Galleria mellonella as an Invertebrate Model for Studying Fungal Infections
Abstract
1. Introduction
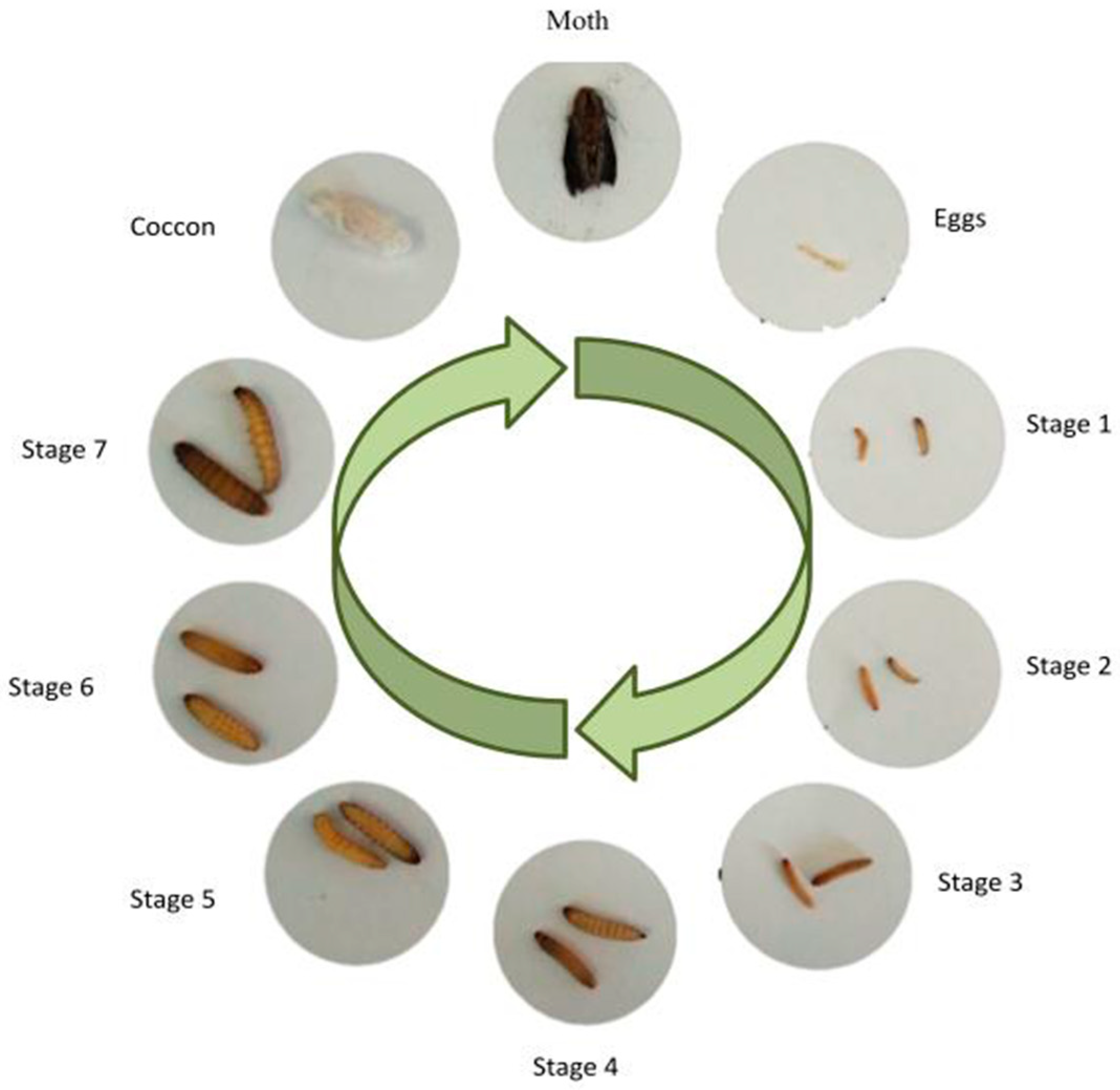
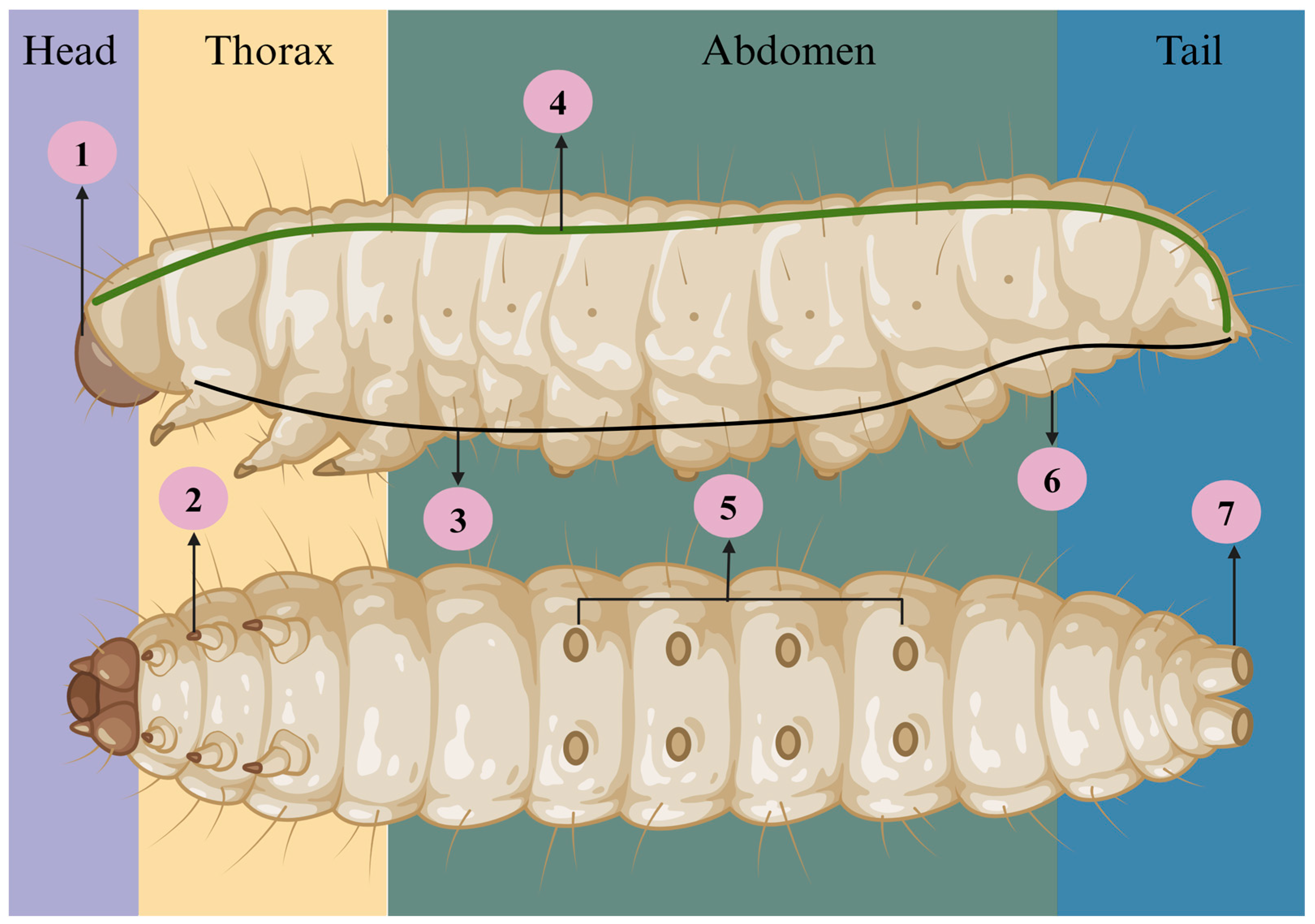
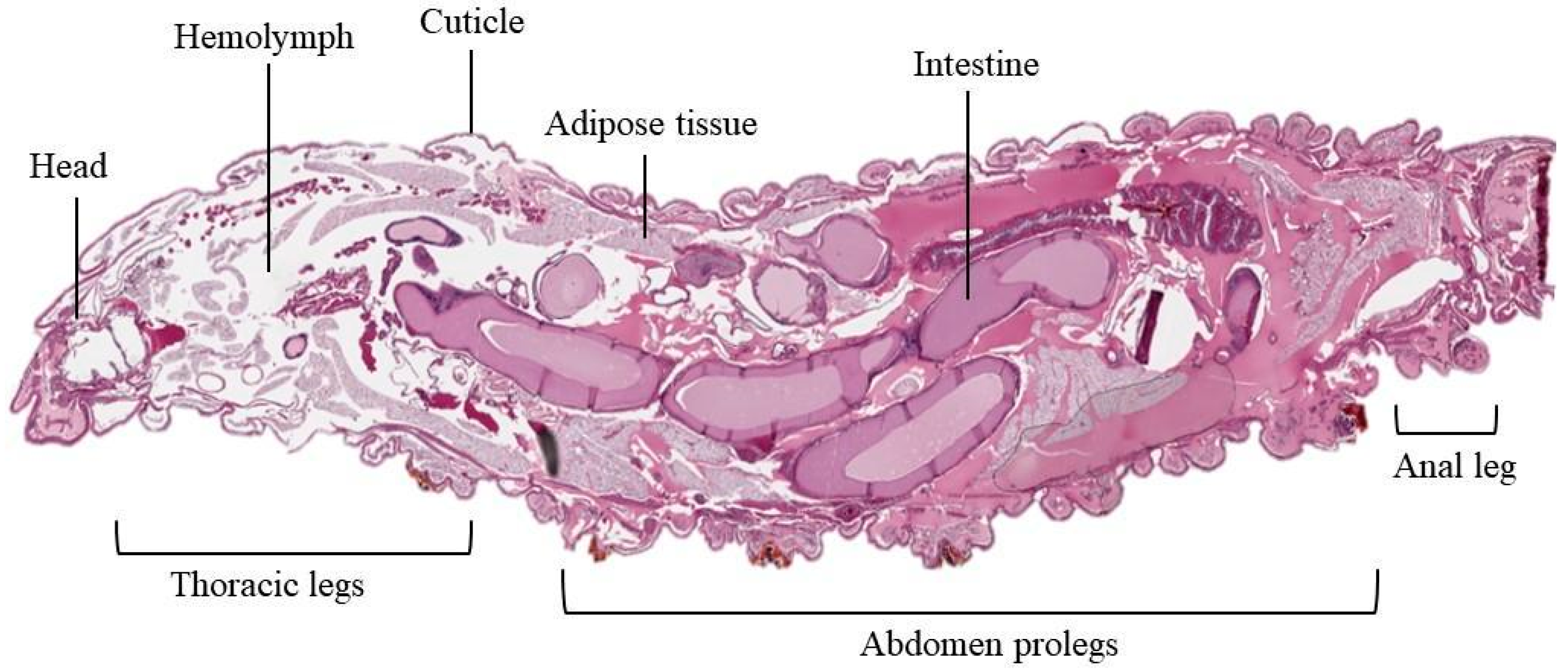
2. Anatomic and Life Cycle of G. mellonella
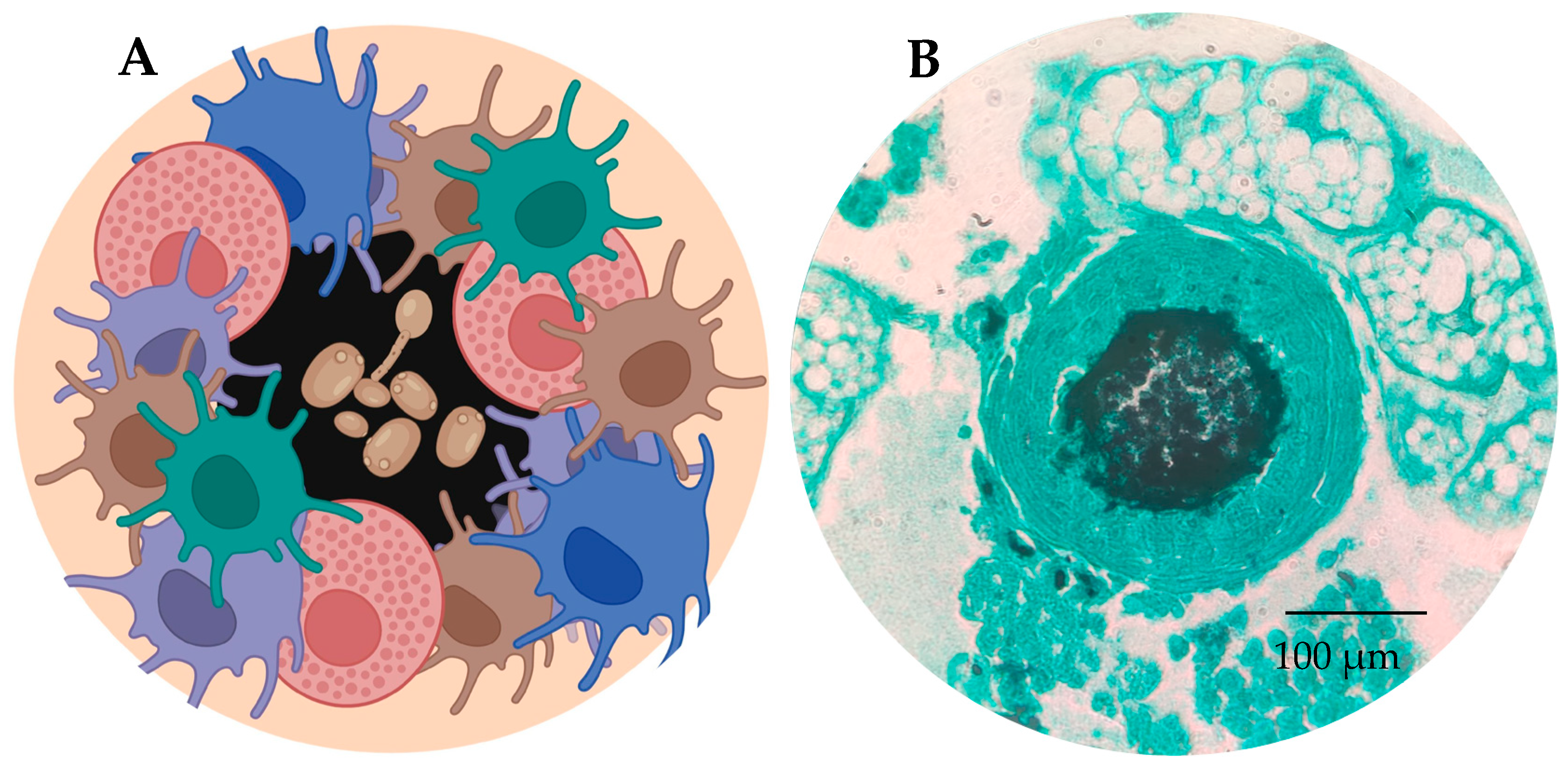
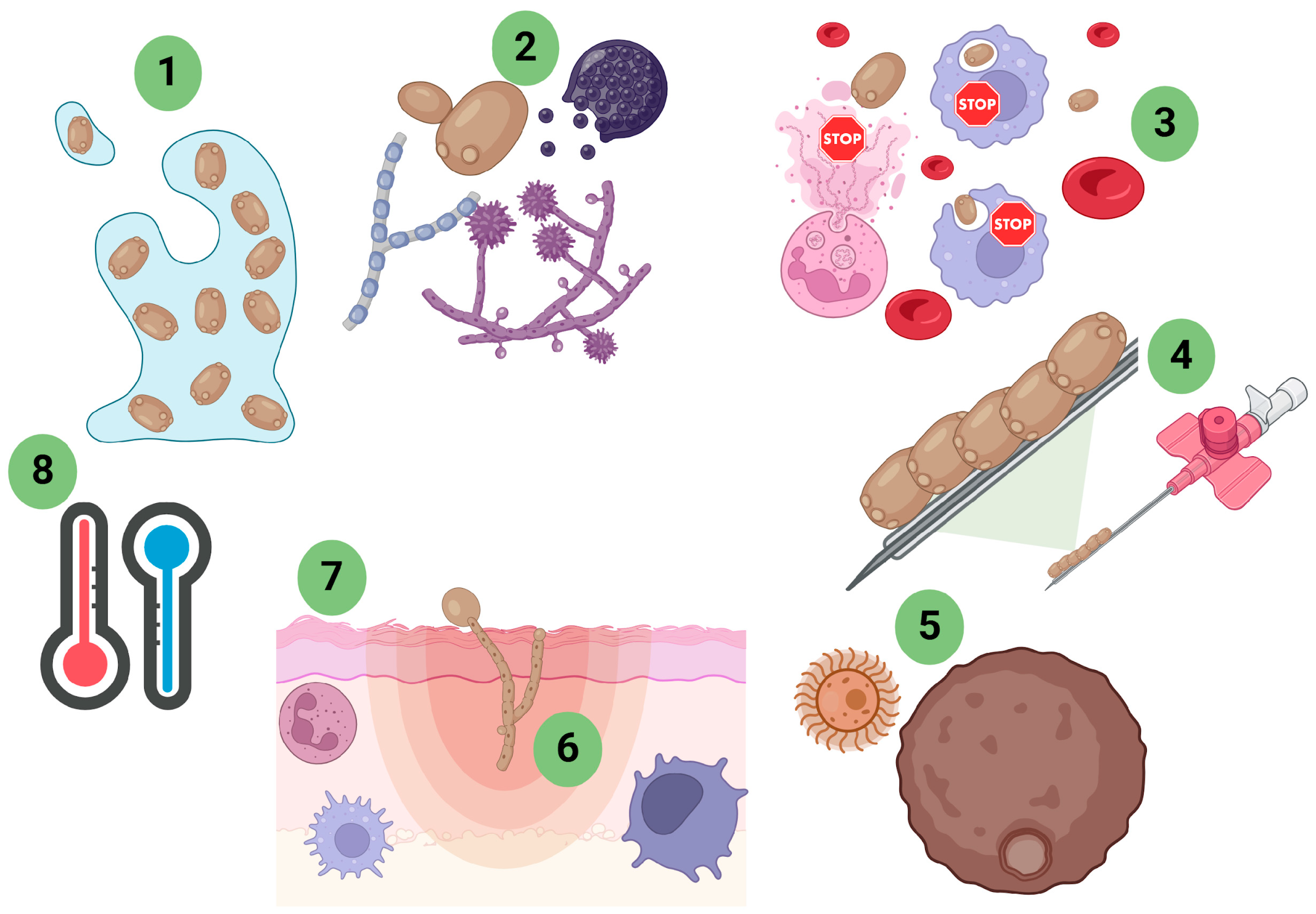
3. Immune System
4. Fungal Virulence Studies in G. mellonella Models
5. Virulence of Yeasts of the Genus Candida
6. Virulence of Non-Candida Yeasts
7. Virulence of Dimorphic Fungal Pathogens in G. mellonella
8. Other Fungi That Cause Opportunistic Infections
| Fungi | Size or Weight of Larva | Inoculum | Quantity | Ref. |
|---|---|---|---|---|
| Candida spp. | 1.2–1.5 cm | 2 × 107 cells/larva | 10 µL | [39] |
| N. glabrata, C. nivariensis and C. bracarensis | 0.3–0.5 g | 1 × 105, 1 × 106 and 1 × 107 cells/larva | 10 µL | [40] |
| Candida sp. | 330 ± 20 mg | 5 × 105 cells/larva (C. albicans and C. tropicalis) and 2 × 106 5 × 106 cells/larva (C. parapsilosis, N. glabrata, and P. kudriavzevii ) | 10 µL | [41] |
| C. auris, C. albicans and C. parapsilosis | 250 to 350 mg | 105 and 106 CFU/Larva | 10 µL | [42] |
| C. auris, C. albicans and C. parapsilosis | 250 to 350 mg | 106 CFU/Larva | 10 µL | [43] |
| C. parapsilosis | - | 5 × 105 cells/larva | 10 µL | [44] |
| M. furfur and M. pachydermatis | 250 and 330 mg | range 1.5 × 106–1.5 × 109 CFU/mL) | 20 μL | [45] |
| C. neoformans | - | 104 cells/larva | 10 μL | [46] |
| C. neoformans and C. albicans | 200–300 mg | 104 to 106 cells/larva | 10 μL | [47] |
| Cryptococcus neoformans var. grubii | 100–200 mg | 5 × 106 cells/larva | 10 μL | [48] |
| S. schenckii sensu stricto and S. brasiliensis | 1 cm | 1 × 105, 1 × 106 or 1 × 107 cells/μL | 10 μL | [49] |
| S. schenckii sensu stricto, S. brasiliensis and S. globosa | 1.2–1.5 cm | 1 × 105 yeast-like cells | 10 μL | [50] |
| S. schenckii sensu stricto and S. brasiliensis | 0.2–0.3 g | 1 × 107 cells/larva | 10 μL | [51] |
| P. brasiliensis and P. lutzii. | 150–200 mg | 5 × 105, 1 × 106 and 5 × 106 cells/larva) | - | [52] |
| P. brasiliensis | 5 × 106 cells/larvae | [53] | ||
| P. brasiliensis | 01–0.2 g | 5 × 106 cells/larvae | 10 μL | [54] |
| P. brasiliensis | 150–200 mg | 5 × 106 cells/larva | 10 μL | [55] |
| C. podassi | 0.2–0.4 g | 103 to 106 spores | 10 μL | [57] |
| C. podassi | 245 mg +/− 25 mg | 104 and 106 total spherules/larva | 8 μL | [58] |
| H. capsulatum | 0.1–0.15 g | (101, 102, 103, 104, 105 and 106 cells/larva | 10 μL | [59] |
| H. capsulatum | 150–200 mg | 1 × 106 yeasts/larvae | 10 μL | [60] |
| P. marneffei | 300–350 mg | 101, 103, 105 and 106 CFU/Larva | 10 μL | [61] |
| A. terreus | 0.3–0.4 g | 1 × 105, 1 × 106 and 1 × 107 conidia/larva | 20 μL | [62] |
| A. Terreus | ~150 mg | - | 10–50 μL | [63] |
| A. fumigatus | - | 8 × 104 conidios | 20 μL | [64] |
| A. niger | - | 1 × 105/larva | 3 μL | [65] |
| A. fumigatus | 0.2–0.3 g | 5 × 105 conidia | 20 μL | [66] |
| A. fumigatus | 0.3 g | - | 10 μL | [67] |
| A. flavus | 160–200 mg | 103 germinated conidia | 10 μL | [68] |
| A. fumigatus | 330 mg | - | 10 μL | [69] |
| A. fumigatus | - | 1 × 108 | 10 μL | [70] |
| A. fumigatus | - | 1 × 108 spore | - | [71] |
| A. fumigatus | 0.4 and 0.5 g | 1 × 107 freshly harvested | 20 μL | [72] |
| A. fumigatus | 275 to 330 mg | 1 × 106/larva | 5 μL | [73] |
| A. leporis, A. hancockii, and A. homomorphus | - | 3000 conidia/μL or, in one experiment, 250 conidia/μL | 20 μL | [74] |
| A. flavus | ~300 mg | 1 × 106, 104 and 103/larva | 5 μL | [75] |
| A. flavus | - | 104 conidia | - | [76] |
| A. flavus | - | 107/mL | 5 μL | [77] |
| A. flavus | - | - | - | [78] |
| F. oxysporum | 0.2–0.3 g | 1.6 × 103 microconidial/larva | 8 μL | [79] |
| F. oxysporum | - | - | - | [80] |
| Rhizopus spp., Rhizomucor spp., Lichtheimia spp., Mucor spp. | 0.3–0.4 g | 1 × 104, 1 × 105, 1 × 106 and 1 × 107 conídios/larva | 20 μL | [81] |
| L. corymbifera | 0.3–0.4 g | 107 spores /larva | 20 μL | [82] |
| M. irregularis, M. hiemalis, L. corymbifera and R. arrhizus | - | 1 × 10 5 esporos/larva | 10 μL | [83] |
| M. lusitanicus | 105 cells/larva | 20 μL | [84] | |
| R. arrhizus and L. corymbifera | 250 ± 50 mg | 101 to 106 sporangiospores or germinated sporangiospores | 10 μL | [85] |
| Pneumocystis murina | 330 ± 25 mg | 4.85 × 105 or 4.85 × 106 cells per larvae | 10 μL | [86] |
| F. monophora | 250–300 mg | 104, 105 and 106 conidia /larva | 20 μL | [87] |
| C. carrionii | 300–500 mg | 104, 105 and 106 conidia /larva | 40 μL | [88] |
