Abstract
Clinical and mycological data are essential for the optimal management of patients with Candida vaginitis (CV), particularly in cases of (i) azole-resistant C. albicans vaginitis, (ii) recurrent CV, and (iii) CV in pregnant women. The present retrospective single-center study investigated the antifungal activity of six commonly used antifungals against randomly selected vaginal isolates recovered from 68 pregnant women in Adana, Türkiye, including C. albicans, petite C. glabrata, non-petite C. glabrata, and C. krusei, using the disk diffusion method at pH 4 and 7. Furthermore, the antifungal activities of fluconazole and itraconazole were also assessed using the broth microdilution method. For all isolates, the mean inhibition zone diameters were narrower for itraconazole and ketoconazole and larger for miconazole at pH 4 than pH 7 (p < 0.05). For nystatin, zone diameters were wider in C. albicans and petite C. glabrata at pH 4 (p < 0.001 and p < 0.001). Remarkably, clotrimazole was more active at pH 4 than at pH 7, except against non-petite C. glabrata isolates. Based on the broth microdilution results, the resistance rate was higher at pH 4 than at pH 7 in all isolates. Candida glabrata petite isolates exhibited MIC values 2 to 5 times higher than those of the non-petite isolates for both fluconazole and itraconazole. This study highlights the potent activity of topical antifungals (miconazole, nystatin, and clotrimazole) for the treatment of CV in pregnant women and highlights the need to identify petite and non-petite mutants of vaginal C. glabrata isolates to obtain more reliable data and for antifungal susceptibility testing prior to decision-making. The results of the two antifungal susceptibility methods were compared for C. albicans and C. glabrata isolates, and the reliability of the disk diffusion test was discussed.
1. Introduction
Candida vaginitis (CV) is one of the most common mycoses globally. Eight out of ten women experience a CV attack, and 40–45% experience two or more episodes [1,2,3]. However, the epidemiological data required to make realistic and sustained estimates of CV are limited [3,4]. CV is almost always treated with fluconazole or topical azole antifungals with fungistatic activity against Candida species. Despite their widespread use in the treatment of acute CV (ACV)/recurrent CV (RCV), azoles are inadequate for treating infections caused by non-albicans Candida species and azole-resistant C. albicans, and their effect decreases at low pH (4–4.5) [2,5,6]. Candida glabrata, the most common cause of non-albicans Candida vaginitis, is resistant to azoles and is on the rise globally; therefore, treatment is challenging, with limited therapeutic options [7,8,9,10]. Although C. albicans remains a common causative agent, the significant decrease in its prevalence is alarming because it can be treated more easily than non-albicans Candida vaginitis. Additionally, the dramatic increase in C. glabrata and C. krusei prevalence causes azole-resistant CV [1,7,11]. The reasons for this increase in non-C. albicans species are thought to include (i) the unnecessary use of azole antifungals, (ii) the widespread use of single-dose treatment options, (iii) low-dose azole maintenance therapy, and (iv) poor adherence of patients to the proper therapeutic regimen [2,7,11]. Overall, there is a shortage of effective oral and topical antifungal compounds for treating CV, particularly in pregnant patients in whom the use of oral antifungals is contraindicated [1,4,7,11].
The current Clinical & Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines do not recommend the routine testing of vaginal isolates. Therefore, in addition to the local antifungal resistance profile, it is important to identify multiple-azole resistant isolates and antifungal drugs that are active at vaginal pH (4–4.5) to effectively manage treatment-resistant and recurrent CV [12]. This is gaining importance in difficult-to-treat CV cases, where resistance to topical antifungals is common [1,2,3,7,11]. To overcome this problem, Dr Sobel’s group [5,13,14,15,16,17] proposed antifungal testing at pH 4.5 using the CLSI broth microdilution method, which simulates the vaginal pH. These leading studies raised awareness for testing vaginal Candida isolates in treatment-resistant cases and RCV and revealed that fluconazole resistance is not as low as expected [15,17]. Hence, antifungal susceptibility testing (AFST) is required to identify a reliable drug-of-choice and to clinically manage patients [5,7,13]. Importantly, CV has limited therapeutic options during pregnancy, and only topical antifungals are recommended. Notably, ketoconazole is no longer recommended for treating CV [18]. Additionally, oral azoles should be avoided in women who are (i) pregnant, (ii) planning pregnancy, or (iii) breastfeeding [18].
Small and slow-growing colonies of C. glabrata “petite variants” are often not considered for AFST by microbiology laboratories. Isolating and identifying such phenotypes is extremely important during treatment, and continuing azole therapy will favor the selection of the pathogen population, which can result in therapeutic failure and poor clinical outcomes [19,20,21]. Consistent with this, we have previously reported that a notable number of clinical C. glabrata isolates are in fact a mixture of both large and petite colonies [20]. Notably, petite isolates also have a high tolerance to echinocandins [20,22]. Therefore, properly characterizing and reporting petite colonies of C. glabrata in clinical samples holds immense clinical importance. A recent study from our hospital revealed no “petite variants” among 27 C. glabrata vaginitis isolates, suggesting that its prevalence varies even within the same hospital [23].
