Fusarium musae Infection in Animal and Plant Hosts Confirms Its Cross-Kingdom Pathogenicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Temperature Assay In Vitro
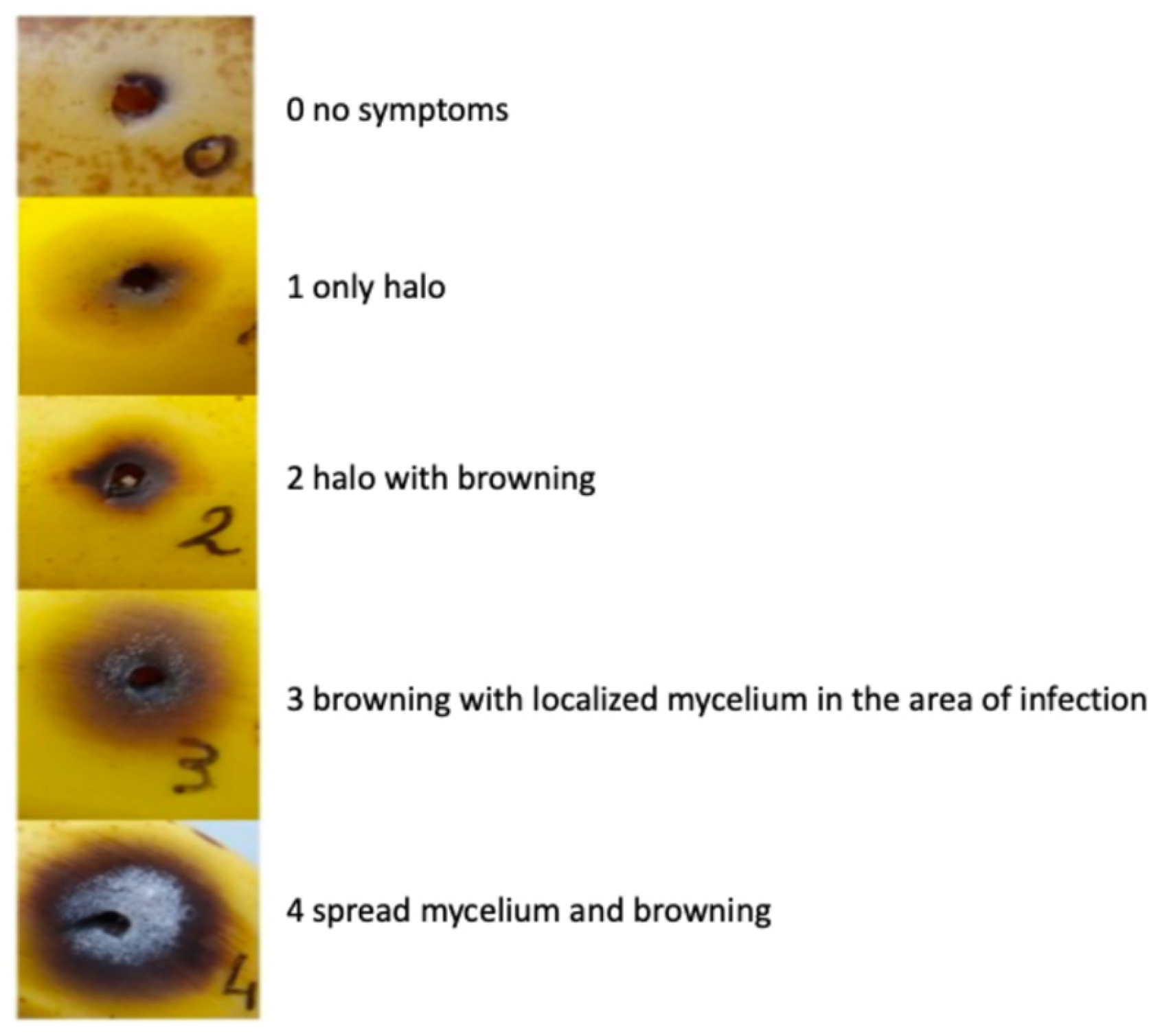
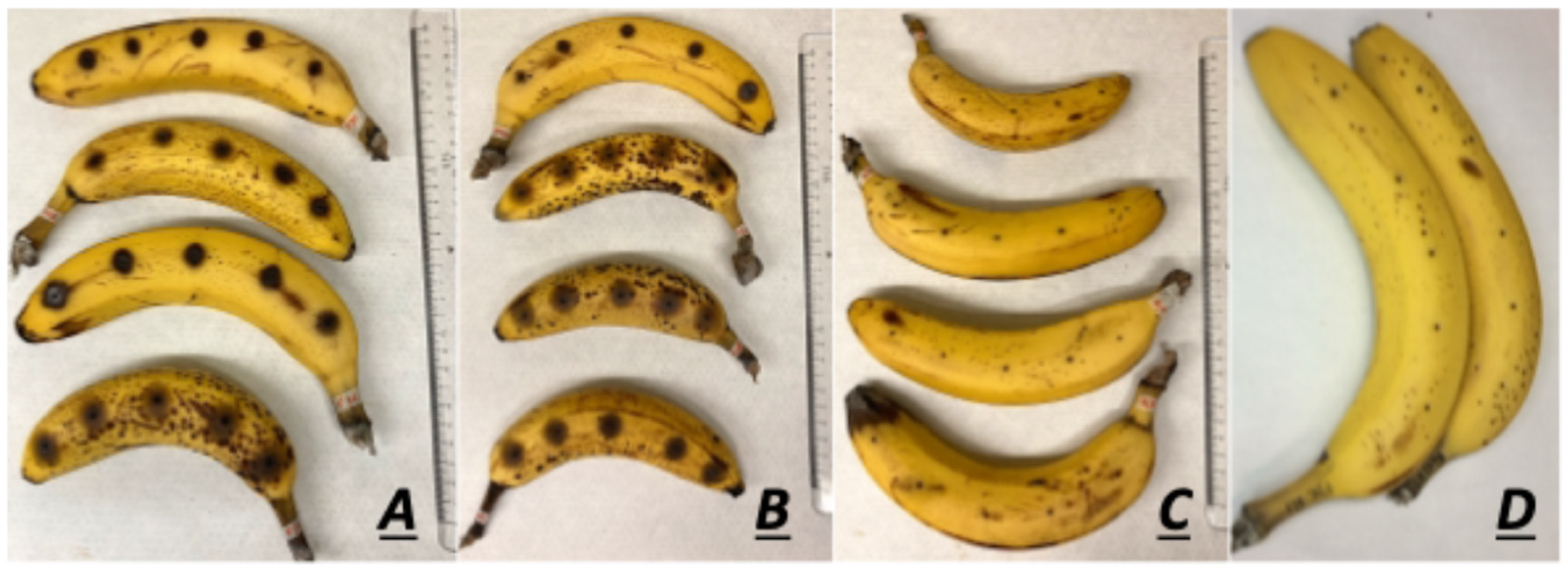
2.3. Banana Fruit Infection
2.4. G. mellonella Larvae Infection Model
2.5. Temperature Assay in G. mellonella Larvae
2.6. Infection Assay in G. mellonella Larvae
2.7. Statistical Analysis
3. Results
3.1. F. musae Can Grow at 24 °C as Well as at 37 °C
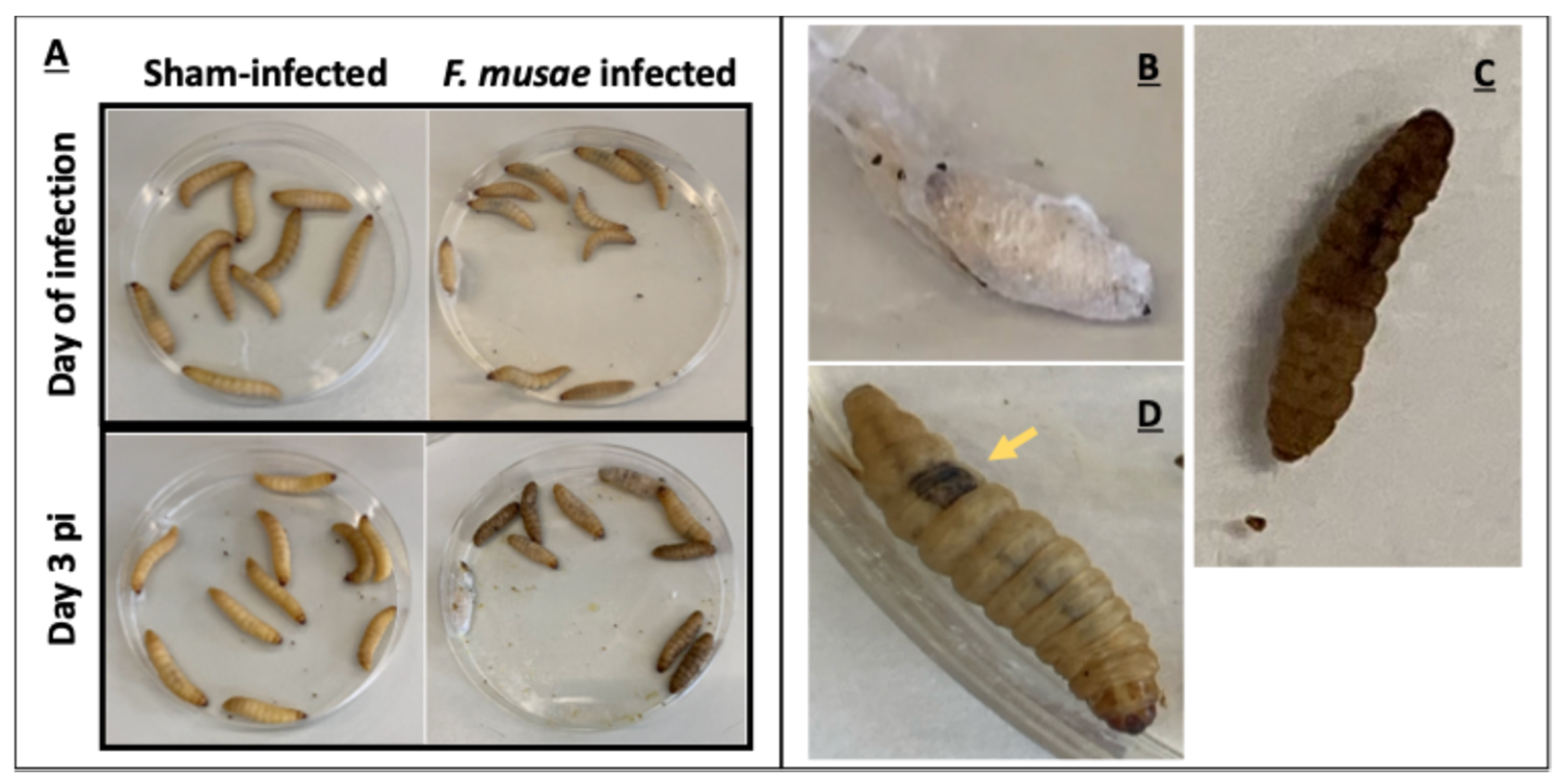
3.2. F. musae Is Pathogenic to Banana Fruits
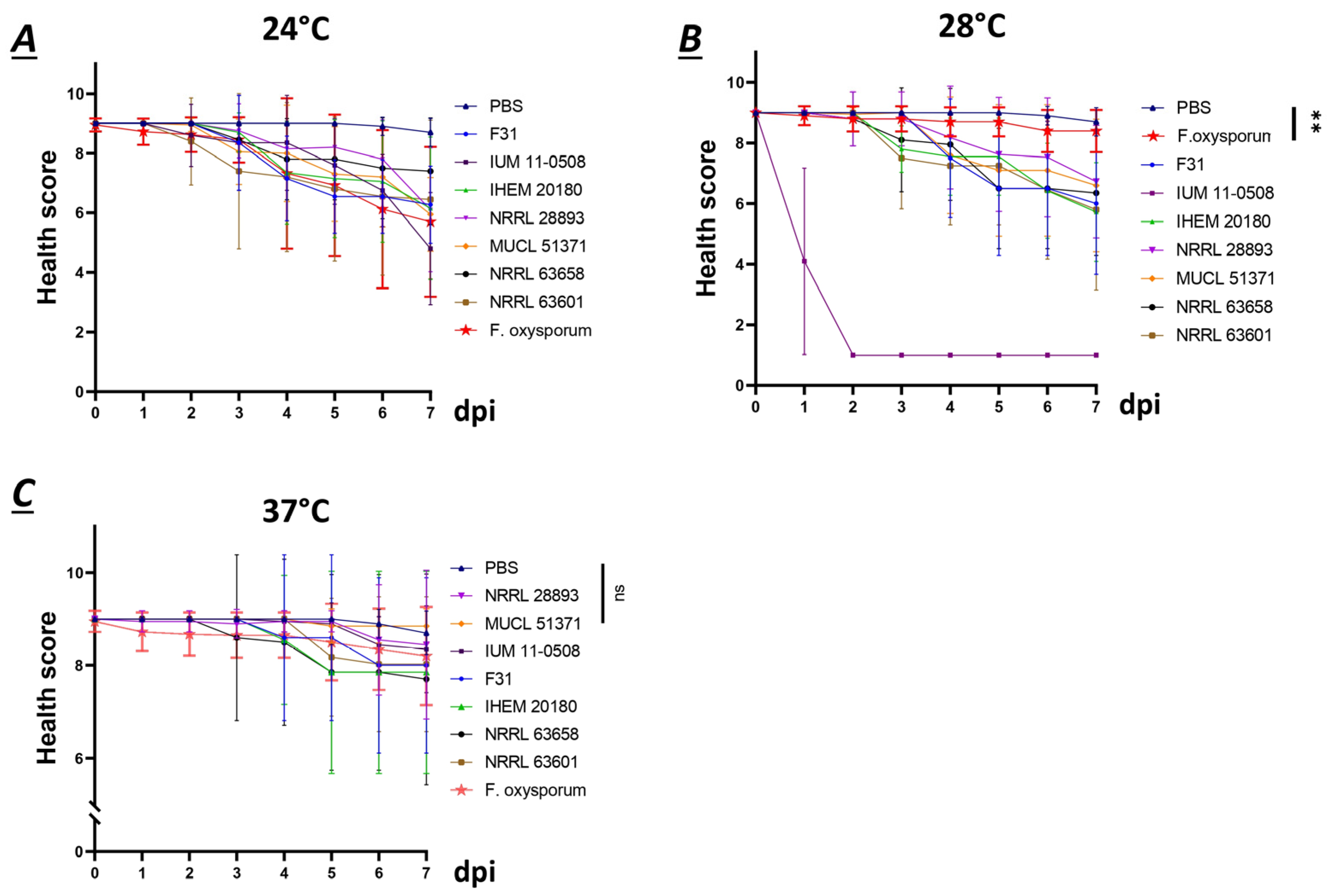
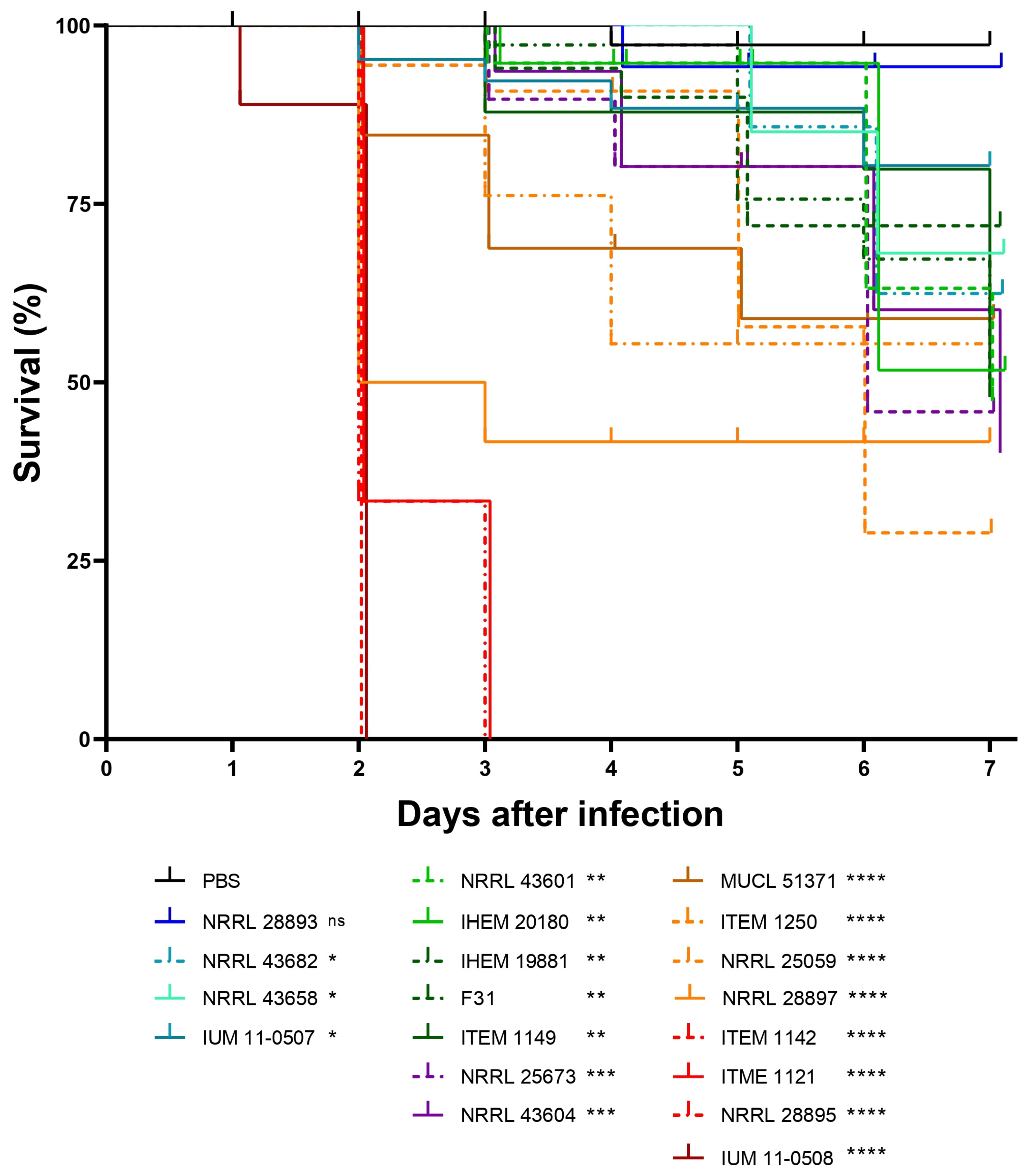
3.3. F. musae Causes Observable Disease in Larvae of G. mellonella at Environmental and Mammalian Physiological Body Temperature
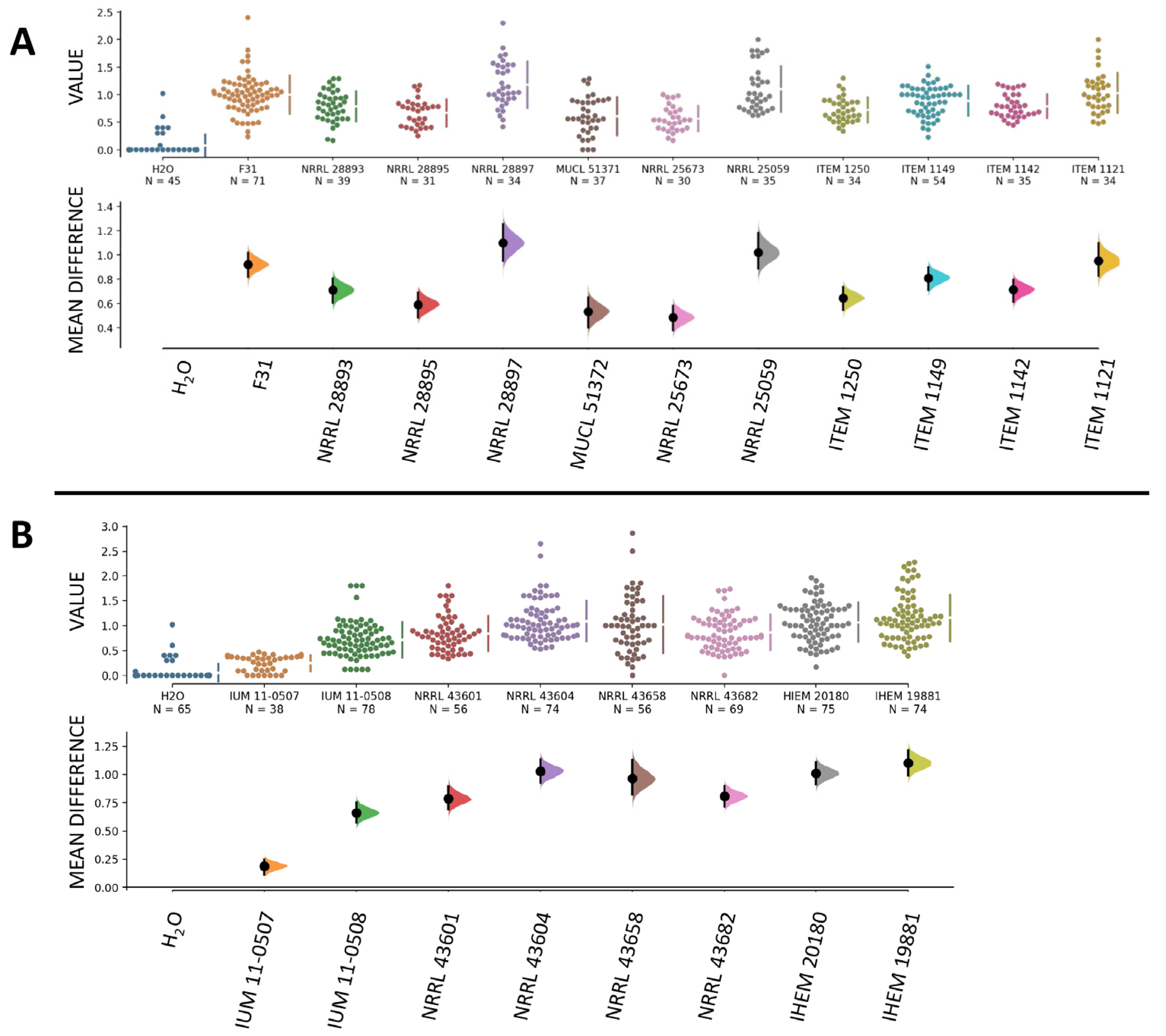
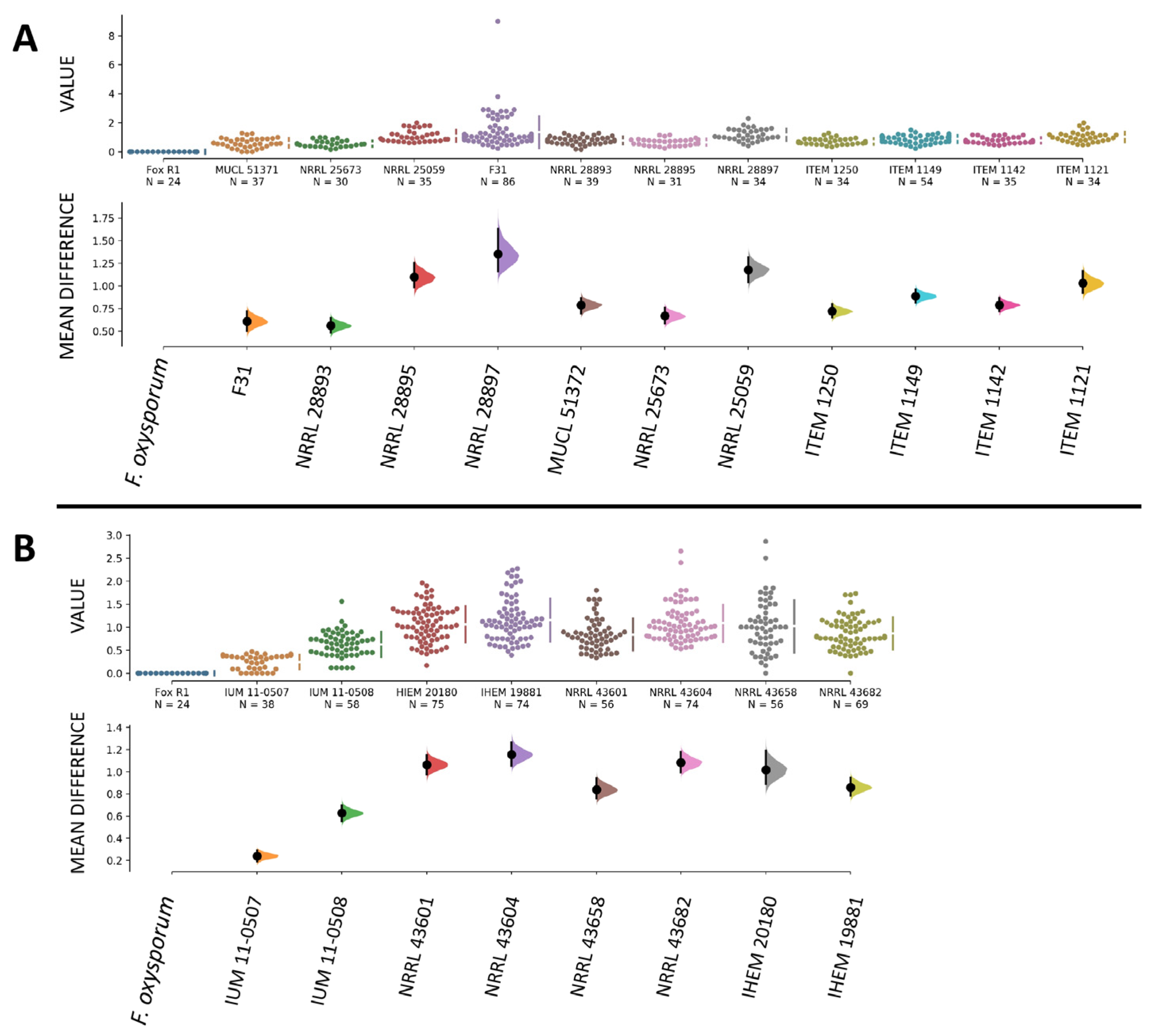
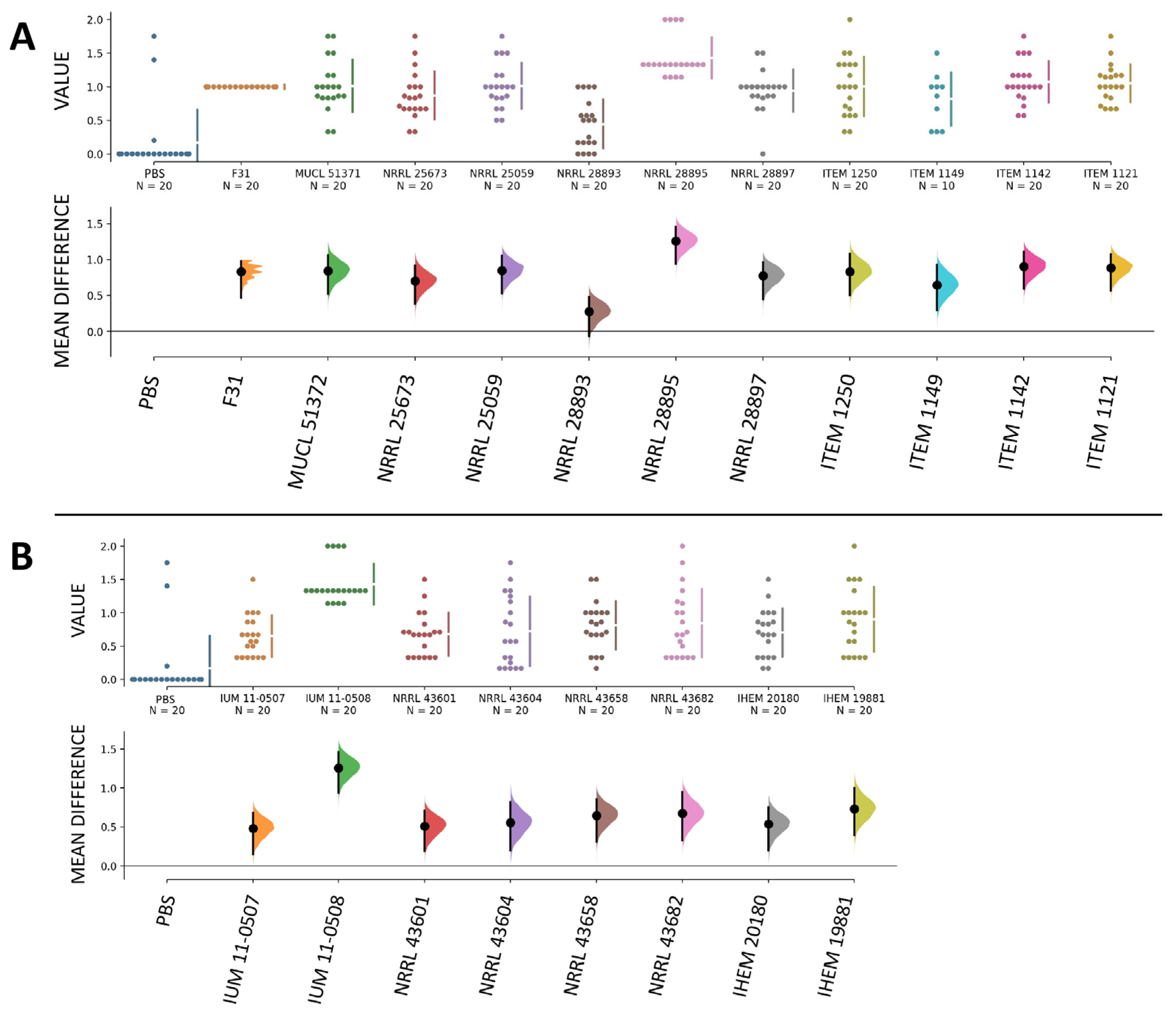
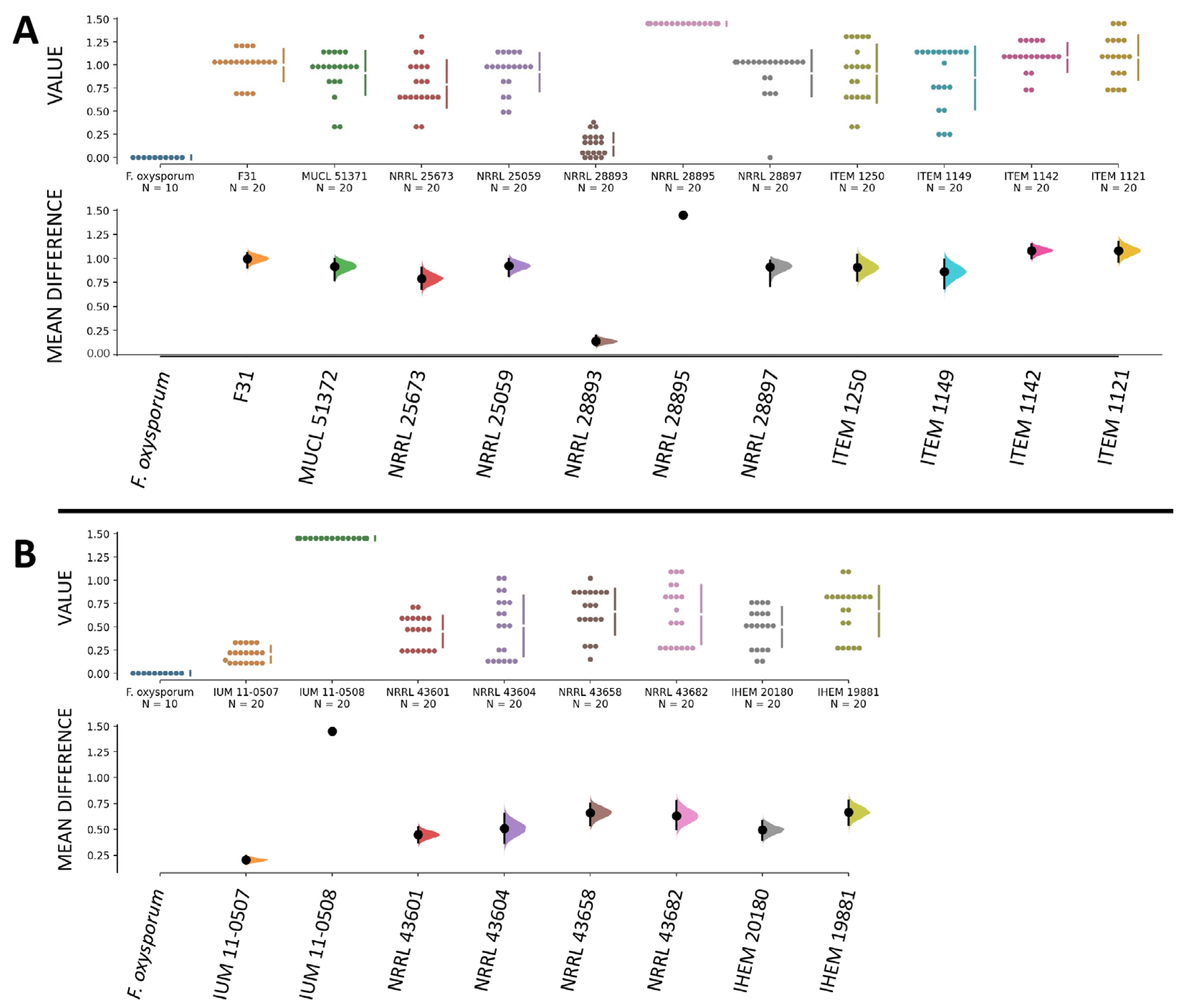
3.4. Human and Plant Strains of F. musae Cause Comparable Levels of Infection in G. mellonella Larvae
3.5. Survival Readouts of G. mellonella Larvae Infected with Human and Plant Strains of F. musae
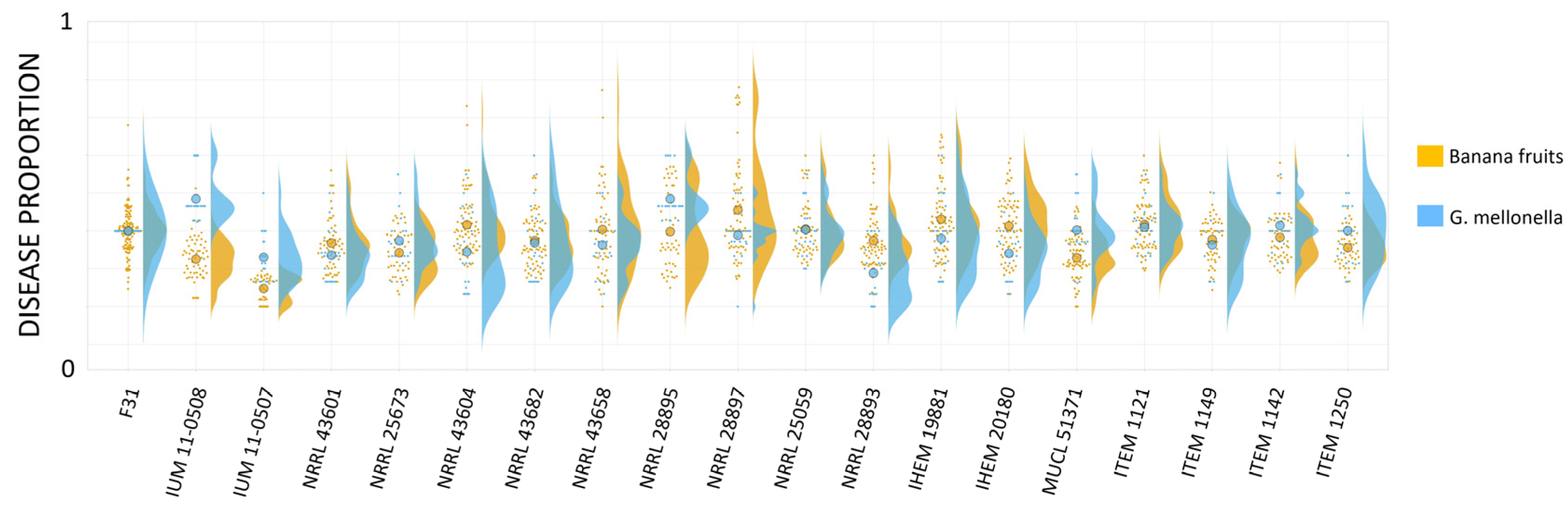
3.6. Quantitative Comparison of Data from Bananas and Larvae Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Hove, F.; Waalwijk, C.; Logrieco, A.; Munaut, F.; Moretti, A. Gibberella musae (Fusarium musae) sp. nov., a recently discovered species from banana is sister to F. verticillioides. Mycologia 2011, 103, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Molnár, O.; Bartók, T.; Szécsi, Á. Occurrence of Fusarium verticillioides and Fusarium musae on banana fruits marketed in Hungary. Acta Microbiol. Immunol. Hung. 2015, 62, 109–119. [Google Scholar] [CrossRef][Green Version]
- Kamel, M.A.M.; Cortesi, P.; Saracchi, M. Etiological agents of crown rot of organic bananas in Dominican Republic. Postharvest Biol. Technol. 2016, 120, 112–120. [Google Scholar] [CrossRef]
