Leukemia: A Case Report. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2023, 8, 105–110. [Google Scholar] [CrossRef]
- Grabau, M.; Pandya, S.; Nanjappa, S.; Shenoy, R.; Aslam, S.; Greene, J.N. Liver Abscess in Patients with Leukemia and Prolonged Neutropenia. Infect. Dis. Clin. Pract. 2017, 25, 193–198. [Google Scholar] [CrossRef]
- Karigane, D.; Kikuchi, T.; Sakurai, M.; Kato, J.; Yamane, Y.; Hashida, R.; Abe, R.; Hatano, M.; Hasegawa, N.; Wakayama, M.; et al. Invasive Hepatic Mucormycosis: A Case Report and Review of the Literature. J. Infect. Chemother. 2019, 25, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Kumaran, V.; Jain, D.; Siraj, F.; Aggarwal, S. Hepatic Mucormycosis in a Patient of Acute Lymphoblastic Leukemia: A Case Report with Literature Review. Indian. J. Hematol. Blood Transfus. 2013, 29, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, S.; Govindasamy, M.; Gupta, M.; Siraj, F.; Varma, V.; Mehta, N.; Kumaran, V.; Mohan, N.; Chopra, P.; Arora, A.; et al. Gastrointestinal Mucormycosis—Four Cases with Different Risk Factors, Involving Different Anatomical Sites. Indian. J. Gastroenterol. 2012, 31, 139–143. [Google Scholar] [CrossRef]
- Li, K.-W. Hepatic Mucormycosis Mimicking Hilar Cholangiocarcinoma: A Case Report and Literature Review. World J. Gastroenterol. 2010, 16, 1039. [Google Scholar] [CrossRef]
- Mehta, C.; Ali, M.T.; Mehta, Y.; Anand, J.S.; George, J.V. A Rare Case of Isolated Hepatic Mucormycosis in Association with Hemophagocytosis Syndrome. J. Acute Med. 2016, 6, 98–101. [Google Scholar] [CrossRef][Green Version]
- Mekeel, K.L.; Hemming, A.W.; Reed, A.I.; Matsumoto, T.; Fujita, S.; Schain, D.C.; Nelson, D.R.; Dixon, L.R.; Fujikawa, T. Hepatic Mucormycosis in a Renal Transplant Recipient. Transplantation 2005, 79, 1636. [Google Scholar] [CrossRef]
- Mezhir, J.J.; Mullane, K.M.; Zarling, J.; Satoskar, R.; Pai, R.K.; Roggin, K.K. Successful Nonoperative Management of Gastrointestinal Mucormycosis: Novel Therapy for Invasive Disease. Surg. Infect. 2009, 10, 447–451. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Battiwalla, M.; Hahn, T.; Ball, D.; Paplham, P.; Brown, K.; Segal, B.H.; McCarthy, P.; Almyroudis, N.G. Two Cases of Hepatic Zygomycosis in Allogeneic Stem Cell Transplant Recipients and Review of Literature. Transplant. Infect. Dis. 2007, 9, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, C.; Cruz-Martinez, R.; Quintero-Quintero, M.; Vilatobá, M. Mucormycosis in Liver Allograft Following Transplant for Secondary Biliary Cirrhosis. Exp. Clin. Transplant. 2024, 22, 314–317. [Google Scholar] [PubMed]
- Peng, X.; Wei, Z.; Wang, L.; Cheng, J. Invasive Splenic Mucormycosis Due to Rhizopus Microsporus during Chemotherapy for Acute Monocytic Leukemia: A Case Report and Literature Review. Front. Oncol. 2023, 13, 1237807. [Google Scholar] [CrossRef]
- Oliver, M.R.; Van Voorhis, W.C.; Boeckh, M.; Mattson, D.; Bowden, R.A. Hepatic Mucormycosis in a Bone Marrow Transplant Recipient Who Ingested Naturopathic Medicine. Clin. Infect. Dis. 1996, 22, 521–524. [Google Scholar] [CrossRef]
- Randi, B.A.; de Oliveira, V.F.; Rapozo, M.M.; Higashino, H.R.; Barbaro del Negro, G.M.; Chaves Magri, M.M.; Rocha, V.; Costa, S.F. Fatal Hepatic Mucormycosis in an Allogeneic Hematopoietic-Stem Cell Transplanted Patient: Case Report of a Rare Presentation and Review of the Literature. J. Infect. Chemother. 2025, 31, 102443. [Google Scholar] [CrossRef]
- Reis, F.P.d.; Campos, S.V.; Aiello, V.D.; Duarte, M.I.S.; Samano, M.N.; Pego-Fernandes, P.M. Gastrointestinal Mucormycosis Post Lung Transplantation. Braz. J. Infect. Dis. 2019, 23, 368–370. [Google Scholar] [CrossRef]
- Rivero, A.; Shaughnessy, M.; Oswald, J.; Goodhope, N.; Oethinger, M. Gastrointestinal Mucormycosis by Mucor Indicus: A Report of Two Cases. Med. Mycol. Case Rep. 2025, 47, 100693. [Google Scholar] [CrossRef]
- Sahu, K.K.; Yanamandra, U.; Kakkar, N.; Malhotra, P. Rare Presentation of Mucormycosis in Aplastic Anaemia: Isolated Hepatic Mucormycosis. Mycopathologia 2019, 184, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Kobayashi, K.; Uldry, E.; Demartines, N.; Golshayan, D.; Halkic, N. Rhizomucor Hepatosplenic Abscesses in a Patient with Renal and Pancreatic Transplantation. Ann. R. Coll. Surg. Engl. 2021, 103, e131–e135. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Nel, J.; Almansouri, A.; Van Duin, D.; Gerber, D.A. Combined Medical and Surgical Management of Hepatic Mucormycosis in an Adult with Acute Myeloid Leukemia: Case Report and Review of the Literature. Mycopathologia 2019, 184, 155–158. [Google Scholar] [CrossRef]
