Half-Century Scientometric Analysis: Unveiling the Excellence of Fungi as Biocontrol Agents and Biofertilisers
Abstract
1. Introduction
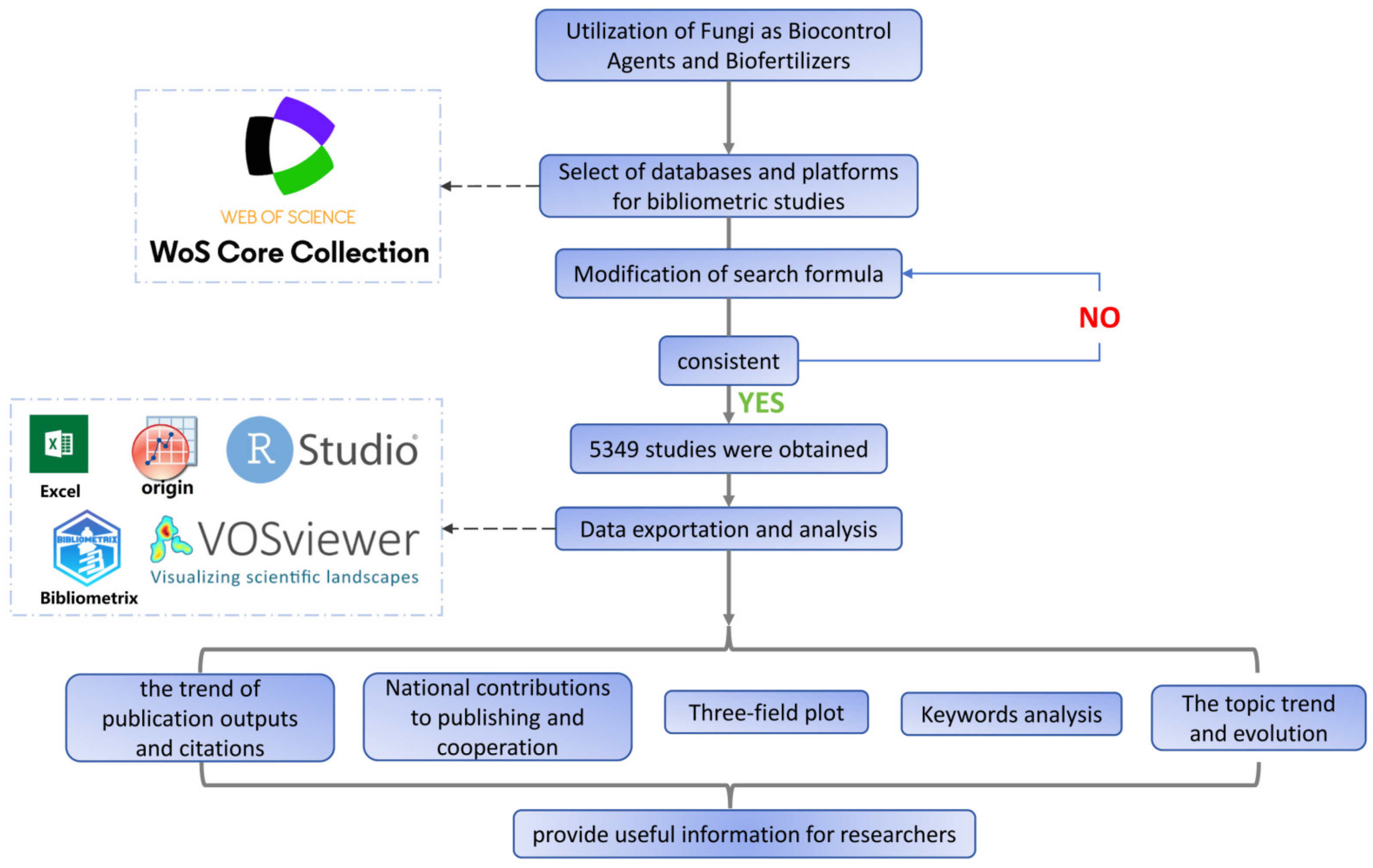
2. Materials and Methods
2.1. Data Collection and Processing