| M. mycetomatis | 300–500 mg | 0.04 to 4 mg wet weight per larvae | 40 μL | [90] |
| M. gypseum, M. canis, T. rubrum, T. mentagrophytes, T. equinium and T. tonsurans | 0.3–0.4 g |
10 6 conídios ml
−1
In medium | 5 μL | [91] |
| M. gypseum, M. canis, T. rubrum, T. mentagrophytes, T. equinium and T. tonsurans | - | - | - | [92] |
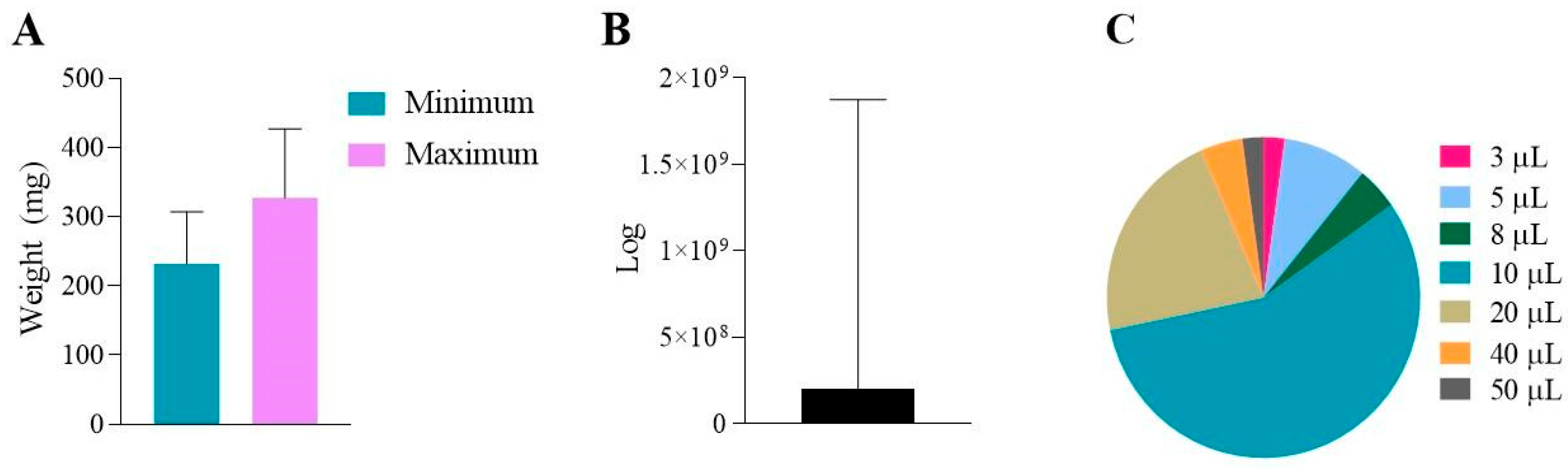
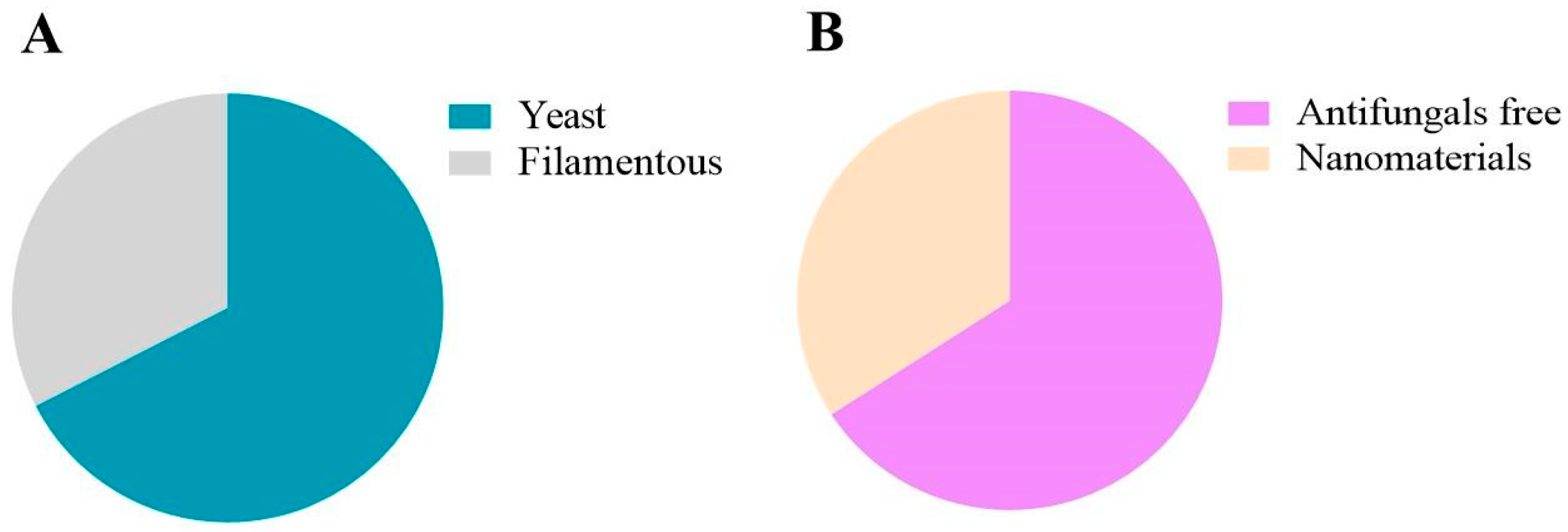
9. Analysis of the Studies Involved
10. Drugs Available or Under Investigation with Future Antifungal Application
11. Antifungal Combinations Available to Fight Infection
| Drugs Candidate | Fungi | Dose/Larva | Ref. |
|---|---|---|---|
| Miltefosine i | C. albicans, C. neoformans and C. gattii | 0.03–16 µg/mL miltefosine | [95] |
| Miltefosine | C. auris | 20 or 40 mg/kg of miltefosine free and 100 mg/kg miltefosine encapsulated | [96] |
| Miltefosine alone or Voriconazole combinate | A. fumigatus and A. flavus | 20 or 40 mg/kg of miltefosine free, 100 mg/kg miltefosine encapsulated, 20 mg/kg of miltefosine + 10 mg/Kg of voriconazole free, and 100 mg/kg miltefosine encapsulated with 10 mg/Kg voriconazole | [97] |
| Atorvastatin | C. albicans | 4.55 or 9.09 mg/kg−1 | [99] |
| Miramistin | C. albicans and A. fumigatus | 16, 160 and 1000 mg/kg−1 | [100] |
| Pilocarpine hydrochloride and acetylcholine | C. albicans | 3.12, 6.25 and 10.5 mM | [101] |
| Miconazole, ravuconazole, oteseconazole, eberconazole, luliconazole, fenbendazole, carbendazim, amorolfine, tafenoquine, alexidine olorofilm and others | M. mycetomatis | 20 μM of compound per larvae | [102] |
| Manogepix | M. mycetomatis | 8.57 mg/kg manogepix and 5.71 mg/kg itraconazole | [103] |
| Azoles + linozoline | C. albicans | FLC (160 μg/mL), ITZ (40 μg/mL), VRC (40 μg/mL), LZD (200 μg/mL), LZD (200 μg/mL) + FLC (160 μg/mL), LZD (200 μg/mL) + ITZ (40 μg/mL), and LZD (200 μg/mL) + VRC (40 μg/mL), | [106] |
| Fluconazole and D-penicillamine | C. albicans | D-penicillamine 40 (μg/mL) and FLU (160 μg/mL) | [107] |
| Verapamil and fluconazole | C. albicans | [108] | |
| Eravacycline and fluconazole | C. albicans | [109] | |
| Teriflunomide and Fluconazole | C. albicans | ERV (2 μg/larva) + FLC (1 μg/larva) | [110] |
| Liposomal amphotericin B and flucytosine | Cryptococcus sp. | [111] | |
| Pedalitin and amphotericin B | C. neoformans | AmB 0.3 mg/kg + PED 10 mg/kg | [112] |
| ethyl acetate extract of poincianella pluviosa with amphotericin B | C. neoformans | 2 MIC and 4 MIC (EAF/AmB: 3.9/0.003, 7.8/0.006, and 15.6/0.015 g/mL, respectively, for two strains) | [113] |
| hydroxychloroquine and itraconazole | C. neoformans | 3 mg/kg ITR, and 6.5 mg/kg HCQ | [114] |
| Minocycline and Fluconazole | C. neoformans | 5.2 mg/kg for the FLU and 6.3 mg/kg MINO | [115] |
| Pyrvinium pamoate, fluconazole, itraconazole, voriconazole, posaconazole or amphotericin B | C. neoformans | 200 mg/L | [116] |
| TOR inhibitor AZD8055, Itraconazole, Voriconazole, fluconazole and Posoconazole | Candida sp., C. neoformans, Aspergillus and E. dermatitidis | - | [117] |
| Voriconazole and terbinafine | A. calidoustus | - | [118] |
| Miltefosine alone or Voriconazole combinate | A. fumigatus and A. flavus | 20 or 40 mg/kg of miltefosine free, 100 mg/kg miltefosine encapsulated, 20 mg/kg of miltefosine + 10 mg/Kg of voriconazole free, and 100 mg/kg miltefosine encapsulated with 10 mg/Kg voriconazole | [97] |
| Minocycline and Itraconazole, voriconazole or posoconazole | Aspergillus sp., Fusarium sp. and E. dermatitidis | - | [119] |
| Chlorhexidine and voriconazole or natamycin | Fusarium sp. | VOR (3 μg/mL), CHL (1.5 μg/mL), and VOR + CHL (3 μg/mL and 1.5 μg/mL, respectively). | [120] |
| Coriconazole (plus amphotericin B, posaconazole and caspofungin | R. microspores, R. oryzae, Syncephalastrum racemosum, Lichthemia corymbifera, L. blakesleeana, and L. ramosa, | 1 mg/kg for AMB and CSF and 10 mg/kg for VRC and PSC | [121] |
| Amphotericin B and Terbinefine or itraconazole | M. mycetomatis | 1 mg/kg of AmB, 5.7 mg/kg ITZ, and 7.14 mg/kg of terbinafine | [122] |
| Alkaloids solenopsins | C. auris | 0.5, 5 and 50 µg/mL of synthetic mixture of solenopsins or natural mixture solenopsins | [123] |
12. Nanomaterials as Promising Vehicles in the Future
| Nanomaterial | Fungi | Drug | Ref. |
|---|---|---|---|
| AN | C. albicans and Cryptococcus sp. | 0.78–600 μg/mL miltefosine encapsulated | [95] |
| LP | C. albicans | Anidulafungin | [126] |
| LP | Rhizopus spp., Rhizomucor spp., Mucor spp., and Lichtheimia spp. | Amphotericin B | [80] |
| LP | C. parapsilosis | Amphotericin B | [127] |