- Shen, M.; Li, Q.; Zeng, Z.; Han, D.; Luo, X. Mucor Indicus Caused Disseminated Infection Diagnosed by Metagenomic Next-Generation Sequencing in an Acute Myeloid Leukemia Patient: A Case Report. Front. Cell Infect. Microbiol. 2023, 13, 1089196. [Google Scholar] [CrossRef]
- Su, H.; Thompson, G.R.; Cohen, S.H. Hepatic Mucormycosis with Abscess Formation. Diagn. Microbiol. Infect. Dis. 2012, 73, 192–194. [Google Scholar] [CrossRef]
- Suh, I.W.; Park, C.S.; Lee, M.S.; Lee, J.H.; Chang, M.S.; Woo, J.H.; Lee, I.C.; Ryu, J.S. Hepatic and Small Bowel Mucormycosis after Chemotherapy in a Patient with Acute Lymphocytic Leukemia. J. Korean Med. Sci. 2000, 15, 351. [Google Scholar] [CrossRef]
- Borg, F.T.; Kuijper, E.J.; Van Der Lelie, H. Fatal Mucormycosis Presenting as an Appendiceal Mass with Metastatic Spread to the Liver during Chemotherapy-Induced Granulocytopenia. Scand. J. Infect. Dis. 1990, 22, 499–501. [Google Scholar] [CrossRef]
- Van Sickels, N.; Hoffman, J.; Stuke, L.; Kempe, K. Survival of a Patient with Trauma-Induced Mucormycosis Using an Aggressive Surgical and Medical Approach. J. Trauma Inj. Infect. Crit. Care 2011, 70, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Trenker, C.; Dohse, M.; Metzelder, S.; Rexin, P.; Mariss, J.; Goerg, C. 71-Year-Old Patient with Chronic Lymphocytic Leukemia (CLL) and Hypoechoic Nodular Spleen and Liver Lesions with Fatal Outcome: Presentation of Mucormycosis in B-Mode Imaging and Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2016, 02, E100–E101. [Google Scholar] [CrossRef][Green Version]
- George Tsaousis, A.K. Liver and Brain Mucormycosis in a Diabetic Patient Type II Successfully Treated with Liposomial Amphotericin, B. Scand. J. Infect. Dis. 2000, 32, 335–337. [Google Scholar] [CrossRef]