These findings motivated us to explore the presence of C. glabrata “petite variants” in our vaginal stock collection from pregnant women and to compare the antifungal testing results with those of their parent isolates. The available C. albicans and non-C. albicans strains were also investigated using the disk diffusion and broth microdilution methods to reveal epidemiological data in Adana, Türkiye. Specifically, both tests were performed at pH 4 and 7, and the results were compared. Additionally, the suitability and reliability of the disk diffusion method were assessed. The findings of the present study could enhance our understanding of the antifungal susceptibility profiles of “petite variants” of C. glabrata in vaginal isolates.
2. Materials and Methods
2.1. Study Group and Identification of Isolates
The population studied and selection of isolates are detailed in Figure 1. Vaginal swab samples were cultured on CHROMagar Candida plates (CHROMagar, Paris, France) [24] and diagnoses of CV were established based on a combination of clinical and mycological findings [25]. All isolates were identified to the species level using MALDI-TOF MS (Bruker Daltonics, Bremen, Germany), the Bruker Biotyper 3.1 software and its database (version 3.1.66) [26] and stored at −20 °C.
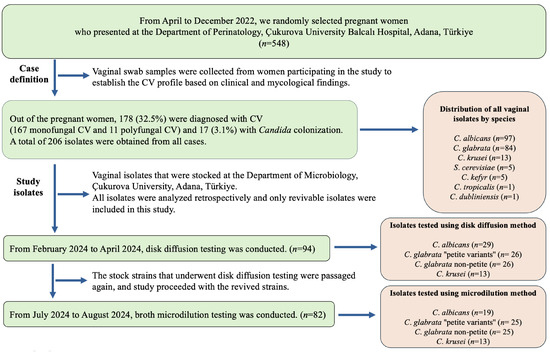
Figure 1.
Flow chart presenting the population studied and selection of isolates.
Subsequently, the isolates were plated onto Sabouraud glucose agar (SGA) plates (Merck, Darmstadt, Germany) for use in the study, and the plates were incubated for 48 h at 37 °C. Only revivable and pure isolates were included in the study.
2.2. Candida glabrata “Petite Variants”
Candida glabrata colonies were sub-cultured onto SGA plates using the single-colony streaking technique. The plates were incubated at 36 °C for 48–72 h, after which colony morphology was evaluated daily. If normal-sized (parent) and small pinpoint colonies grew on the same SGA plate, the colonies were individually subcultured onto SGA [21]. For the final identification, C. glabrata colonies were subcultured on yeast–peptone–glycerol (YPG) agar supplemented with 10 g/L yeast extract (Merck), 20 g/L peptone (Merck), and 20 g/L glycerol (Isolab, İstanbul, Türkiye) and incubated at 36 °C for 72 h. Parent colonies were grown on YPG agar; however, small pinpoint colonies that could not grow on YPG (because these colonies could not ferment glycerol) were considered “petite mutants” [20].
2.3. Antifungal Testing
2.3.1. Disk Diffusion Method
Antifungal susceptibility tests were performed using the disk diffusion method, based on the CLSI M44-A2 document. For this, Mueller–Hinton agar (Merck) containing 2% glucose (Merck) and 0.5 µg/mL methylene blue (Merck) was prepared [27]. The pH of the prepared medium was adjusted to 4 or 7 using NaOH (Isolab) or HCl (Isolab), as required [28]. For each isolate, a 0.5 McFarland turbidity yeast suspension was prepared in approximately 5 mL of sterile saline using 24 h fresh cultures passaged on SGA. Yeast suspensions were spread homogeneously on two separate media plates prepared at pH 4 and 7. Fluconazole (FLU; 25 μg, Bioanalyse, Ankara, Türkiye), itraconazole (ITR; 10 μg, Bioanalyse), ketoconazole (KTC; 50 μg, Bioanalyse), clotrimazole (CLT; 50 μg, Bioanalyse), miconazole (MCZ; 50 μg, Bioanalyse), and nystatin (NY; 100 U, Bioanalyse) disks were added to the test media. The diameters of the zones of inhibition determined at the end of the incubation period (24–48 h at 36 °C) were recorded.
The resistance cut-off values for fluconazole were evaluated as susceptible (S), dose-dependently susceptible (S-DD), and resistant (R) according to CLSI M44-A2 [27]. Other antifungal drugs were reported according to the values presented in Table S1, which were adapted from the literature as resistance cut-off values have not yet been reported [29,30]. The quality control strains C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were included in all assays. All tests were performed in duplicate.