| LP | C. albicans | EFG1 | [128] |
| NE | C. auris | Amphotericin B | [94] |
| NE | C. auris | Micafungin | [33] |
| NE | C. auris, C. albicans and C. parapsilosis | Amphotericin B | [19] |
| NE | C. auris, C. albicans and C. parapsilosis | Micafungin | [130] |
| NLC | S. schenckii and C. albicans | Itraconazole | [132] |
| Nq | C. albicans | Farnesol | [133] |
| Mn | C. albicans | Zinc oxide Mn | [134] |
| Nanocarreador | C. neoformans | Propolis | [135] |
| Nanoparticles PLGA | A. brasiliensis | Pterostilbene | [136] |
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Loizou, G.D. Editorial: 3Rs—Strategies for reduction and refinement of animal studies. Front. Pharmacol. 2023, 14, 1200965. [Google Scholar] [CrossRef] [PubMed]
- Titball, R.W.; Senior, N.J. Isolation and primary culture of Galleria mellonella hemocytes for infection studies. F1000Research 2020, 9, 1392. [Google Scholar]
- Giammarino, A.; Bellucci, N.; Angiolella, L. Galleria mellonella as a Model for the Study of Fungal Pathogens: Advantages and Disadvantages. Pathogens 2024, 13, 233. [Google Scholar] [CrossRef]
- Santos Júnior SRDos Barbalho, F.V.; Nosanchuk, J.D.; Amaral, A.C.; Taborda, C.P. Biodistribution and Adjuvant Effect of an Intranasal Vaccine Based on Chitosan Nanoparticles against Paracoccidioidomycosis. J. Fungi 2023, 9, 245. [Google Scholar] [CrossRef]
- Asai, M.; Li, Y.; Newton, S.M.; Robertson, B.D.; Langford, P.R. Galleria mellonella-intracellular bacteria pathogen infection models: The ins and outs. FEMS Microbiol. Rev. 2023, 47, fuad011. [Google Scholar] [CrossRef]
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.Y. Galleria mellonella as a Suitable Model of Bacterial Infection: Past, Present and Future. Front. Cell. Infect. Microbiol. 2021, 11, 782733. [Google Scholar] [CrossRef]
- Fuchs, B.B.; O’Brien, E.; Khoury, J.B.E.; Mylonakis, E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010, 1, 475–482. [Google Scholar] [CrossRef]
- Burd, B.S.; Mussagy, C.U.; Singulani, J.d.L.; Tanaka, J.L.; Scontri, M.; Brasil, G.S.P.; Guerra, N.B.; Assato, P.A.; Abreu, A.P.D.S.; Bebber, C.C.; et al. Galleria Mellonella Larvae as an Alternative to Low-Density Polyethylene and Polystyrene Biodegradation. J. Polym. Environ. 2023, 31, 1232–1241. [Google Scholar] [CrossRef]
- De Jong, A.W.; van Veldhuizen, D.; Groot, A.T.; Hagen, F. Standardized methods to rear high-quality Galleria mellonella larvae for the study of fungal pathogens. Entomol. Exp. Appl. 2022, 170, 1073–1080. [Google Scholar] [CrossRef]
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi LM, A.; Cristina APrata, M.; Jorge AO, C.; Junqueira, J.C. From moths to caterpillars: Ideal conditions for Galleria mellonella rearing for in vivo microbiological studies. Virulence 2018, 9, 383–389. [Google Scholar] [CrossRef]
- Durieux, M.-F.; Melloul, É.; Jemel, S.; Roisin, L.; Dardé, M.-L.; Guillot, J.; Dannaoui, É.; Botterel, F. Galleria mellonella as a screening tool to study virulence factors of Aspergillus fumigatus. Virulence 2021, 12, 818–834. [Google Scholar] [CrossRef]
- Singkum, P.; Suwanmanee, S.; Pumeesat, P.; Luplertlop, N. A powerful in vivo alternative model in scientific research: Galleria mellonella. Acta Microbiol. Immunol. Hung. 2019, 66, 31–55. [Google Scholar] [CrossRef]
- Vilmos, P.; Va Kurucz, E. Mini-review Insect immunity: Evolutionary roots of the mammalian innate immune system. Immunol. Lett. 1998, 62, 59–66. [Google Scholar] [CrossRef]
- Salzet, M. Vertebrate innate immunity resembles a mosaic of invertebrate immune responses. Trends Immunol. 2001, 22, 285–288. [Google Scholar] [CrossRef]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect. Biochem. Mol. Biol. 2002, 32, 1295–1309. Available online: www.elsevier.com/locate/ibmb (accessed on 1 January 2025). [CrossRef]
- Genç, T.T.; Kaya, S.; Günay, M.; Çakaloğlu, Ç. Humoral immune response of Galleria mellonella after mono- and co-injection with Hypericum perforatum extract and Candida albicans. APMIS 2024, 132, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Marinacci, B.; Pellegrini, B.; Cataldi, A.; Dindo, M.L.; Carradori, S.; Grande, R. Immunophenotyping of hemocytes from infected Galleria mellonella larvae as an innovative tool for immune profiling, infection studies and drug screening. Sci. Rep. 2024, 14, 759. [Google Scholar] [CrossRef] [PubMed]