- Vikum, D.; Nordøy, I.; Torp Andersen, C.; Fevang, B.; Line, P.D.; Kolrud, F.K.; Aukrust, P.; Buanes, T. A Young, Immunocompetent Woman with Small Bowel and Hepatic Mucormycosis Successfully Treated with Aggressive Surgical Debridements and Antifungal Therapy. Case Rep. Infect. Dis. 2017, 2017, 4173246. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, S.; Waqas, O.; Munir, J.; Sultan, F.; Hameed, A. Hatosplenic Mucormycosis Post Autologous Stem Cell Transplant. Pak. J. Med. Sci. 2017, 33, 776. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O. A Global Analysis of Mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54, S35–S43. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef]
- Chamilos, G.; Marom, E.M.; Lewis, R.E.; Lionakis, M.S.; Kontoyiannis, D.P. Predictors of Pulmonary Zygomycosis versus Invasive Pulmonary Aspergillosis in Patients with Cancer. Clin. Infect. Dis. 2005, 41, 60–66. [Google Scholar] [CrossRef]
- Boutin, C.-A.; Durocher, F.; Beauchemin, S.; Ziegler, D.; Abou Chakra, C.N.; Dufresne, S.F. Breakthrough Invasive Fungal Infections in Patients with High-Risk Hematological Disorders Receiving Voriconazole and Posaconazole Prophylaxis: A Systematic Review. Clin. Infect. Dis. 2024, 79, 151–160. [Google Scholar] [CrossRef]
- Caramalho, R.; Tyndall, J.D.A.; Monk, B.C.; Larentis, T.; Lass-Flörl, C.; Lackner, M. Intrinsic Short-Tailed Azole Resistance in Mucormycetes Is Due to an Evolutionary Conserved Aminoacid Substitution of the Lanosterol 14α-Demethylase. Sci. Rep. 2017, 7, 15898. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Breakthrough Invasive Mold Infections in the Hematology Patient: Current Concepts and Future Directions. Clin. Infect. Dis. 2018, 67, 1621–1630. [Google Scholar] [CrossRef]
- Lamaris, G.A.; Ben-Ami, R.; Lewis, R.E.; Chamilos, G.; Samonis, G.; Kontoyiannis, D.P. Increased Virulence of Zygomycetes Organisms Following Exposure to Voriconazole: A Study Involving Fly and Murine Models of Zygomycosis. J. Infect. Dis. 2009, 199, 1399–1406. [Google Scholar] [CrossRef]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and Sites of Involvement of Invasive Fungal Infections in Patients with Haematological Malignancies: A 20-Year Autopsy Study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef]