- Hirata, T.; Kimishima, E.; Aoki, T.; Nirenberg, H.I.; O’Donnell, K. Morphological and molecular characterization of Fusarium verticillioides from rotten banana imported into Japan. Mycoscience 2001, 42, 155–166. [Google Scholar] [CrossRef]
- Shi, W.; Tan, Y.; Wang, S.; Gardiner, D.M.; De Saeger, S.; Liao, Y.; Wang, C.; Fan, Y.; Wang, Z.; Wu, A. Mycotoxigenic potentials of Fusarium species in various culture matrices revealed by mycotoxin profiling. Toxins 2017, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Esposto, M.C.; Prigitano, A.; Tortorano, A.M. Fusarium musae as cause of superficial and deep-seated human infections. J. Mycol. Médicale 2016, 26, 403–405. [Google Scholar] [CrossRef]
- Triest, D. Banana fruits affected by Fusarium post-harvest disease as source of human fusariosis. Acta Microbiol. Immunol. Hung. 2016, 63, 359–360. [Google Scholar] [CrossRef]
- Triest, D.; Hendrickx, M. Postharvest disease of banana caused by Fusarium musae: A public health concern? PLoS Pathog. 2016, 12, e1005940. [Google Scholar] [CrossRef]
- Triest, D.; Piérard, D.; De Cremer, K.; Hendrickx, M. Fusarium musae infected banana fruits as potential source of human fusariosis: May occur more frequently than we might think and hypotheses about infection. Commun. Integr. Biol. 2016, 9, e1162934. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sarver, B.A.J.; Brandt, M.; Chang, D.C.; Noble-Wang, J.; Park, B.J.; Sutton, D.A.; Benjamin, L.; Lindsley, M.; Padhye, A.; et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 2007, 45, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.J. Koch’s Postulates—Then and Now. Microbe Mag. 2006, 1, 223–228. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phillips, A.J.L.; Jayawardena, R.S.; Promputtha, I.; Hyde, K.D. Importance of molecular data to identify fungal plant pathogens and guidelines for pathogenicity testing based on Koch’s postulates. Pathogens 2021, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Van Baarlen, P.; Van Belkum, A.; Summerbell, R.C.; Crous, P.W.; Thomma, B.P.H.J. Molecular mechanisms of pathogenicity: How do pathogenic microorganisms develop cross-kingdom host jumps? FEMS Microbiol. Rev. 2007, 31, 239–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Yoon, S.