- Marena, G.D.; Ruiz-Gaitán, A.; Garcia-Bustos, V.; Tormo-Mas, M.Á.; Pérez-Royo, J.M.; López, A.; Bernarbe, P.; Ruiz, M.D.P.; Macian, L.Z.; Saez, C.V.; et al. Nanoemulsion Increases the Antifungal Activity of Amphotericin B against Four Candida auris Clades: In Vitro and In Vivo Assays. Microorganisms 2023, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.Q.; Dragotakes, Q.; Kulkarni, M.; Hardwick, J.M.; Casadevall, A. Galleria mellonella immune melanization is fungicidal during infection. Commun. Biol. 2022, 5, 1364. [Google Scholar] [CrossRef]
- Smith, D.F.Q.; Casadevall, A. Fungal immunity and pathogenesis in mammals versus the invertebrate model organism Galleria mellonella. Pathog. Dis. 2021, 79, ftab013. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Zaragoza, O. Immune response of Galleria mellonella against human fungal pathogens. J. Fungi 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Márquez, I.A.; Manrique-Moreno, M.; Patiño-González, E.; Jemioła-Rzemińska, M.; Strzałka, K. Effect of antimicrobial peptides from Galleria mellonella on molecular models of Leishmania membrane. Thermotropic and fluorescence anisotropy study. J. Antibiot. 2018, 71, 642–652. [Google Scholar] [CrossRef]
- Cytryńska, M.; Mak, P.; Zdybicka-Barabas, A.; Suder, P.; Jakubowicz, T. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 2007, 28, 533–546. [Google Scholar] [CrossRef]
- Brown, S.E.; Howard, A.; Kasprzak, A.B.; Gordon, K.H.; East, P.D. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect. Biochem. Mol. Biol. 2009, 39, 792–800. [Google Scholar] [CrossRef]
- Curtis, A.; Binder, U.; Kavanagh, K. Galleria mellonella Larvae as a Model for Investigating Fungal—Host Interactions. Front. Fungal Biol. 2022, 3, 893494. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Ding, Y.; Yi, Y. Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva: Cell types and their role in the innate immunity. Tissue Cell 2016, 48, 297–304. [Google Scholar] [CrossRef]
- Sowa-Jasiłek, A.; Zdybicka-Barabas, A.; Stączek, S.; Wydrych, J.; Skrzypiec, K.; Mak, P.; Deryło, K.; Tchórzewski, M.; Cytryńska, M. Galleria mellonella lysozyme induces apoptotic changes in Candida albicans cells. Microbiol. Res. 2016, 193, 121–131. [Google Scholar] [CrossRef]
- Siscar-Lewin, S.; Hube, B.; Brunke, S. Emergence and evolution of virulence in human pathogenic fungi. Trends Microbiol. 2022, 30, 693–704. [Google Scholar] [CrossRef]
- Ratcliffe, N.A. Invertebrate immunity—A primer for the non-specialist. Immunol. Lett. 1985, 10, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Angiolella, L. Virulence Regulation and Drug-Resistance Mechanism of Fungal Infection. Microorganisms 2022, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Marena, G.D.; Carvalho, G.C.; Dos Santos Ramos, M.A.; Chorilli, M.; Bauab, T.M. Anti-Candida auris activity in vitro and in vivo of micafungin loaded nanoemulsions. Med. Mycol. 2023, 61, myac090. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, challenges, and promising strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agriculture (Pol’nohospodarstvo) 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Greeff-Laubscher, M.R.; Beukes, I.; Marais, G.J.; Jacobs, K. Mycotoxin production by three different toxigenic fungi genera on formulated abalone feed and the effect of an aquatic environment on fumonisins. Mycology 2020, 11, 105–117. [Google Scholar] [CrossRef]
- García-Carnero, L.C.; Clavijo-Giraldo, D.M.; Gómez-Gaviria, M.; Lozoya-Pérez, N.E.; Tamez-Castrellón, A.K.; López-Ramírez, L.A.; Mora-Montes, H.M. Early virulence predictors during the candida species—Galleria mellonella interaction. J. Fungi 2020, 6, 152. [Google Scholar] [CrossRef]
- Hernando-Ortiz, A.; Eraso, E.; Quindós, G.; Mateo, E. Candidiasis by Candida glabrata, Candida nivariensis and Candida bracarensis in Galleria mellonella: Virulence and therapeutic responses to echinocandins. J. Fungi 2021, 7, 998. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Bordallo-Cardona, M.Á.; Borghi, E.; Falleni, M.; Tosi, D.; Muñoz, P.; Escribano, P.; Guinea, J. Candida isolates causing candidemia show different degrees of virulence in Galleria mellonella. Med. Mycol. 2020, 58, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, V.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.; Sigona-Giangreco, I.A.; Cabañero-Navalon, M.D.; Sabalza-Baztán, O.; Salavert-Lletí, M.; Tormo, M.Á.; Pemán, J. Characterization of the Differential Pathogenicity of Candida auris in a Galleria mellonella Infection Model. Microbiol. Spectr. 2021, 9, e0001321. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, V.; Pemán, J.; Ruiz-Gaitán, A.; Cabañero-Navalon, M.D.; Cabanilles-Boronat, A.; Fernández-Calduch, M.; Marcilla-Barreda, L.; Sigona-Giangreco, I.A.; Salavert, M.; Tormo-Mas, M.