- Nesher, L.; Rolston, K.V.I. Neutropenic Enterocolitis, a Growing Concern in the Era of Widespread Use of Aggressive Chemotherapy. Clin. Infect. Dis. 2013, 56, 711–717. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global Guideline for the Diagnosis and Management of Mucormycosis: An Initiative of the European Confederation of Medical Mycology in Cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Ismail, M.H.; Hodkinson, H.J.; Setzen, G.; Sofianos, C.; Hale, M.J. Gastric Mucormycosis. Trop. Gastroenterol. 1990, 11, 103–105. [Google Scholar] [PubMed]
- Cole, G.T.; Halawa, A.A.; Anaissie, E.J. The Role of the Gastrointestinal Tract in Hematogenous Candidiasis: From the Laboratory to the Bedside. Clin. Infect. Dis. 1996, 22, S73–S88. [Google Scholar] [CrossRef]
- Masood, A.; Sallah, S. Chronic Disseminated Candidiasis in Patients with Acute Leukemia: Emphasis on Diagnostic Definition and Treatment. Leuk. Res. 2005, 29, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Safiia, J.; Díaz, M.A.; Alshaker, H.; Atallah, C.J.; Sakr, P.; Moshovitis, D.G.; Nawlo, A.; Franceschi, A.E.; Liakos, A.; Koo, S. Recent Advances in Diagnostic Approaches for Mucormycosis. J. Fungi 2024, 10, 727. [Google Scholar] [CrossRef]
- Lamoth, F.; Kontoyiannis, D.P. PCR Diagnostic Platforms for Non-Aspergillus Mold Infections: Ready for Routine Implementation in the Clinic? Expert. Rev. Mol. Diagn. 2024, 24, 273–282. [Google Scholar] [CrossRef]
- Bellanger, A.-P.; Gbaguidi-Haore, H.; Berceanu, A.; Gouzien, L.; El Machhour, C.; Bichard, D.; Lanternier, F.; Scherer, E.; Millon, L.; Chouaki, T.; et al. Use of the Mucorales QPCR on Blood to Screen High-Risk Hematology Patients Is Associated with Better Survival. Med. Mycol. 2024, 62, myae030. [Google Scholar] [CrossRef]
- Legrand, M.; Gits-Muselli, M.; Boutin, L.; Garcia-Hermoso, D.; Maurel, V.; Soussi, S.; Benyamina, M.; Ferry, A.; Chaussard, M.; Hamane, S.; et al. Detection of Circulating Mucorales DNA in Critically Ill Burn Patients: Preliminary Report of a Screening Strategy for Early Diagnosis and Treatment. Clin. Infect. Dis. 2016, 63, 1312–1317. [Google Scholar] [CrossRef]
- Millon, L.; Caillot, D.; Berceanu, A.; Bretagne, S.; Lanternier, F.; Morio, F.; Letscher-Bru, V.; Dalle, F.; Denis, B.; Alanio, A.; et al. Evaluation of Serum Mucorales Polymerase Chain Reaction (PCR) for the Diagnosis of Mucormycoses: The MODIMUCOR Prospective Trial. Clin. Infect. Dis. 2022, 75, 777–785. [Google Scholar] [CrossRef]