-J.; Park, Y.-J.; Kim, S.-Y.; Ryu, C.-M. Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 2020, 22, 2485–2495. [Google Scholar] [CrossRef]
- Fallon, J.; Kelly, J.; Kavanagh, K. Galleria mellonella as a model for fungal pathogenicity testing. In Host-Fungus Interactions; Brand, A.C., MacCallum, D.M., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 845, pp. 469–485. [Google Scholar] [CrossRef]
- Pereira, T.C.; Rossoni, R.D.; Ribeiro, F.d.C.; de Menezes, R.T.; Junqueira, J.C.; Scorzoni, L. Recent Advances in the Use of Galleria mellonella model to study immune responses against human pathogens. J. Fungi 2018, 4, 128. [Google Scholar] [CrossRef]
- Singkum, P.; Suwanmanee, S.; Pumeesat, P.; Luplertlop, N. A powerful in vivo alternative model in scientific research: Galleria mellonella. Acta Microbiol. Immunol. Hung. 2019, 66, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Vanhoffelen, E.; Michiels, L.; Brock, M.; Lagrou, K.; Reséndiz-Sharpe, A.; Vande Velde, G. Powerful and Real-Time Quantification of Antifungal Efficacy against Triazole-Resistant and-Susceptible Aspergillus fumigatus Infections in Galleria mellonella by longitudinal bioluminescence imaging. Microbiol. Spectr. 2023, 11, e00825-23. [Google Scholar] [CrossRef] [PubMed]
- Vanhoffelen, E.; Vermoesen, L.; Michiels, L.; Lagrou, K.; Reséndiz-Sharpe, A.; Vande Velde, G. Sensitive bioluminescence imaging of cryptococcosis in Galleria mellonella improves antifungal screening under in vivo conditions. Virulence 2024, 15, 2327883. [Google Scholar] [CrossRef]
- Moretti, A.; Mule, G.; Susca, A.; González-Jaén, M.; Logrieco, A. Toxin Profile, Fertility and AFLP Analysis of Fusarium verticillioides from Banana Fruits. Eur. J. Plant Pathol. 2004, 110, 601–609. [Google Scholar] [CrossRef]