Á.; et al. Host–pathogen interactions upon Candida auris infection: Fungal behaviour and immune response in Galleria mellonella. Emerg. Microbes Infect. 2022, 11, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Pál, S.E.; Tóth, R.; Nosanchuk, J.D.; Vágvölgyi, C.; Németh, T.; Gácser, A. A candida parapsilosis overexpression collection reveals genes required for pathogenesis. J. Fungi 2021, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Pinzón, E.N.; Rey, F.M.; Martinez, H.; Parra Giraldo, C.M.; Celis Ramírez, A.M. Galleria mellonella as a Novelty in vivo Model of Host-Pathogen Interaction for Malassezia furfur CBS 1878 and Malassezia pachydermatis CBS 1879. Front. Cell. Infect. Microbiol. 2020, 10, 199. [Google Scholar] [CrossRef]
- García-Rodas, R.; Casadevall, A.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS ONE 2011, 6, e24485. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Herrero-Fernández, I.; García-Barbazán, I.; Scorzoni, L.; Rueda, C.; Rossi, S.A.; García-Rodas, R.; Zaragoza, O. Cryptococcus neoformans induces antimicrobial responses and behaves as a facultative intracellular pathogen in the non mammalian model Galleria mellonella. Virulence 2014, 6, 66–74. [Google Scholar] [CrossRef]
- Benaducci, T.; Sardi, J.d.C.O.; Lourencetti, N.M.S.; Scorzoni, L.; Gullo, F.P.; Rossi, S.A.; Derissi, J.B.; Prata, M.C.d.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Virulence of Cryptococcus sp. biofilms in vitro and in vivo using Galleria mellonella as an alternative model. Front. Microbiol. 2016, 7, 290. [Google Scholar] [CrossRef]
- Clavijo-Giraldo, D.M.; Matínez-Alvarez, J.A.; Lopes-Bezerra, L.M.; Ponce-Noyola, P.; Franco, B.; Almeida, R.S.; Mora-Montes, H.M. Analysis of Sporothrix schenckii sensu stricto and Sporothrix brasiliensis virulence in Galleria mellonella. J. Microbiol. Methods 2016, 122, 73–77. [Google Scholar] [CrossRef]
- Lozoya-Pérez, N.E.; Clavijo-Giraldo, D.M.; Martínez-Duncker, I.; García-Carnero, L.C.; López-Ramírez, L.A.; Niño-Vega, G.A.; Mora-Montes, H.M. Influences of the Culturing Media in the Virulence and Cell Wall of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. J. Fungi 2020, 6, 323. [Google Scholar] [CrossRef]
- Reis, N.F.; de Jesus, M.C.S.; Souza, L.C.d.S.V.d.; Alcântara, L.M.; Rodrigues, J.A.d.C.; Brito, S.C.P.; Penna, P.d.A.; Vieira, C.S.; Silva, J.R.S.; Penna, B.d.A.; et al. Sporothrix brasiliensis Infection Modulates Antimicrobial Peptides and Stress Management Gene Expression in the Invertebrate Biomodel Galleria mellonella. J. Fungi 2023, 9, 1053. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; De Paula e Silva, A.C.A.; Singulani, J.D.L.; Leite, F.S.; De Oliveira, H.C.; Moraes da Silva, R.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Comparison of virulence between Paracoccidioides brasiliensis and Paracoccidioides lutzii using Galleria mellonella as a host model. Virulence 2015, 6, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; De Paula E Silva, A.C.A.; De Oliveira, H.C.; Marcos, C.M.; Singulani, J.D.L.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Can passage in Galleria mellonella activate virulence factors of Paracoccidioides brasiliensis as in the murine model? Med. Mycol. 2018, 56, 374–377. [Google Scholar] [CrossRef]
- Marcos, C.M.; Tamer, G.; de Oliveira, H.C.; Assato, P.A.; Scorzoni, L.; Santos, C.T.; de Lacorte Singulani, J.; da Silva, J.d.F.; de Almeida, R.; de Paula e Silva, A.C.A.; et al. Down-regulation of TUFM impairs host cell interaction and virulence by Paracoccidioides brasiliensis. Sci. Rep. 2019, 9, 17206. [Google Scholar] [CrossRef]
- De Souza Pitangui, N.; Fernandes, F.F.; Aparecido da Silva, T.; Gonçales, R.A.; Roque-Barreira, M.C. Role of paracoccin on Paracoccidioides brasiliensis virulence and susceptibility to antifungal drugs in the Galleria mellonella larvae model. Virulence 2023, 14, 2150455. [Google Scholar] [CrossRef]
- Gonçales, R.A.; Ricci-Azevedo, R.; Vieira, V.C.S.; Fernandes, F.F.; de O Thomaz, S.M.; Carvalho, A.; Vendruscolo, P.E.; Cunha, C.; Roque-Barreira, M.C.; Rodrigues, F. Paracoccin Overexpression in Paracoccidioides brasiliensis Enhances Fungal Virulence by Remodeling Chitin Properties of the Cell Wall. J. Infect. Dis. 2021, 224, 164–174. [Google Scholar] [CrossRef]
- Mendoza Barker, M.; Saeger, S.; Campuzano, A.; Yu, J.J.; Hung, C.Y. Galleria mellonella Model of Coccidioidomycosis for Drug Susceptibility Tests and Virulence Factor Identification. J. Fungi 2024, 10, 131. [Google Scholar] [CrossRef]