- Moreno, A.; Mah, J.; Budvytiene, I.; Ho, D.Y.; Schwenk, H.T.; Banaei, N. Dynamics and Prognostic Value of Plasma Cell-Free DNA PCR in Patients with Invasive Aspergillosis and Mucormycosis. J. Clin. Microbiol. 2024, 62, e00394-24. [Google Scholar] [CrossRef] [PubMed]
- Lieu, A.; Zimmet, A.N.; Pozdol, J.; Kushner, L.E.; Ho, D.; Banaei, N. Concordance of Non-Invasive Plasma Cell-Free DNA with Invasive Diagnostics for Diagnosis of Invasive Fungal Disease. Clin. Infect. Dis. 2025, ciaf021. [Google Scholar] [CrossRef] [PubMed]
- Huygens, S.; Schauwvlieghe, A.; Wlazlo, N.; Moors, I.; Boelens, J.; Reynders, M.; Chong, G.-L.; Klaassen, C.H.W.; Rijnders, B.J.A. Diagnostic Value of Microbial Cell-Free DNA Sequencing for Suspected Invasive Fungal Infections: A Retrospective Multicenter Cohort Study. Open Forum Infect. Dis. 2024, 11, ofae252. [Google Scholar] [CrossRef]
- Babady, N.E.; Chiu, C.Y.; Craney, A.; Gaston, D.C.; Hicklen, R.S.; Hogan, C.A.; John, T.M.; Stewart, A.G. Diagnosis and Management of Invasive Fungal Diseases by Next-Generation Sequencing: Are We There Yet? Expert. Rev. Mol. Diagn. 2024, 24, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E. How I Treat Mucormycosis. Blood 2011, 118, 1216–1224. [Google Scholar] [CrossRef]
- Sigera, L.S.M.; Denning, D.W. A Systematic Review of the Therapeutic Outcome of Mucormycosis. Open Forum Infect. Dis. 2024, 11, ofad704. [Google Scholar] [CrossRef]
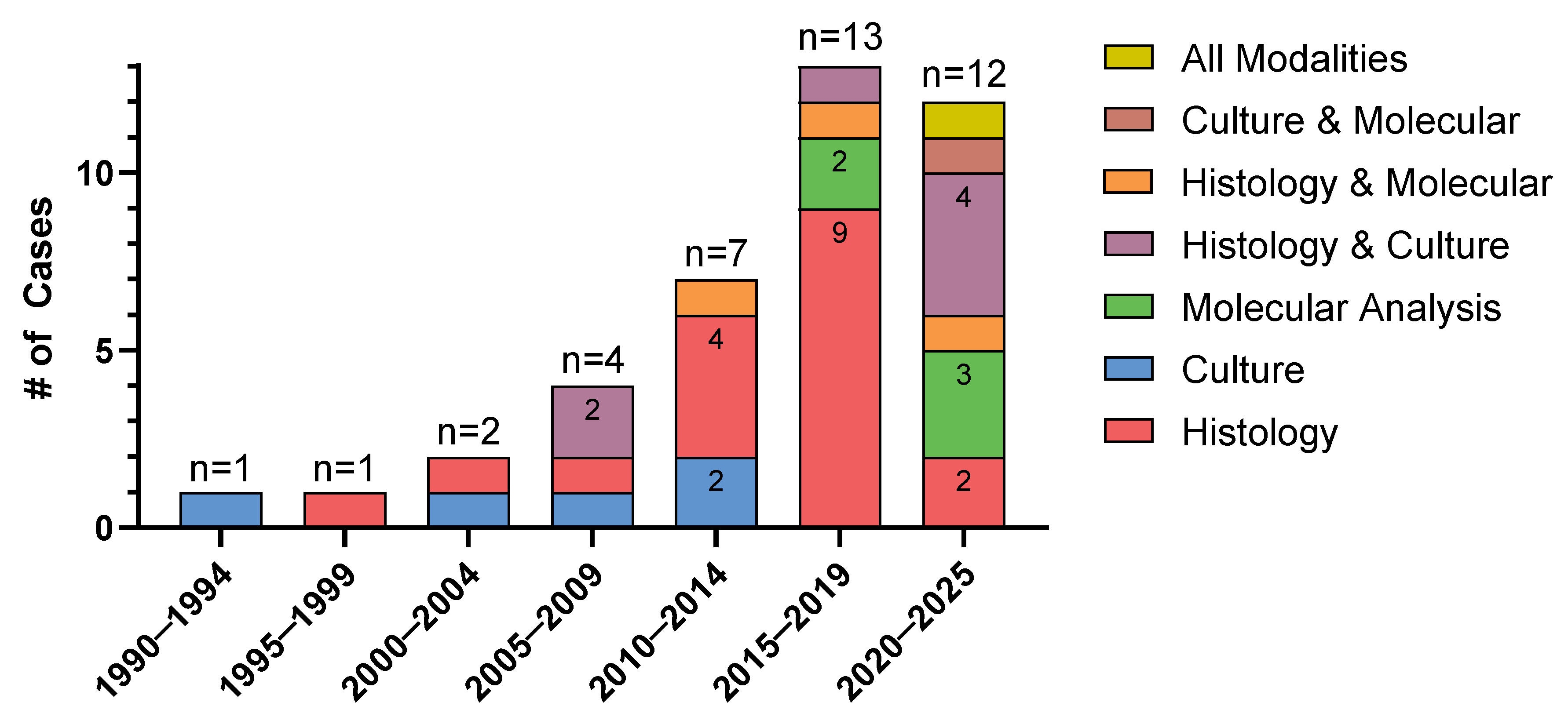
| Demographics | |
| Sex, male | 29 (73%) |
| Age, median years (range) | 39 (19–75) |
| Underlying conditions a | |
| Hematologic malignancy | 22 (55%) |
| Acute myeloid leukemia | 10 |
| Acute lymphoblastic leukemia | 5 |
| Lymphoma | 3 |
| Myelodysplastic syndrome | 2 |
| Chronic lymphocytic leukemia | 1 |
| Myelofibrosis | 1 |
| Solid organ transplant | 12 (30%) |
| Renal | 5 |
| Liver | 5 |
| Pulmonary | 1 |
| Pancreatic and renal | 1 |
| Presenting symptoms | |
| Fever | 28 (70%) |
| Abdominal pain | 25 (63%) |
| Elevated liver function test | 14 (35%) |
| Antifungal prophylaxis | 11 (28%) |
| Fluconazole | 6 |
| Voriconazole | 2 |
| Posaconazole | 2 |
| Ketoconazole | 1 |
| Hepatic imaging b | |
| Solitary lesion | 11/39 (28%) |
| Multiple lesions | 28/39 (72%) |