- Loh, J.M.; Adenwalla, N.; Wiles, S.; Proft, T. Galleria mellonella larvae as an infection model for group A streptococcus. Virulence 2013, 4, 419–428. [Google Scholar] [CrossRef]
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond P Values: Data Analysis with Estimation Graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, J. SuperPlotsOfData—A web app for the transparent display and quantitative comparison of continuous data from different conditions. Mol. Biol. Cell 2021, 32, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.M.; Keller, N.P. Crossover fungal pathogens: The biology and pathogenesis of fungi capable of crossing kingdoms to infect plants and humans. Fungal Genet. Biol. 2013, 61, 146–157. [Google Scholar] [CrossRef]
- Tava, V.; Prigitano, A.; Cortesi, P.; Esposto, M.C.; Pasquali, M. Fusarium musae from diseased bananas and human patients: Susceptibility to fungicides used in clinical and agricultural settings. J. Fungi 2021, 7, 784. [Google Scholar] [CrossRef]
- Degradi, L.; Tava, V.; Prigitano, A.; Esposto, M.C.; Tortorano, A.M.; Saracchi, M.; Kunova, A.; Cortesi, P.; Pasquali, M. Exploring mitogenomes diversity of Fusarium musae from banana fruits and human patients. Microorganisms 2022, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Degradi, L.; Tava, V.; Kunova, A.; Cortesi, P.; Saracchi, M.; Pasquali, M. Telomere to telomere genome assembly of Fusarium musae F31, causal agent of crown rot disease of banana. Mol. Plant Microbe Interact. 2021, 34, 1455–1457. [Google Scholar] [CrossRef] [PubMed]
- Capilla, J.; Clemons, K.V.; Stevens, D.A. Animal models: An important tool in mycology. Med. Mycol. 2007, 45, 657–684. [Google Scholar] [CrossRef]
- Fuchs, B.B.; O’Brien, E.; El Khoury, J.B.; Mylonakis, E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010, 1, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Desalermos, A.; Fuchs, B.B.; Mylonakis, E. Selecting an invertebrate model host for the study of fungal pathogenesis. PLoS Pathog. 2012, 8, e1002451. [Google Scholar] [CrossRef]