2.3.2. Broth Microdiution Method
Susceptibility testing for fluconazole and itraconazole was conducted on viable isolates using standard broth microdilution methods, following the CLSI M27-A3 and CLSI M60 guidelines [31,32]. The tests were performed in RPMI 1640 medium (Merck) buffered with MOPS (Merck) and supplemented with 0.2% glucose, and the cultures were incubated at 35 °C for 24 and 48 h. The pH of the prepared medium was adjusted to 4 or 7 using NaOH or HCl, as in the disk diffusion method [28]. Fluconazole resistance breakpoints were evaluated according to the CLSI M27-A3 and M27-S4 guidelines [31,33]. Since the CLSI does not provide specific resistance breakpoints for itraconazole, epidemiological cut-off values were utilized [31,34] (Table S2).
2.4. Statistical Analysis
Categorical measurements are summarized as numbers and percentages, and numerical measurements are summarized as the mean and standard deviation. The sensitivity or resistance of microorganisms to antifungals and their compatibility or incompatibility at different pH values (4 and 7) were examined based on McNemar’s test. Whether the inhibition zone diameters met the assumption of a normal distribution was tested with the Shapiro–Wilk test. A t-test of dependent groups was used to compare the inhibition diameters at pH 4 and pH 7. One-way analysis of variance was used for a general comparison of inhibition diameters according to microorganisms. Games-Howell tests were used in pairwise comparisons of groups for situations found to be significant in this analysis. To assess the relationships between the inhibition zone diameters at pH 4 and pH 7 for all isolates, Pearson’s correlation coefficient was used. To control the influence of microorganisms on the correlations between the inhibition zone diameters at pH 4 and 7, a partial correlation analysis was employed. IBM SPSS Statistics 20 (IBM Corp., Armonk, NY, USA) was used to statistically analyze the data. The statistical significance level was set at 0.05 for all tests.
2.5. Ethics Statement
This study was conducted in accordance with the local legislation and institutional requirements. The experimental protocols were approved by the Ethics Committee of Çukurova University Faculty of Medicine after thorough scrutiny of the proposed procedures (no. 14.02.2020/87).
3. Results
The performance of the reference strains evaluated with the two AFST methods was within the expected range. The susceptibility profiles of C. albicans (n = 29), petite C. glabrata (n = 26), non-petite C. glabrata (n = 26), and C. krusei (n = 13) isolates to six antifungal drugs (fluconazole, itraconazole, clotrimazole, ketoconazole, miconazole, and nystatin) at pH 7 and pH 4 are presented in Figure 2, and the mean inhibition zone diameter values are presented in Table S3. Table S4 presents a correlation analysis between the variables (pH 4 and pH 7) to show the consistency of each isolate’s pH changes within the species. For all isolates, the mean inhibition zone diameters were narrower for itraconazole and ketoconazole and larger for miconazole at pH 4 than at pH 7 (p < 0.05). The mean inhibition zone diameter of fluconazole decreased at pH 4 compared with that at pH 7 (C. albicans, petite C. glabrata, and non-petite C. glabrata, p < 0.001, p = 0.039, and p = 0.01, respectively), except for C. krusei (p < 0.01), whereas the average inhibition zone diameter of clotrimazole increased at pH 4 compared with that at pH 7 (C. albicans, petite C. glabrata and C. krusei, p = 0.037, p < 0.001, and p < 0.001, respectively), except for non-petite C. glabrata (p = 0.277). The broth microdilution susceptibility results and MIC values for fluconazole and itraconazole are shown in Figure S1 and Table 1. According to the broth microdilution test performed at pH 4, the resistance rate was higher compared to that at pH 7 in all isolates. Furthermore, while evaluating the broth microdilution tests, we observed that MIC determination at pH 4 was clearer and easier to interpret.
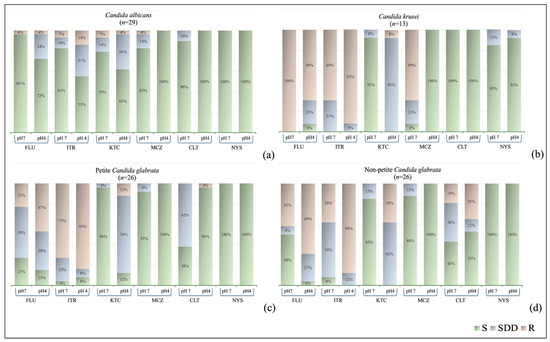
Figure 2.
Disk diffusion method results of vaginal Candida isolates at pH 4 and pH 7: (a) Candida albicans, (b) Candida krusei, (c) petite Candida glabrata, (d) non-petite Candida glabrata. S, Susceptible; SDD, Susceptible-dose-dependent; R, Resistant. FLU, Fluconazole; ITR, Itraconazole, CLT; Clotrimazole, KTC, Ketoconazole; MCZ, Miconazole; NY, Nystatin.