2.2. Data Analysis
3. Results
3.1. The Increasing Number of Publications Signifies That BCAs and Biofertilisers Have Emerged as Focal Research Topics
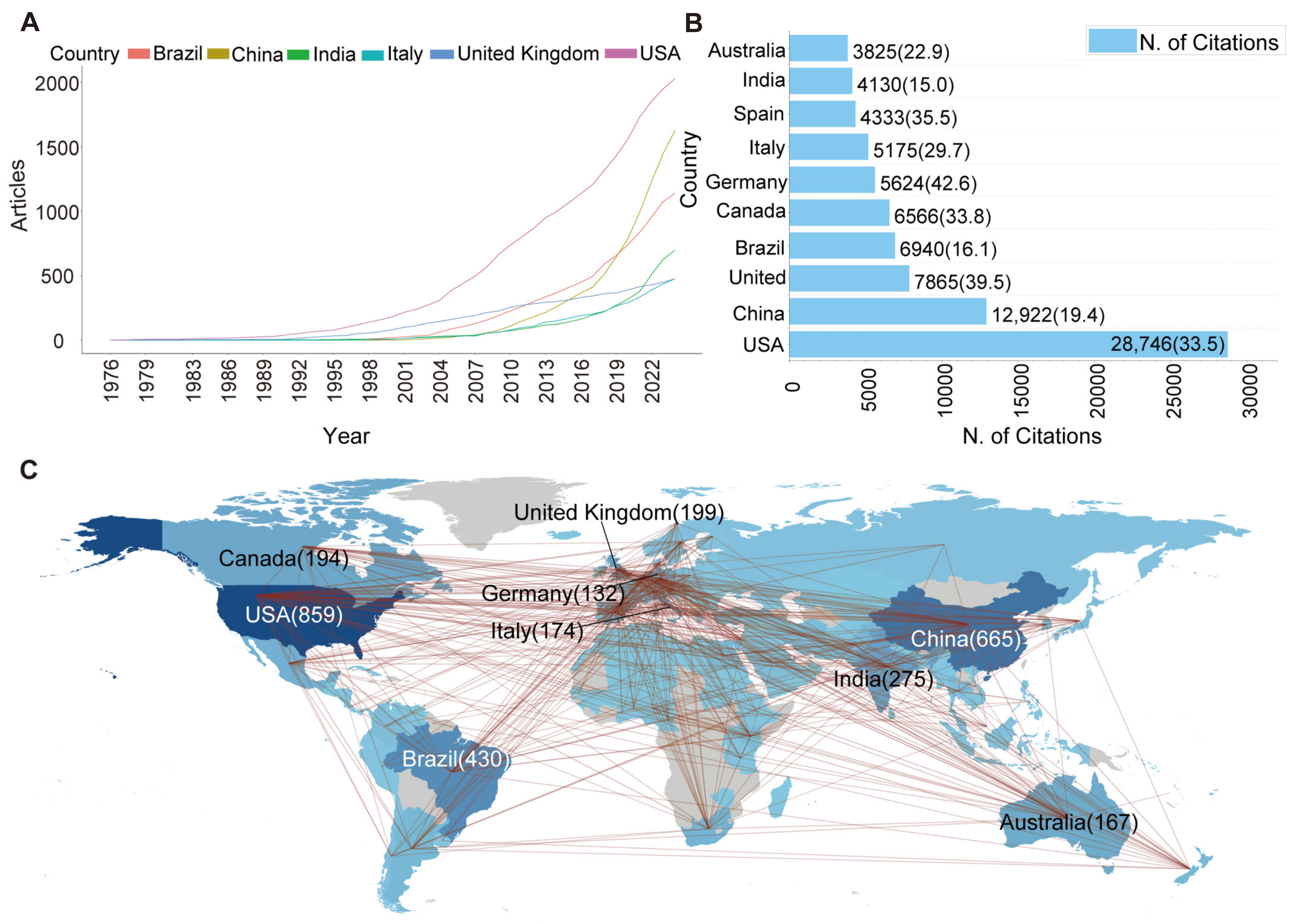