- Swearengen, J.R. Choosing the right animal model for infectious disease research. Anim. Models Exp. Med. 2018, 1, 100–108. [Google Scholar] [CrossRef]
- Junqueira, J.; Mylonakis, E. Current status and trends in alternative models to study fungal pathogens. J. Fungi 2019, 5, 12. [Google Scholar] [CrossRef]
- Brennan, M.; Thomas, D.Y.; Whiteway, M.; Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002, 34, 153–157. [Google Scholar] [CrossRef]
- Mylonakis, E.; Moreno, R.; El Khoury, J.B.; Idnurm, A.; Heitman, J.; Calderwood, S.B.; Ausubel, F.M.; Diener, A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 2005, 73, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.; Fallon, J.P. Galleria mellonella larvae as models for studying fungal virulence. Fungal Biol. Rev. 2010, 24, 79–83. [Google Scholar] [CrossRef]
- Slater, J.L.; Gregson, L.; Denning, D.W.; Warn, P.A. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med. Mycol. 2011, 49, S107–S113. [Google Scholar] [CrossRef] [PubMed]
- Trevijano-Contador, N.; Zaragoza, O. Immune response of Galleria mellonella against human fungal pathogens. J. Fungi 2018, 5, 3. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Forastiero, A.; Cendejas-Bueno, E.; Gregson, L.; Mellado, E.; Howard, S.; Livermore, J.; Hope, W.; Cuenca-Estrella, M. An invertebrate model to evaluate virulence in Aspergillus fumigatus: The role of azole resistance. Med. Mycol. 2014, 52, 311–319. [Google Scholar] [CrossRef]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Galleria mellonella: An invertebrate model to study pathogenicity in correctly defined fungal species. Fungal Biol. 2016, 120, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Champion, O.L.; Wagley, S.; Titball, R.W. Galleria mellonella as a model host for microbiological and toxin research. Virulence 2016, 7, 840–845. [Google Scholar] [CrossRef]