Table 1.
In vitro antifungal susceptibility metrics of vaginal Candida isolates, using CLSI M27-A3 standard.
Candida albicans isolates (n = 29) demonstrated a significant correlation in susceptibility to fluconazole, itraconazole, and ketoconazole between pH 4 and pH 7 (p < 0.01). Although all C. albicans isolates were susceptible to miconazole and clotrimazole at pH 4, their susceptibilities to miconazole and clotrimazole were lower at pH 7 (24 isolates for miconazole and 26 for clotrimazole) (p > 0.05). Furthermore, all C. albicans isolates were susceptible to nystatin at both pH 7 and 4, with larger mean inhibition zone diameters at pH 4 (p < 0.001). In C. albicans isolates, both disk diffusion and broth microdilution test results were consistent for fluconazole, whereas inconsistent results were observed for itraconazole. Furthermore, in C. albicans isolates, the minimum inhibitory concentration (MIC) ranges for fluconazole at pH 4 were similar to those at pH 7, whereas the MIC ranges for itraconazole were narrower; the MIC values inhibiting the growth of 90% of isolates (MIC90) for both drugs were found to be two dilutions lower. The antifungal susceptibility profiles of petite (n = 26) and non-petite (n = 26) C. glabrata isolates are presented in Figure 2. In petite C. glabrata isolates, susceptibility results for fluconazole and itraconazole exhibited a significant correlation between pH 4 and pH 7, whereas in non-petite C. glabrata isolates, the correlation between itraconazole and nystatin was significant (p < 0.01). Although all C. glabrata colonies were susceptible to miconazole at pH 4, the mean inhibition zone diameter was greater at pH 4 than at pH 7 (p < 0.001, Figure 3). Clotrimazole was more effective against petite C. glabrata isolates at pH 4 (96.2%, 25/26) than at pH 7 (38.5%, 10/26) (p < 0.05), whereas it was only effective against 42.3% (11/26) and 50% (13/26) of non-petite C. glabrata isolates, respectively (p > 0.05). All isolates were sensitive to nystatin at both pH values. Furthermore, for all tested antifungals, the mean inhibition zone diameters were greater in petite colonies than those in non-petite colonies at pH 4. Consistent results were observed in both the disk diffusion and broth microdilution tests for fluconazole in petite and non-petite C. glabrata strains; the itraconazole results agreed with the disk methods results at pH 7, while the pH 4 results were inconsistent with these test outcomes. Additionally, petite strains of C. glabrata exhibited MIC values 2 to 5 times higher than those of the non-petite strains for both fluconazole and itraconazole. Furthermore, the MIC ranges observed for both petite and non-petite C. glabrata at pH 4 were at least three dilutions lower than those at pH 7, and the MIC90 values were at least one dilution higher.
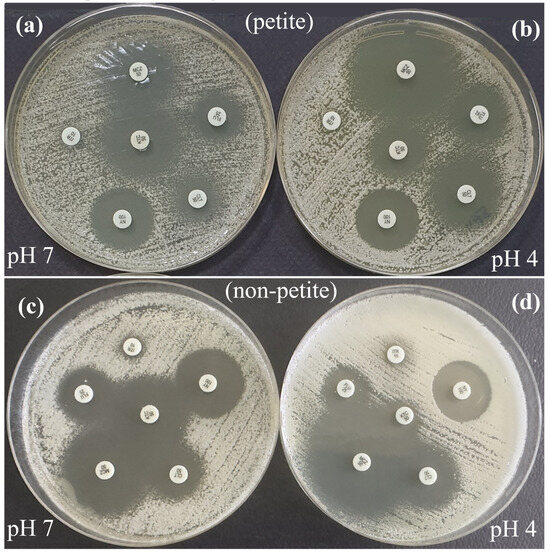
Figure 3.
Antifungal susceptibility zone diameters of C. glabrata petite (a,b) and non-petite (c,d) colonies on Mueller–Hinton agar, pH 7 (a,c) and pH 4 (b,d), using the disk diffusion method. In petite colonies, the zone diameters for KTC at pH 7 and MCZ, CLT, and FLU at pH 4 were larger. In non-petite colonies, the zone diameters for KTC, FLU, and NY were larger at pH 7, while MCZ and CLT were larger at pH 4. FLU, Fluconazole; ITR, Itraconazole, CLT; Clotrimazole, KTC, Ketoconazole; MCZ, Miconazole; NY, Nystatin.