- Garcia, J.A.; Vu, K.; Thompson, G.R.; Gelli, A. Characterization of the Growth and Morphology of a BSL-2 Coccidioides posadasii Strain That Persists in the Parasitic Life Cycle at Ambient CO2. J. Fungi 2022, 8, 455. [Google Scholar] [CrossRef]
- Thomaz, L.; García-Rodas, R.; Guimarães, A.J.; Taborda, C.P.; Zaragoza, O.; Nosanchuk, J.D. Galleria mellonella as a model host to study Paracoccidioides Lutzii and Histoplasma Capsulatum. Virulence 2013, 4, 139–146. [Google Scholar] [CrossRef]
- Fregonezi, N.F.; Oliveira, L.T.; Singulani, J.d.L.; Marcos, C.M.; dos Santos, C.T.; Taylor, M.L.; Mendes-Giannini, M.J.S.; de Oliveira, H.C.; Fusco-Almeida, A.M. Heat Shock Protein 60, Insights to Its Importance in Histoplasma capsulatum: From Biofilm Formation to Host-Interaction. Front. Cell. Infect. Microbiol. 2021, 10, 591950. [Google Scholar] [CrossRef]
- Huang, X.; Li, D.; Xi, L.; Mylonakis, E. Galleria mellonella Larvae as an Infection Model for Penicillium marneffei. Mycopathologia 2015, 180, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Maurer, E.; Browne, N.; Surlis, C.; Jukic, E.; Moser, P.; Kavanagh, K.; Lass-Flörl, C.; Binder, U. Galleria mellonella as a host model to study Aspergillus terreus virulence and amphotericin B resistance. Virulence 2015, 6, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Won, E.J.; Choi, M.J.; Shin, J.H.; Park, Y.-J.; Byun, S.A.; Jung, J.S.; Kim, S.H.; Shin, M.G.; Suh, S.-P. Diversity of clinical isolates of Aspergillus terreus in antifungal susceptibilities, genotypes and virulence in Galleria mellonella model: Comparison between respiratory and ear isolates. PLoS ONE 2017, 12, e0186086. [Google Scholar] [CrossRef] [PubMed]
- Bakti, F.; Sasse, C.; Heinekamp, T.; Pócsi, I.; Braus, G.H. Heavy metal-induced expression of PcaA provides cadmium tolerance to Aspergillus fumigatus and supports its virulence in the Galleria mellonella model. Front. Microbiol. 2018, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Stączek, S.; Zdybicka-Barabas, A.; Pleszczyńska, M.; Wiater, A.; Cytryńska, M. Aspergillus niger α-1,3-glucan acts as a virulence factor by inhibiting the insect phenoloxidase system. J. Invertebr. Pathol. 2020, 171, 107341. [Google Scholar] [CrossRef]
- Curtis, A.; Walshe, K.; Kavanagh, K. Prolonged Subculturing of Aspergillus fumigatus on Galleria Extract Agar Results in Altered Virulence and Sensitivity to Antifungal Agents. Cells 2023, 12, 1065. [Google Scholar] [CrossRef]
- Li, X.; Feng, R.; Luo, P.; Zhang, Y.; Lu, L. Synergistic effects of putative Ca2+-binding sites of calmodulin in fungal development, temperature stress and virulence of Aspergillus fumigatus. Virulence 2024, 15, 2290757. [Google Scholar] [CrossRef]
- Rudhra, O.; Gnanam, H.; Sivaperumal, S.; Namperumalsamy, V.P.; Prajna, L.; Kuppamuthu, D. Melanin depletion affects Aspergillus flavus conidial surface proteins, architecture, and virulence. Appl. Microbiol. Biotechnol. 2024, 108, 291. [Google Scholar] [CrossRef]
- Wassano, N.S.; da Silva, G.B.; Reis, A.H.; AGerhardt, J.; Antoniel, E.P.; Akiyama, D.; Rezende, C.P.; Neves, L.X.; Vasconcelos, E.J.; de Figueiredo, F.L.; et al. Sirtuin E deacetylase is required for full virulence of Aspergillus fumigatus. Commun. Biol. 2024, 7, 704. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, J.; Zhou, Z.; Goldman, G.H.; Lu, L.; Zhang, Y. Histone acetyltransferase Sas3 contributes to fungal development, cell wall integrity, and virulence in Aspergillus fumigatus. Appl. Environ. Microbiol. 2024, 90, e0188523. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Ye, J.; Lu, L. The fungal-specific histone acetyltransferase Rtt109 regulates development, DNA damage response, and virulence in Aspergillus fumigatus. Mol. Microbiol. 2021, 115, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Dietl, A.M.; Binder, U.; Bauer, I.; Shadkchan, Y.; Osherov, N.; Haas, H. Arginine auxotrophy affects siderophore biosynthesis and attenuates virulence of Aspergillus fumigatus. Genes 2020, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Pereira da Costa Filho, A.; Thomaz Pereira Brancini, G.; Alves de Castro, P.; Valero, C.; Alves Ferreira Filho, J.; Pereira Silva, L.; Rocha, M.C.; Malavazi, I.; Pontes, J.G.D.M.; Fill, T.; et al. Aspergillus fumigatus G-Protein Coupled Receptors GprM and GprJ Are Important for the Regulation of the Cell Wall Integrity Pathway, Secondary Metabolite Production, and Virulence. 2020. Available online: https://journals.asm.org/doi/10.1128/mbio.02458-20 (accessed on 1 January 2025).