| Disseminated disease | |
| Clinical and/or radiologic suspicion of extrahepatic manifestations c | 18 (45%) |
| No suspicious findings for extrahepatic involvement | 9 (23%) |
| No extra-hepatic intra-abdominal pathology noted and no extra-abdominal imaging performed | 11 (28%) |
| No data on extra-hepatic manifestations | 2 (5%) |
| Diagnosis of mucormycosis (some patients had multiple overlapping methods) | |
| Histopathology | 29 (73%) |
| Molecular | 10 (25%) |
| Culture | 14 (36%) |
| Mucorales genus identified (including via molecular methods) | |
| Rhizopus | 8 (20%) |
| Rhizomucor | 4 (10%) |
| Mucor | 4 (10%) |
| Lichtheimia | 1 (3%) |
| Co-infections | |
| Co-pathogen identified d | 8/29 (28%) |
| Therapy | |
| Mucorales-active antifungals as part of initial therapy | 2 (5%) |
| Mucorales-active antifungal therapy at some point during hospitalization | 34 (85%) |
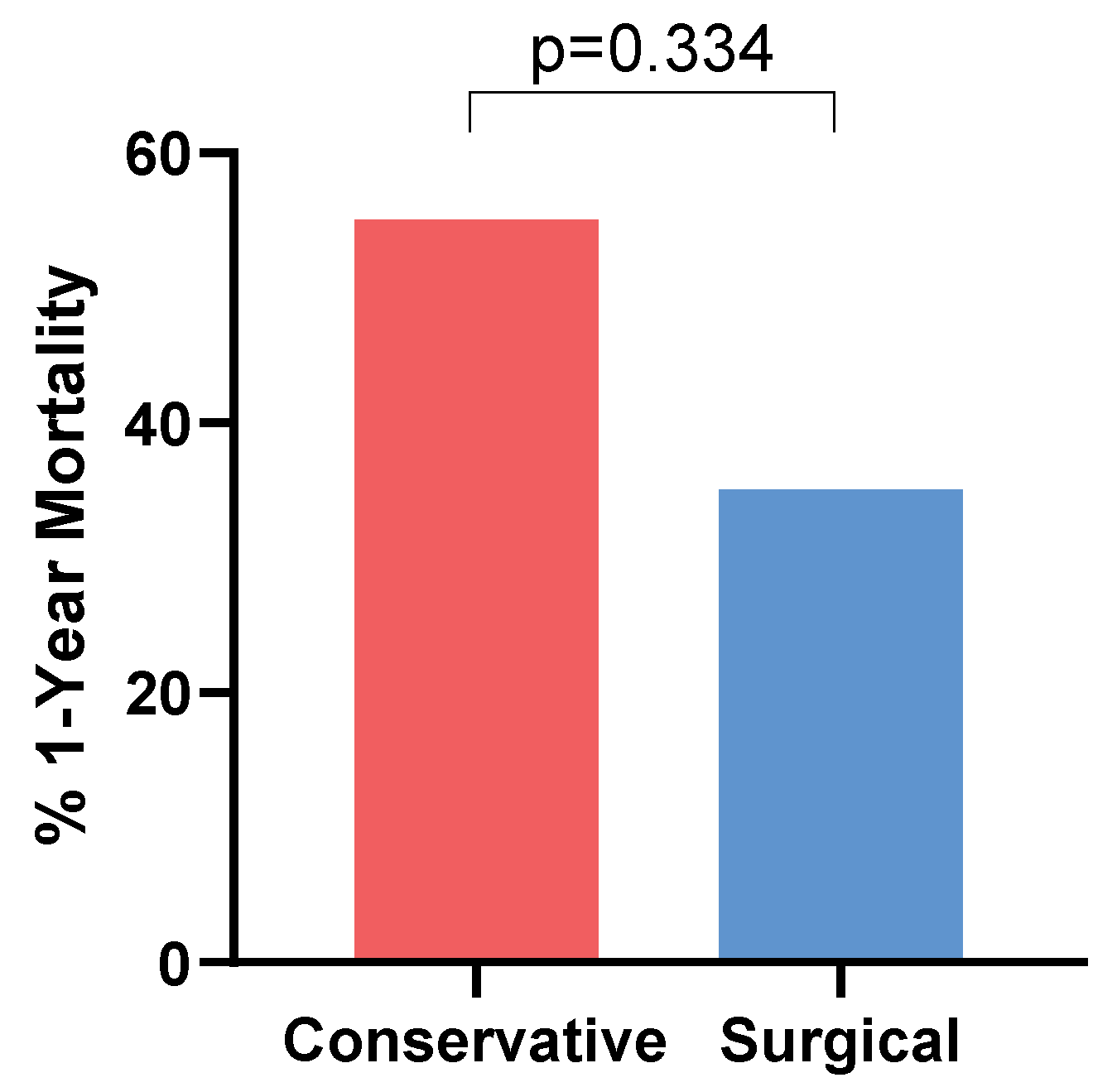
| Surgery | 18 (45%) |
| Outcomes | |
| Radiographic resolution e | 11/19 (58%) |
| 1-year all-cause mortality f | 18/39 (46%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allos, H.; Hicklen, R.S.; Matsuo, T.; Adachi, J.; Wurster, S.; Kontoyiannis, D.P. The Heterogenous Presentation of Hepatic Mucormycosis in Adults: A Case Report and Review of the Literature. J. Fungi 2025, 11, 408. https://doi.org/10.3390/jof11060408
Allos H, Hicklen RS, Matsuo T, Adachi J, Wurster S, Kontoyiannis DP. The Heterogenous Presentation of Hepatic Mucormycosis in Adults: A Case Report and Review of the Literature. Journal of Fungi. 2025; 11(6):408. https://doi.org/10.3390/jof11060408
Chicago/Turabian StyleAllos, Hazim, Rachel S. Hicklen, Takahiro Matsuo, Javier Adachi, Sebastian Wurster, and Dimitrios P. Kontoyiannis. 2025. "The Heterogenous Presentation of Hepatic Mucormycosis in Adults: A Case Report and Review of the Literature" Journal of Fungi 11, no. 6: 408. https://doi.org/10.3390/jof11060408
APA StyleAllos, H., Hicklen, R. S., Matsuo, T., Adachi, J., Wurster, S., & Kontoyiannis, D. P. (2025). The Heterogenous Presentation of Hepatic Mucormycosis in Adults: A Case Report and Review of the Literature. Journal of Fungi, 11(6), 408. https://doi.org/10.3390/jof11060408