- Champion, O.L.; Titball, R.W.; Bates, S. Standardization of G. mellonella larvae to provide reliable and reproducible results in the study of fungal pathogens. J. Fungi 2018, 4, 108. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Whitten, M.M.A.; Tyurin, M.V.; Ficken, K.J.; Greig, C.; Melo, N.R.; Glupov, V.V.; Dubovskiy, I.M.; Butt, T.M. Fungal infection dynamics in response to temperature in the lepidopteran insect Galleria mellonella. Insect Sci. 2018, 25, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, S.K.; Alvarado, M.; Rodríguez, Y.M.; Parra-Giraldo, C.M.; Varón, C.; Morales-López, S.E.; Rodríguez, J.Y.; Gómez, B.L.; Escandón, P. Pathogenicity Assessment of Colombian Strains of Candida auris in the Galleria mellonella Invertebrate Model. J. Fungi 2021, 7, 401. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Muhammed, M.; Kasperkovitz, P.V.; Vyas, J.M.; Mylonakis, E. Fusarium pathogenesis investigated using Galleria mellonella as a heterologous host. Fungal Biol. 2011, 115, 1279–1289. [Google Scholar] [CrossRef]
- Navarro Velasco, G.; Prados-Rosales, R.; Ortiz-Urquiza, A.; Quesada-Moraga, E.; Pietro, A. Galleria mellonella as model host for the trans-kingdom pathogen Fusarium oxysporum. Fungal Genet. Biol. 2011, 48, 1124–1129. [Google Scholar] [CrossRef]
- Khalaf, M. Screening of Fusarium isolates pathogenicity in vitro by using the larvae of Galleria mellonella L. Decis. Sci. 2023, 38, 19–28. [Google Scholar]
- Kessel, L.; Johnson, L.; Arvidsson, H.; Larsen, M. The relationship between body and ambient temperature and corneal temperature. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6593–6597. [Google Scholar] [CrossRef]
- Keizer, E.M.; Valdes, I.D.; Forn-Cuni, G.; Klijn, E.; Meijer, A.H.; Hillman, F.; Wösten, H.A.B.; de Cock, H. Variation of virulence of five Aspergillus fumigatus isolates in four different infection models. PLoS ONE 2021, 16, e0252948. [Google Scholar] [CrossRef]