Although all C. krusei isolates (n = 13) were resistant to fluconazole at pH 7, only 69.2% (9/13) were resistant to fluconazole at pH 4. The susceptibility to miconazole was 30.8% (4/13) at pH 7, and all isolates (100%, 13/13) were susceptible at pH 4 (p < 0.05). All C. krusei isolates were susceptible to clotrimazole (100%, 13/13) at both pH 4 and 7; however, the mean inhibition zone diameters were larger at pH 4 (p < 0.001; Table 1). The disk diffusion and broth microdilution susceptibility test results were inconsistent for itraconazole (Figure S1).
4. Discussion
The antifungal susceptibility profiles of vaginal Candida isolates to six antifungal compounds were tested, and the available options for treating CV during pregnancy were highlighted. Additionally, petite and non-petite C. glabrata isolates derived from the same patients were further examined and compared. The results indicated that resistance to topical antifungal compounds was uncommon. Miconazole and nystatin were specifically more active against C. albicans and C. glabrata isolates at pH 4 than at pH 7. Notably, topical nystatin is not commercially available in Türkiye. In the present study, 51% of all isolates (48/94) were susceptible to fluconazole at pH 7, whereas 29% (27/94) were susceptible to fluconazole at pH 4 (p < 0.05). Only 29% (27/94) and 20% (19/94) of the isolates were susceptible to itraconazole at pH 7 and 4, respectively (p > 0.05). Therefore, mycological information is required to reliably treat CV in pregnant women. The present study also revealed that miconazole and clotrimazole are the best therapeutic options for CV in pregnant women in Adana, Türkiye.
An ARTEMIS survey indicated that when using invasive infection isolates, the fluconazole disk diffusion test is especially reliable for detecting resistant Candida species isolates through reference MIC testing, which has been performed with increasing accuracy worldwide [35]. Importantly, the level of agreement between disk diffusion and MIC results in these studies was the highest for C. albicans and lowest for C. glabrata. The latter was almost entirely due to major errors (resistance in disk diffusion and susceptibility in the broth microdilution test) [35,36]. Our study showed a high level of agreement for C. albicans and C. glabrata isolates, but significant discrepancies in itraconazole susceptibility were noted for petite C. glabrata and C. krusei isolates. Given the limited number of isolates, these findings should be interpreted with caution and require further validation with a larger sample size to rule out potential random errors. The disk diffusion method has been compared with the broth microdilution method, and several advantages (easier, inexpensive, and easy-to-interpret) and disadvantages (does not provide MICs) have been noted [35,36].
Sobel and Akins [15] tested 125 vaginal C. albicans isolates against fluconazole. At pH 7, 42.3% (n = 54) were initially considered susceptible to fluconazole; however, at pH 4.5, 31% (17/54) of the isolates were considered resistant, and tests performed at an acidic pH were more reliable. Notably, fluconazole susceptibility in vaginal C. albicans isolates longitudinally obtained from women with RCV remained predominantly stable despite azole avoidance, and only 5.2–10.5% of the susceptible isolates become resistant or vice versa [16]. A 10-year U.S. study noted that C. albicans constituted at least 71.8% of CV isolates, and 44 out of 193 (22.8%) isolates were reported to be fluconazole-resistant (MIC ≥ 8 µg/mL). Furthermore, 52% and 38% of the C. albicans isolates exhibited susceptibility at pH 7 and 4.5, respectively [17]. In our previous study at the same hospital, 207 vaginal isolates recovered from pregnant women were tested against 13 antifungal drugs using the CLSI broth microdilution method (M27-A3). Clotrimazole, ketoconazole, and miconazole were 90–98% active against the strains. Briefly, 1.9% of the isolates were fluconazole-resistant, and 6.8% exhibited S-DD [37]. In the present study, the susceptibility profiles to fluconazole, itraconazole, and ketoconazole were lower at pH 4 than pH 7.
Boikov et al. [13] tested 108 vaginal isolates—60 C. albicans and 48 non-albicans Candida strains—against a new echinocandin, CD101, and found that it has potent activity at both pH 4 and 7. Many fluconazole-resistant isolates, particularly C. glabrata, are resistant to itraconazole. In another study, ibrexafungerp was found to be effective against 187 vaginal Candida isolates [fluconazole-resistant (n = 52), fluconazole-susceptible C. albicans (n = 30), and non-albicans Candida (n = 100)] at pH 4 and 7, and the MIC did not change with pH [14].