- Jones, A.M.; Panaccione, D.G. Ergot Alkaloids Contribute to the Pathogenic Potential of the Fungus Aspergillus leporis. Appl. Environ. Microbiol. 2023, 89, e0041523. [Google Scholar] [CrossRef] [PubMed]
- Hatmaker, E.A.; Rangel-Grimaldo, M.; Raja, H.A.; Pourhadi, H.; Knowles, S.L.; Fuller, K.; Adams, E.M.; Lightfoot, J.D.; Bastos, R.W.; Goldman, G.H.; et al. Genomic and Phenotypic Trait Variation of the Opportunistic Human Pathogen Aspergillus flavus and Its Close Relatives. Microbiol. Spectr. 2022, 10, e0306922. [Google Scholar] [CrossRef]
- Usman, S.; Du, C.; Qin, Q.; Odiba, A.S.; He, R.; Wang, B.; Jin, C.; Fang, W. Phosphomannose Isomerase Is Involved in Development, Stress Responses, and Pathogenicity of Aspergillus flavus. Microbiol. Spectr. 2022, 10, e0202722. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, D.; Yang, S.; Fu, W.; Ma, D.; Yao, Y.; Lin, H.; Yuan, J.; Yang, Y.; Zhuang, Z. Regulation of Fungal Morphogenesis and Pathogenicity of Aspergillus flavus by Hexokinase AfHxk1 through Its Domain Hexokinase_2. J. Fungi 2023, 9, 1077. [Google Scholar] [CrossRef]
- Djenontin, E.; Debourgogne, A.; Mousavi, B.; Delhaes, L.; Cornet, M.; Valsecchi, I.; Adebo, M.; Guillot, J.; Botterel, F.; Dannaoui, E. Azole resistance in Aspergillus flavus and associated fitness cost. Mycoses 2024, 67, e13766. [Google Scholar] [CrossRef]
- Navarro-Velasco, G.Y.; Prados-Rosales, R.C.; Ortíz-Urquiza, A.; Quesada-Moraga, E.; Di Pietro, A. Galleria mellonella as model host for the trans-kingdom pathogen Fusarium oxysporum. Fungal Genet. Biol. 2011, 48, 1124–1129. [Google Scholar] [CrossRef]
- López-Berges, M.S. ZafA-mediated regulation of zinc homeostasis is required for virulence in the plant pathogen Fusarium oxysporum. Mol. Plant Pathol. 2020, 21, 244–249. [Google Scholar] [CrossRef]
- Maurer, E.; Hörtnagl, C.; Lackner, M.; Grässle, D.; Naschberger, V.; Moser, P.; Segal, E.; Semis, M.; Lass-Flörl, C.; Binder, U. Galleria mellonella as a model system to study virulence potential of mucormycetes and evaluation of antifungal treatment. Med. Mycol. 2019, 57, 351–362. [Google Scholar] [CrossRef]
- Hassan, M.I.A.; Keller, M.; Hillger, M.; Binder, U.; Reuter, S.; Herold, K.; Telagathoti, A.; Dahse, H.-M.; Wicht, S.; Trinks, N.; et al. The impact of episporic modification of Lichtheimia corymbifera on virulence and interaction with phagocytes. Comput. Struct. Biotechnol. J. 2021, 19, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhang, M.; Xu, W.; Wang, X.; Zheng, H.; Mei, H.; Liu, W. Characterization of mitogenomes from four Mucorales species and insights into pathogenicity. Mycoses 2022, 65, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Szebenyi, C.; Gu, Y.; Gebremariam, T.; Kocsubé, S.; Kiss-Vetráb, S.; Jáger, O.; Patai, R.; Spisák, K.; Sinka, R.; Binder, U.; et al. cotH Genes Are Necessary for Normal Spore Formation and Virulence in Mucor lusitanicus. mBio 2023, 14, e0338622. [Google Scholar] [CrossRef] [PubMed]
- Samdavid Thanapaul, R.J.R.; Roberds, A.; Rios, K.E.; Walsh, T.J.; Bobrov, A.G. Hyphae of Rhizopus arrhizus and Lichtheimia corymbifera Are More Virulent and Resistant to Antifungal Agents Than Sporangiospores In Vitro and in Galleria mellonella. J. Fungi 2023, 9, 958. [Google Scholar] [CrossRef]
- Fuchs, B.B.; Bishop, L.R.; Kovacs, J.A.; Mylonakis, E. Galleria mellonella are Resistant to Pneumocystis murina Infection. Mycopathologia 2011, 171, 273–277. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Liu, H.; Xi, L.; Cooper, C.R. Corrigendum: Increased virulence of albino mutant of Fonsecaea monophora in Galleria mellonella. Med. Mycol. 2019, 57, 1018–1023. [Google Scholar] [CrossRef]
- Shi, D.; Yang, Z.; Liao, W.; Liu, C.; Zhao, L.; Su, H.; Wang, X.; Mei, H.; Chen, M.; Song, Y.; et al. Galleria mellonella in vitro model for chromoblastomycosis shows large differences in virulence between isolates. IMA Fungus 2024, 15, 5. [Google Scholar] [CrossRef]
- Ahmed, A.O.A.; Van Leeuwen, W.; Fahal, A.; Van De Sande, W.; Verbrugh, H.; Van Belkum, A. Mycetoma caused by Madurella mycetomatis: A neglected infectious burden. Lancet Infect. Dis. 2004, 4, 566–574. [Google Scholar] [CrossRef]
- Kloezen, W.; van Helvert-van Poppel, M.; Fahal, A.H.; van de Sande, W.W.J. A madurella mycetomatis grain model in Galleria mellonella larvae. PLoS Neglected Trop. Dis. 2015, 9, e0003926. [Google Scholar] [CrossRef]
- Achterman, R.R.; Smith, A.R.; Oliver, B.G.; White, T.C. Sequenced dermatophyte strains: Growth rate, conidiation, drug susceptibilities, and virulence in an invertebrate model. Fungal Genet. Biol. 2011, 48, 335–341. [Google Scholar] [CrossRef]
- Li, W.; Metin, B.; White, T.C.; Heitman, J. Organization and evolutionary trajectory of the mating type (MAT) locus in dermatophyte and dimorphic fungal pathogens. Eukaryot. Cell 2010, 9, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Utilising Galleria mellonella larvae for studying in vivo activity of conventional and novel antimicrobial agents. Pathog. Dis. 2020, 78, ftaa059. [Google Scholar] [CrossRef] [PubMed]
- Marena, G.D.; Ramos, M.A.D.S.; Lima, L.C.; Chorilli, M.; Bauab, T.M. Galleria mellonella for systemic assessment of anti-Candida auris using amphotericin B loaded in nanoemulsion. Sci. Total Environ. 2021, 807, 151023. [Google Scholar] [CrossRef] [PubMed]
- de Castro Spadari, C.; da Silva de Bastiani, F.W.M.; Lopes, L.B.; Ishida, K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. Int. J. Nanomed. 2019, 14, 5187–5199. [Google Scholar] [CrossRef]
- Barreto, T.L.; Rossato, L.; de Freitas, A.L.D.; Meis, J.F.; Lopes, L.B.; Colombo, A.L.; Ishida, K. Miltefosine as an alternative strategy in the treatment of the emerging fungus Candida auris. Int. J. Antimicrob. Agents 2020, 56, 106049. [Google Scholar] [CrossRef]
- Barreto, T.L.; Lopes, L.B.; Melo, A.S.A.; Ishida, K. In vivo synergism of free miltefosine or in alginate-based nanocarrier combined with voriconazole on aspergillosis. Future Microbiol. 2021, 16, 1153–1160. [Google Scholar] [CrossRef]
- Nyilasi, I.; Kocsubé, S.; Krizsán, K.; Galgóczy, L.; Pesti, M.; Papp, T.; Vágvölgyi, C. In vitro synergistic interactions of the effects of various statins and azoles against some clinically important fungi. FEMS Microbiol. Lett. 2010, 307, 175–184. [Google Scholar] [CrossRef]
- Ajdidi, A.; Sheehan, G.; Elteen, K.A.; Kavanagh, K. Assessment of the in vitro and in vivo activity of atorvastatin against Candida albicans. J. Med. Microbiol. 2019, 68, 1497–1506. [Google Scholar] [CrossRef]
- Osmanov, A.; Wise, A.; Denning, D.W. In vitro and in vivo efficacy of miramistin against drug-resistant fungi. J. Med. Microbiol. 2019, 68, 1047–1052. [Google Scholar] [CrossRef]
- Nile, C.; Falleni, M.; Cirasola, D.; Alghamdi, A.; Anderson, O.F.; Delaney, C.; Ramage, G.; Ottaviano, E.; Tosi, D.; Bulfamante, G.; et al. Repurposing Pilocarpine Hydrochloride for Treatment of Candida albicans Infections. mSphere 2019, 4, e00689-18. [Google Scholar] [CrossRef]
- Lim, W.; Nyuykonge, B.; Eadie, K.; Konings, M.; Smeets, J.; Fahal, A.; Bonifaz, A.; Todd, M.; Perry, B.; Samby, K.; et al. Screening the pandemic response box identified benzimidazole carbamates, Olorofim and ravuconazole as promising drug candidates for the treatment of eumycetoma. PLoS Neglected Trop. Dis. 2022, 16, e0010159. [Google Scholar] [CrossRef] [PubMed]
- Konings, M.; Eadie, K.; Strepis, N.; Nyuykonge, B.; Fahal, A.H.; Verbon, A.; van de Sande, W.W.J. The combination of manogepix and itraconazole is synergistic and inhibits the growth of Madurella mycetomatis in vitro but not in vivo. Med. Mycol. 2023, 61, myad118. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Johnson, C.; Berman, J.; Coste, A.T.; Cuomo, C.A.; Perlin, D.S.; Bicanic, T.; Harrison, T.S.; Wiederhold, N.; Bromley, M.; et al. The importance of antimicrobial resistance in medical mycology. Nat. Commun. 2022, 13, 5352. [Google Scholar] [CrossRef]
- Marena, G.D.; Carvalho, G.C.; Ruiz-Gaitán, A.; Onisto, G.S.; Bugalho, B.C.M.; Genezini, L.M.V.; Dos Santos, M.O.; Blanco, A.L.; Chorilli, M.; Bauab, T.M. Potential Activity of Micafungin and Amphotericin B Co-Encapsulated in Nanoemulsion against Systemic Candida auris Infection in a Mice Model. J. Fungi 2024, 10, 253. [Google Scholar] [CrossRef]
- Lu, M.; Yang, X.; Yu, C.; Gong, Y.; Yuan, L.; Hao, L.; Sun, S. Linezolid in combination with azoles induced synergistic effects against Candida albicans and protected Galleria mellonella against experimental candidiasis. Front. Microbiol. 2019, 10, 3142. [Google Scholar]
- Li, Y.; Jiao, P.; Li, Y.; Gong, Y.; Chen, X.; Sun, S. The Synergistic Antifungal Effect and Potential Mechanism of D-Penicillamine Combined With Fluconazole Against Candida albicans. Front. Microbiol. 2019, 10, 2853. [Google Scholar] [CrossRef]
- Vega-Chacón, Y.; de Albuquerque, M.C.; Pavarina, A.C.; Goldman, G.H.; de Oliveira Mima, E.G. Verapamil inhibits efflux pumps in Candida albicans, exhibits synergism with fluconazole, and increases survival of Galleria mellonella. Virulence 2021, 12, 231–243. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Duan, X.; Cui, M.; Xing, W.; Zheng, S. Synergistic effect of eravacycline combined with fluconazole against resistant Candida albicans in vitro and in vivo. Get. Expert Rev. Anti Infect. Ther. 2023, 11, 1259–1267. [Google Scholar] [CrossRef]
- Li, X.; Kong, B.; Sun, Y.; Sun, F.; Yang, H.; Zheng, S. Synergistic potential of teriflunomide with fluconazole against resistant Candida albicans in vitro and in vivo. Front. Cell. Infect. Microbiol. 2023, 13, 1282320. [Google Scholar] [CrossRef]
- Word Health Organization. Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease Among Adults, Adolescents and Children Living with HIV [Internet]. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581832/ (accessed on 19 October 2024).