- Binder, U.; Arastehfar, A.; Schnegg, L.; Hörtnagl, C.; Hilmioğlu-Polat, S.; Perlin, D.S.; Lass-Flörl, C. Efficacy of LAMB against emerging azole- and multidrug-resistant Candida parapsilosis isolates in the Galleria mellonella model. J. Fungi 2020, 6, 377. [Google Scholar] [CrossRef]
- Sexton, A.C.; Howlett, B.J. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot. Cell 2006, 5, 1941–1949. [Google Scholar] [CrossRef]
- Wright, C.L.; Kavanagh, O. Galleria mellonella as a novel in vivo model to screen natural product-derived modulators of innate immunity. Appl. Sci. 2022, 12, 6587. [Google Scholar] [CrossRef]
- Nag, P.; Paul, S.; Shriti, S.; Das, S. Defence response in plants and animals against a common fungal pathogen, Fusarium oxysporum. Curr. Res. Microb. Sci. 2022, 3, 100135. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, V.; Alvarez-Moreno, C.; Pape, P.L.; Restrepo, S.; Guarro, J.; Ramírez, A.M.C. A One Health perspective to recognize Fusarium as important in clinical practice. J. Fungi 2020, 6, 235. [Google Scholar] [CrossRef] [PubMed]
| Strain | Country | Host (Tissue) | Reference |
|---|---|---|---|
| F31 f (DSMZ 112727) | Dominican Republic | Banana (fruit) | [3] |
| IUM 11-0507 f | Greece | Human (blood) | [6] |
| IUM 11-0508 f | Greece | Human (cornea) | [6] |
| NRRL 28893 a | Mexico | Banana (fruit) | [1] |
| NRRL 28895 a | Mexico | Banana | [4] |
| NRRL 28897 a | Mexico | Banana | [4] |
| NRRL 43601 b | Maryland, USA | Human (skin) | [10] |
| NRRL 43604 b | Ohio, USA | Human (nasal sinus) | [10] |
| NRRL 43658 b | Minnesota, USA | Human (contact lens) | [10] |
| NRRL 43682 b | Minnesota, USA | Human (cornea) | [10] |
| NRRL 25673 a | Guatemala | Banana (fruit) | [1] |
| (MUCL 53204) | |||
| NRRL 25059 a | Honduras | Banana (fruit) | [1] |
| (CBS 624.87, MUCL 52574) | |||
| IHEM 20180 c | Brussels, Belgium | Human (sinus biopsy) | [5] |
| IHEM 19881 c | Brest, France | Human (shoulder biopsy) | [9] |
| ITEM 1121 e | Panama | Banana (fruit) | [9] |
| (MUCL 52573) | |||