Notably, antifungal susceptibility tests have not been standardized or validated for non-albicans Candida species. Therefore, empiric therapy is recommended for at least 30–40% of CV cases [11]. Recently, Dunaiski et al. [9] contributed to our in-depth-understanding of why C. glabrata vaginitis causes treatment-resistant infections using whole-genome sequencing, identifying a clone that is predominant in women with C. glabrata vaginitis and harbors various mutations in resistance-associated genes. Interestingly, in our study, the susceptibility profiles of petite and non-petite C. glabrata isolates potentially varied at different pH values, and clotrimazole was more effective against petite isolates than non-petite (parent) isolates recovered from the same patient. This finding contradicts earlier findings reporting that petite isolates are more resistant than non-petite isolates, as we observed that petite strains exhibited higher MIC values for fluconazole and itraconazole than non-petite strains [20,21]. We suggest that the susceptibility profiles of mitochondrial mutants in C. glabrata remain poorly understood. However, according to our stock records, 51.2% of vaginal C. glabrata isolates are “petite variants”, warranting a closer investigation of “petite variants” associated with recurrent and recalcitrant infections, and poor prognosis. The present study and recent work by our group [23] revealed that the prevalence of “petite variants” in vaginal isolates varies from 0% to 51.2%; thus, determining “petite variants” and their clinical relevance is important.
AFST is crucial for effective treatment, but its access is limited in resource-constrained regions such as Africa, the Asia–Pacific, and Latin America, like many laboratories in our country [38]. Various methods of detecting antifungal susceptibility profiles are available, including phenotypic, molecular, and automated systems. Once their validity is confirmed, cost-effective methods that do not require specialized equipment or extensive experience are preferred [38]. Broth microdilution methods are reference methods in the CLSI and EUCAST guidelines, but they are not for day-to-day use and not readily accessible due to the high costs associated with certain test guidelines, such as those of the CLSI, as well as significant equipment requirements [31,38]. Simple disk tests provide rapid and reliable results for many common Candida species [35,36]. However, owing to the limited sample size and potential discrepancies for certain isolates, the reliability of the disk diffusion method should be interpreted with caution, as noted in the present study. Moreover, easily accessible media for this method, such as Mueller–Hinton agar, reduce the cost of the method and ensure reproducibility [27,38]. Two of the timeframe problems with AFST are (i) a need to know the fungal species before an interpretation can be issued (for the CLSI and/or EUCAST) and (ii) isolate transport and regrowing delays in obtaining the answer, as observed in our study. The present study had several limitations, primarily (i) a limited number of study isolates, which represents a significant constraint on the generalizability of the findings and impedes the comparability of the methods. Additionally (ii) its retrospective nature and (iii) data are limited to in vitro test findings, and data on clinical treatment and outcomes are lacking. Further studies can explore the steps necessary to address the limitations of the current study.
5. Conclusions
This study highlights the importance of examining susceptibility differences between petite and non-petite C. glabrata variants, as well as comparing them with other Candida species, in managing CV. These findings are particularly crucial for pregnant patients who cannot use oral antifungals, emphasizing the need for alternative treatment strategies. Overall, AFST is vital for patient care guidance, and this will prevent inappropriate therapy and lead to rapid recovery from CV. In addition, to assess the size of the resistance problem at the regional or national level, understanding the current profile is encouraged. Importantly, disk diffusion is an applicable method for improving access to resource-constrained laboratories. Therefore, in addition to the local antifungal resistance profile, it is important to identify azole-resistant isolates and antifungal drugs that are active at vaginal pH (4–4.5). Owing to the high prevalence of CV and its severe morbidity, clinicians and clinical mycologists should work collaboratively to manage this global disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof11020092/s1, Table S1. Threshold values for interpreting disk diffusion tests used in this study. Table S2. Threshold values for interpreting broth microdilution tests in this study. Table S3. Mean inhibition zone diameters of vaginal Candida isolates for six antifungals at pH 4 and pH 7. Table S4. Correlation and partial correlation coefficients between the inhibition zone diameters at pH 4 and 7 for all isolates. Figure S1. Broth microdilution method results for vaginal Candida isolates at pH 4 and pH 7.
Author Contributions
Conceptualization, M.S. and M.I.; methodology, N.Ü., A.S.K., İ.Ş., D.Y.M. and M.I.; formal analysis, O.B., F.H. and İ.Ü.; investigation, M.S., A.S.K., O.B., F.H. and F.İ.U.; resources, N.Ü., A.S.K. and M.I.; data curation, M.S., N.Ü., A.S.K., İ.Ş., O.B., F.H., F.İ.U., D.Y.M. and İ.Ü.; writing—original draft preparation, A.S.K., D.Y.M. and M.I.; writing—review and editing, M.I.; visualization, N.Ü., O.B. and F.H.; supervision, M.I.; project administration, M.I.; funding acquisition, M.S. and N.Ü. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Çukurova University Scientific Research Projects (TSA-2021-12961).
Institutional Review Board Statement
This study was conducted according to the local legislation and institutional requirements. The experimental protocols were approved by the Ethics Committee of Çukurova University Faculty of Medicine after thoroughly scrutinizing of the proposal procedures (Date: 14 February 2020; No: 87).