- Sangalli-Leite, F.; Scorzoni, L.; de Paula e Silva, A.C.A.; de Fátimada Silva, J.; de Oliveira, H.C.; de Lacorte Singulani, J.; Gullo, F.P.; da Silva, R.M.; Regasini, L.O.; da Silva, D.H.S.; et al. Synergistic effect of pedalitin and amphotericin B against Cryptococcus neoformans by in vitro and in vivo evaluation. Int. J. Antimicrob. Agents 2016, 48, 504–511. [Google Scholar] [CrossRef]
- Andriani, G.M.; Morguette, A.E.B.; Spoladori, L.F.A.; Pereira, P.M.L.; Cabral, W.R.C.; Fernandes, B.T.; Tavares, E.R.; Almeida, R.S.; Lancheros, C.A.C.; Nakamura, C.V.; et al. Antifungal Combination of Ethyl Acetate Extract of Poincianella pluviosa (DC.) L. P. Queiros Stem Bark With Amphotericin B in Cryptococcus neoformans. Front. Microbiol. 2021, 12, 660645. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Long, X.; Jia, S.; Zhu, J.; Zhou, Z.; Ahmed, S.; Jiang, Y.; Jiang, Y. In vitro and in vivo synergistic effects of hydroxychloroquine and itraconazole on Cryptococcus neoformans. Folia Microbiol. 2023, 68, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Cao, Z.; Lv, N.; Zhang, H.; Liu, Y.; Hu, L.; Li, J. Minocycline and Fluconazole Have a Synergistic Effect Against Cryptococcus neoformans Both in vitro and in vivo. Front. Microbiol. 2020, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Chen, M.; Xiao, J.; Fang, H. Synergistic effect of pyrvinium pamoate and posaconazole against Cryptococcus neoformans in vitro and in vivo. Front. Cell. Infect. Microbiol. 2022, 12, 1074903. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, L.; Yao, Z.; Gao, L.; Yang, J.; Zeng, T. In Vitro and In Vivo Interactions of TOR Inhibitor AZD8055 and Azoles Against Pathogenic Fungi [Internet]. 2022. Available online: https://journals.asm.org/journal/spectrum (accessed on 1 January 2025).
- Glampedakis, E.; Coste, A.T.; Aruanno, M.; Bachmann, D.; Delarze, E.; Erard, V.; Lamoth, F. Efficacy of antifungal monotherapies and combinations against Aspergillus calidoustus. Antimicrob. Agents Chemother. 2018, 62, e01137-18. [Google Scholar] [CrossRef]
- Gao, L.; Sun, Y.; Yuan, M.; Li, M.; Zeng, T. In Vitro and In Vivo Study on the Synergistic Effect of Minocycline and Azoles against Pathogenic Fungi [Internet]. Antimicrob. Agents Chemother. 2020, 64, e00290-20. [Google Scholar] [CrossRef]
- Jiang, T.; Tang, J.; Wu, Z.; Sun, Y.; Tan, J.; Yang, L. The combined utilization of Chlorhexidine and Voriconazole or Natamycin to combat Fusarium infections. BMC Microbiol. 2020, 20, 275. [Google Scholar] [CrossRef]
- Macedo, D.; Leonardelli, F.; Dudiuk, C.; Vitale, R.G.; Del Valle, E.; Giusiano, G.; Gamarra, S.; Garcia-Effron, G. In vitro and in vivo evaluation of voriconazole-containing antifungal combinations against mucorales using a Galleria mellonella model of mucormycosis. J. Fungi 2019, 5, 5. [Google Scholar] [CrossRef]
- Eadie, K.; Parel, F.; Helvert-van Poppel, M.; Fahal, A.; van de Sande, W. Combining two antifungal agents does not enhance survival of Galleria mellonella larvae infected with Madurella mycetomatis. Trop. Med. Int. Health 2017, 22, 696–702. [Google Scholar] [CrossRef]
- Honorato, L.; Bonilla, J.J.A.; da Silva, L.R.; Kornetz, J.; Zamith-Miranda, D.; Valdez, A.F.; Nosanchuk, J.D.; Fox, E.G.P.; Nimrichter, L. Alkaloids solenopsins from fire ants display in vitro and in vivo activity against the yeast Candida auris. Virulence 2024, 15, 2413329. [Google Scholar] [CrossRef]
- Marena, G.D.; Ramos, M.A.d.S.; Carvalho, G.C.; Junior, J.A.P.; Resende, F.A.; Corrêa, I.; Ono, G.Y.B.; Araujo, V.H.S.; de Camargo, B.A.F.; Bauab, T.M.; et al. Natural product-based nanomedicine applied to fungal infection treatment: A review of the last 4 years. Phytotherapy Res. 2022, 36, 2710–2745. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Vera-González, N.; Bailey-Hytholt, C.M.; Langlois, L.; de Camargo Ribeiro, F.; de Souza Santos, E.L.; Junqueira, J.C.; Shukla, A. Anidulafungin liposome nanoparticles exhibit antifungal activity against planktonic and biofilm Candida albicans. J. Biomed. Mater. Res. Part A 2020, 108, 2263–2276. [Google Scholar] [CrossRef] [PubMed]
- Binder, U.; Arastehfar, A.; Schnegg, L.; Hörtnagl, C.; Hilmioğlu-Polat, S.; Perlin, D.S.; Lass-Flörl, C. Efficacy of lamb against emerging azole-and multidrug-resistant Candida parapsilosis isolates in the Galleria mellonella model. J. Fungi 2020, 6, 377. [Google Scholar] [CrossRef]
- Araújo, D.; Gaspar, R.; Mil-Homens, D.; Henriques, M.; Silva, B.F.B.; Silva, S. Cationic lipid-based formulations for encapsulation and delivery of anti-EFG1 2’ OMethylRNA oligomer. Med. Mycol. 2022, 60, myac030. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Marena, G.D.; López, A.; Carvalho, G.C.; Marín, M.d.P.; Ruiz, M.D.P.; Pérez-Royo, J.M.; Tormo-Mas, M.Á.; Bernabé, P.; Valentín, E.; Bauab, T.M.; et al. Sunflower Oil and Cholesterol Nanoemulsion: A Novel Carrier for Micafungin to Combat Multi-Resistant Candida auris. Pathogens 2024, 13, 549. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Passos, J.S.; Martino LC de Dartora, V.F.C.; Araujo GLB de Ishida, K.; Lopes, L.B. Development, skin targeting and antifungal efficacy of topical lipid nanoparticles containing itraconazole. Eur. J. Pharm. Sci. 2020, 149, 105296. [Google Scholar] [CrossRef]
- Costa, A.F.; da Silva, J.T.; Martins, J.A.; Rocha, V.L.; de Menezes, L.B.; Amaral, A.C. Chitosan nanoparticles encapsulating farnesol evaluated in vivo against Candida albicans. Braz. J. Microbiol. 2024, 55, 143–154. [Google Scholar] [CrossRef]
- Xu, M.-N.; Li, L.; Pan, W.; Zheng, H.-X.; Wang, M.-L.; Peng, X.-M.; Dai, S.-Q.; Tang, Y.-M.; Zeng, K.; Huang, X.-W. Zinc Oxide Nanoparticles Prime a Protective Immune Response in Galleria mellonella to Defend Against Candida albicans. Front. Microbiol. 2021, 12, 766138. [Google Scholar] [CrossRef] [PubMed]
- Thammasit, P.; Tharinjaroen, C.S.; Tragoolpua, Y.; Rickerts, V.; Georgieva, R.; Bäumler, H.; Tragoolpua, K. Targeted Propolis-Loaded Poly (Butyl) Cyanoacrylate Nanoparticles: An Alternative Drug Delivery Tool for the Treatment of Cryptococcal Meningitis. Front. Pharmacol. 2021, 12, 723727. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, A.; Palocci, C.; Chronopoulou, L.; De Angelis, G.; Badiali, C.; Petruccelli, V.; D’angeli, S.; Pasqua, G.; Simonetti, G. Poly-(lactic-co-glycolic) Acid Nanoparticles Entrapping Pterostilbene for Targeting Aspergillus Section Nigri. Molecules 2022, 27, 5424. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marena, G.D.; Thomaz, L.; Nosanchuk, J.D.; Taborda, C.P. Galleria mellonella as an Invertebrate Model for Studying Fungal Infections. J. Fungi 2025, 11, 157. https://doi.org/10.3390/jof11020157
Marena GD, Thomaz L, Nosanchuk JD, Taborda CP. Galleria mellonella as an Invertebrate Model for Studying Fungal Infections. Journal of Fungi. 2025; 11(2):157. https://doi.org/10.3390/jof11020157
Chicago/Turabian StyleMarena, Gabriel Davi, Luciana Thomaz, Joshua Daniel Nosanchuk, and Carlos Pelleschi Taborda. 2025. "Galleria mellonella as an Invertebrate Model for Studying Fungal Infections" Journal of Fungi 11, no. 2: 157. https://doi.org/10.3390/jof11020157
APA StyleMarena, G. D., Thomaz, L., Nosanchuk, J. D., & Taborda, C. P. (2025). Galleria mellonella as an Invertebrate Model for Studying Fungal Infections. Journal of Fungi, 11(2), 157. https://doi.org/10.3390/jof11020157