| ITEM 1142 e | Ecuador | Banana (fruit) | [1] |
| (MUCL 53196) | |||
| ITEM 1149 e | Panama | Banana (fruit) | [1] |
| (MUCL 52201) | |||
| ITEM 1250 e | Canary Islands | Banana (fruit) | [1] |
| (MUCL 53203) | |||
| MUCL 51371 d | Philippines | Banana (fruit) | [1] |
| Category | Description | Score |
|---|---|---|
| Activity | No activity | 0 |
| Minimal activity on stimulation | 1 | |
| Active when stimulated | 2 | |
| Active without stimulation | 3 | |
| Cocoon formation | No cocoon | 0 |
| Partial cocoon | 0.5 | |
| Full cocoon | 1 | |
| Melanization | Complete melanization (black) | 0 |
| Dark spots on brown wax worm | 1 | |
| >3 spots on beige wax worm | 2 | |
| <3 spots on beige wax worm | 3 | |
| No melanization | 4 | |
| Survival | Dead | 0 |
| Alive | 1 | |
| TOTAL | SUM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tava, V.; Reséndiz-Sharpe, A.; Vanhoffelen, E.; Saracchi, M.; Cortesi, P.; Lagrou, K.; Velde, G.V.; Pasquali, M. Fusarium musae Infection in Animal and Plant Hosts Confirms Its Cross-Kingdom Pathogenicity. J. Fungi 2025, 11, 90. https://doi.org/10.3390/jof11020090
Tava V, Reséndiz-Sharpe A, Vanhoffelen E, Saracchi M, Cortesi P, Lagrou K, Velde GV, Pasquali M. Fusarium musae Infection in Animal and Plant Hosts Confirms Its Cross-Kingdom Pathogenicity. Journal of Fungi. 2025; 11(2):90. https://doi.org/10.3390/jof11020090
Chicago/Turabian StyleTava, Valeria, Agustin Reséndiz-Sharpe, Eliane Vanhoffelen, Marco Saracchi, Paolo Cortesi, Katrien Lagrou, Greetje Vande Velde, and Matias Pasquali. 2025. "Fusarium musae Infection in Animal and Plant Hosts Confirms Its Cross-Kingdom Pathogenicity" Journal of Fungi 11, no. 2: 90. https://doi.org/10.3390/jof11020090
APA StyleTava, V., Reséndiz-Sharpe, A., Vanhoffelen, E., Saracchi, M., Cortesi, P., Lagrou, K., Velde, G. V., & Pasquali, M. (2025). Fusarium musae Infection in Animal and Plant Hosts Confirms Its Cross-Kingdom Pathogenicity. Journal of Fungi, 11(2), 90. https://doi.org/10.3390/jof11020090