Informed Consent Statement
Informed consent was obtained from all participants, and written consent for publication was provided.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Amir Arastehfar for his help in investigating petite mutants of Candida glabrata.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Foxman, B.; Muraglia, R.; Dietz, J.P.; Sobel, J.D.; Wagner, J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: Results from an internet panel survey. J. Low. Genit. Tract. Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef]
- Danby, C.S.; Boikov, D.; Rautemaa, R.; Sobel, J.D. Effect of pH on in vitro susceptibility of Candida glabrata and Candida albicans to eleven antifungal agents—Implications for clinical use. Antimicrob. Agents Chemother. 2012, 56, 1403–1406. [Google Scholar] [CrossRef]
- Arechavala, A.; Negroni, R.; Santiso, G.; Depardo, R.; Bonvehi, P. Chronic recurrent vulvovaginitis is not only due to Candida. Rev. Iberoam. Micol. 2021, 38, 132–137. [Google Scholar] [CrossRef]
- Sobel, J.D.; Sobel, R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin. Pharmacother. 2018, 19, 971–977. [Google Scholar] [CrossRef]
- Fernandes, Â.; Azevedo, N.; Valente, A.; Dias, M.; Gomes, A.; Nogueira-Silva, C.; Henriques, M.; Silva, S.; Gonçalves, B. Vulvovaginal candidiasis and asymptomatic vaginal colonization in Portugal: Epidemiology, risk factors and antifungal pattern. Med. Mycol. 2022, 60, myac029. [Google Scholar] [CrossRef]
- Dunaiski, C.M.; Kock, M.M.; Chan, W.Y.; Ismail, A.; Peters, R.P.H. Molecular epidemiology and antimicrobial resistance of vaginal Candida glabrata isolates in Namibia. Med. Mycol. 2024, 62, myae009. [Google Scholar] [CrossRef]
- Sobel, J.D.; Chaim, W.; Nagappan, V.; Leaman, D. Treatment of vaginitis caused by Candida glabrata: Use of topical boric acid and flucytosine. Am. J. Obstet. Gynecol. 2003, 189, 1297–1300. [Google Scholar] [CrossRef]
- Sobel, J.D. Treatment of vaginitis caused by non-albicans Candida species. Expert Rev. Anti Infect. Ther. 2024, 22, 289–296. [Google Scholar] [CrossRef]
- Linhares, I.M.; Summers, P.R.; Larsen, B.; Giraldo, P.C.; Witkin, S.S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 2011, 204, 120.e1–120.e5. [Google Scholar] [CrossRef]
- Boikov, D.A.; Locke, J.B.; James, K.D.; Bartizal, K.; Sobel, J.D. In vitro activity of the novel echinocandin CD101 at pH 7 and 4 against Candida spp. isolates from patients with vulvovaginal candidiasis. J. Antimicrob. Chemother. 2017, 72, 1355–1358. [Google Scholar] [CrossRef]
- Sobel, J.D.; Borroto-Esoda, K.; Azie, N.; Angulo, D. In vitro pH activity of ibrexafungerp against fluconazole-susceptible and -resistant Candida isolates from women with vulvovaginal candidiasis. Antimicrob. Agents Chemother. 2021, 65, e0056221. [Google Scholar] [CrossRef]
- Sobel, J.D.; Akins, R. Determining susceptibility in Candida vaginal isolates. Antimicrob. Agents Chemother. 2022, 66, e0236621. [Google Scholar] [CrossRef]
- Sobel, J.D.; Sebastian, S.; Boikov, D.A. A longitudinal study on fluconazole resistance in Candida albicans vaginal isolates. Mycoses 2023, 66, 563–565. [Google Scholar] [CrossRef]
- Sobel, J.D. Resistance to fluconazole of Candida albicans in vaginal isolates: A 10-year study in a clinical referral center. Antimicrob. Agents Chemother. 2023, 67, e0018123. [Google Scholar] [CrossRef]
- Saxon, C.; Edwards, A.; Rautemaa-Richardson, R.; Owen, C.; Nathan, B.; Palmer, B.; Wood, C.; Ahmed, H. British Association for Sexual Health and HIV national guideline for the management of vulvovaginal candidiasis (2019). Int. J. STD AIDS 2020, 31, 1124–1144. [Google Scholar] [CrossRef]
- Posteraro, B.; Tumbarello, M.; La Sorda, M.; Spanu, T.; Trecarichi, E.M.; De Bernardis, F.; Scoppettuolo, G.; Sanguinetti, M.; Fadda, G. Azole resistance of Candida glabrata in a case of recurrent fungemia. J. Clin. Microbiol. 2006, 44, 3046–3047. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Hovhannisyan, H.; Fuentes, D.; Cabrera, N.; Quinteros, C.; Ilkit, M.; Ünal, N.; Hilmioğlu-Polat, S.; Jabeen, K.; et al. Overlooked Candida glabrata petites are echinocandin tolerant, induce host inflammatory responses, and display poor in vivo fitness. mBio 2023, 14, e0118023. [Google Scholar] [CrossRef]
- Badrane, H.; Cheng, S.; Dupont, C.L.; Hao, B.; Driscoll, E.; Morder, K.; Liu, G.; Newbrough, A.; Fleres, G.; Kaul, D.; et al. Genotypic diversity and unrecognized antifungal resistance among populations of Candida glabrata from positive blood cultures. Nat. Commun. 2023, 14, 5918. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; Jimenez-Ortigosa, C.; DeGregorio, L.; Quinteros, C.; Shor, E.; Perlin, D.S. Multifactorial role of mitochondria in echinocandin tolerance revealed by transcriptome analysis of drug-tolerant cells. mBio 2021, 12, e0195921. [Google Scholar] [CrossRef]
- Karakoyun, A.S.; Unal, N.; Sucu, M.; Bingöl, O.; Unal, I.; Ilkit, M. Integrating clinical and microbiological expertise to improve vaginal candidiasis management. Mycopathologia 2024, 189, 96. [Google Scholar] [CrossRef]
- Pincus, D.H.; Orenga, S.; Chatellier, S. Yeast identification—Past, present, and future methods. Med. Mycol. 2007, 45, 97–121. [Google Scholar] [CrossRef]
- Guzel, A.B.; Ilkit, M.; Burgut, R.; Urunsak, I.F.; Ozgunen, F.T. An evaluation of risk factors in pregnant women with Candida vaginitis and the diagnostic value of simultaneous vaginal and rectal sampling. Mycopathologia 2011, 172, 25–36. [Google Scholar] [CrossRef]
- Normand, A.C.; Gabriel, F.; Riat, A.; Cassagne, C.; Bourgeois, N.; Huguenin, A.; Chauvin, P.; De Geyter, D.; Bexkens, M.; Rubio, E.; et al. Optimization of MALDI-ToF mass spectrometry for yeast identification: A multicenter study. Med. Mycol. 2020, 58, 639–649. [Google Scholar] [CrossRef]
- CLSI M44-A2; Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. 2nd ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009.
- Liu, W.; Zhang, X.; Liu, Z.; Luo, X. Impact of pH on the antifungal susceptibility of vaginal Candida albicans. Int. J. Gynaecol. Obstet. 2011, 114, 278–280. [Google Scholar] [CrossRef]
- Dota, K.F.D.; Freitas, A.R.; Consolaro, M.E.L.; Svidzinski, T.I.E. A challenge for clinical laboratories: Detection of antifungal resistance in Candida species causing vulvovaginal candidiasis. Lab. Med. 2011, 42, 87–93. [Google Scholar] [CrossRef]
- Khan, M.; Ahmed, J.; Gul, A.; Ikram, A.; Lalani, F.K. Antifungal susceptibility testing of vulvovaginal Candida species among women attending antenatal clinic in tertiary care hospitals of Peshawar. Infect. Drug Resist. 2018, 11, 447–456. [Google Scholar] [CrossRef]
- CLSI M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 3rd ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- CLSI M60; Performance Standards for Antifungal Susceptibility Testing of Yeasts. 1st ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- CLSI M27-S4; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 4th Supp. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Pfaller, M.A.; Espinel-Ingroff, A.; Canton, E.; Castanheira, M.; Cuenca-Estrella, M.; Diekema, D.J.; Fothergill, A.; Fuller, J.; Ghannoum, M.; Jones, R.N.; et al. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B, flucytosine, and itraconazole and Candida spp. as determined by CLSI broth microdilution. J. Clin. Microbiol. 2012, 50, 2040–2046. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Hazen, K.C.; Messer, S.A.; Boyken, L.; Tendolkar, S.; Hollis, R.J.; Diekema, D.J. Comparison of results of fluconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS global antifungal surveillance program. J. Clin. Microbiol. 2004, 42, 3607–3612. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Messer, S.A.; Boyken, L.; Hollis, R.J. Activities of fluconazole and voriconazole against 1586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: Report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J. Clin. Microbiol. 2003, 41, 1440–1446. [Google Scholar] [CrossRef]
- Kalkanci, A.; Güzel, A.B.; Khalil, I.I.; Aydin, M.; Ilkit, M.; Kuştimur, S. Yeast vaginitis during pregnancy: Susceptibility testing of 13 antifungal drugs and boric acid and the detection of four virulence factors. Med. Mycol. 2012, 50, 585–593. [Google Scholar] [CrossRef]
- Kwizera, R.; Abdolrasouli, A.; Garcia-Effron, G.; Denning, D.W. Antifungal susceptibility testing: Applicability of methods and strategies for improving access in resource-constrained settings. Lancet Infect. Dis. 2024, 24, e782–e793. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).