3.2. The USA and China Dominate the Field of BCAs and Biofertilisers
3.3. Sankey Analysis Links the Institutes from Countries, Keywords, and Journals
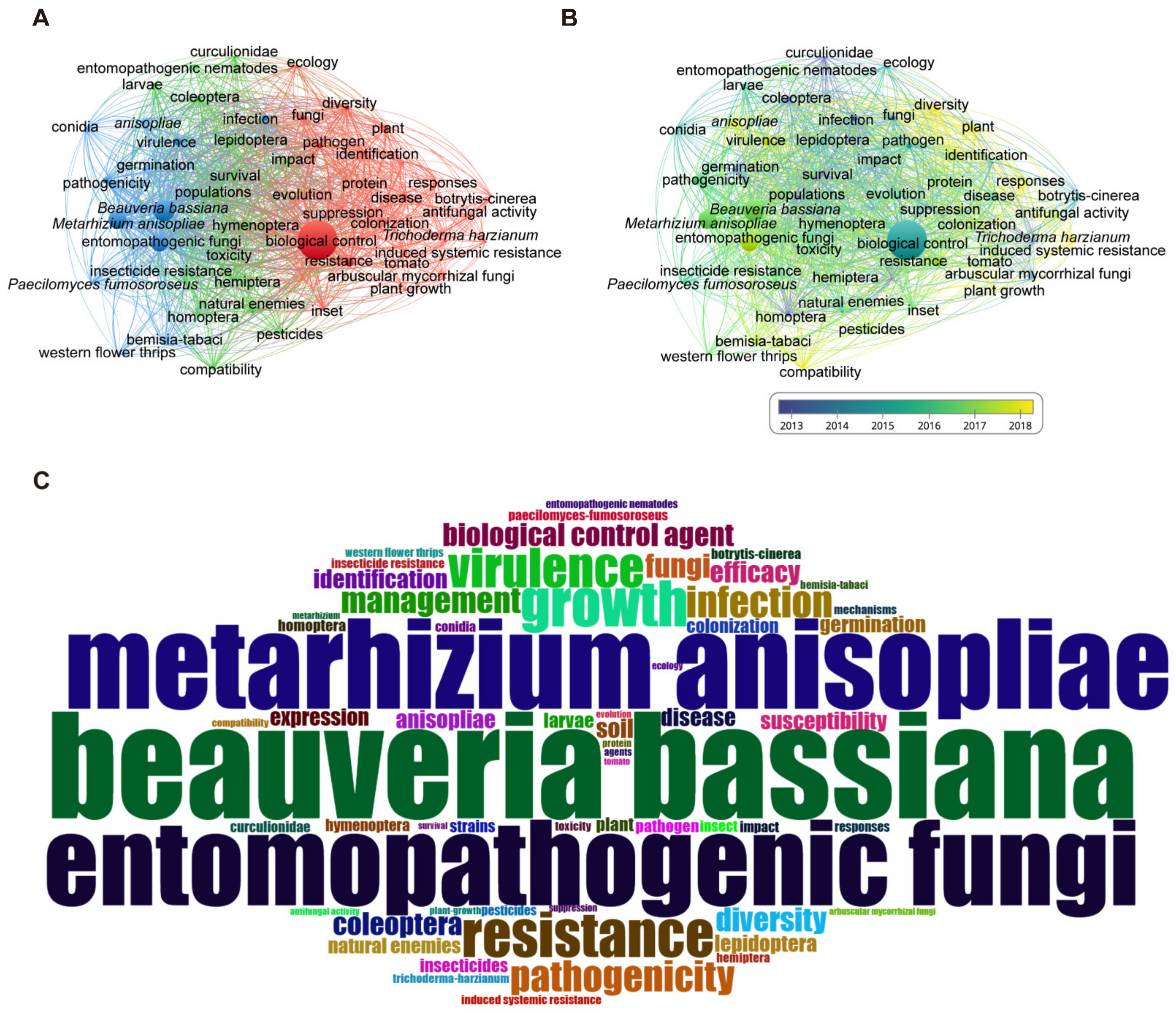
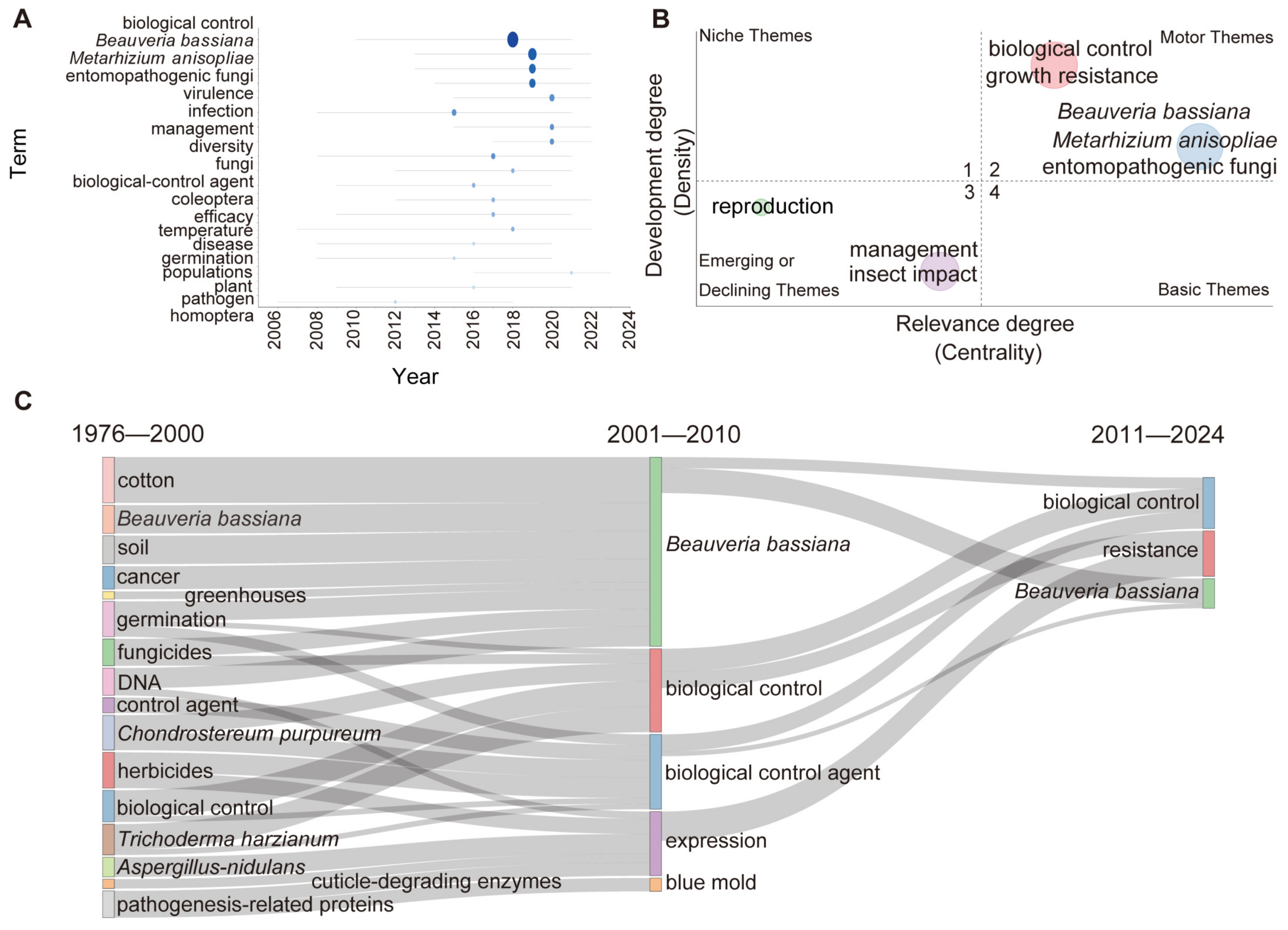
3.4. High-Frequency Keywords Show That B. bassiana and M. anisopliae Are the Key Fungi Used as BCAs and Biofertilisers
3.5. The Evolution of Keywords Highlights B. bassiana as a Prominent Candidate for Future Biocontrol
4. Discussion
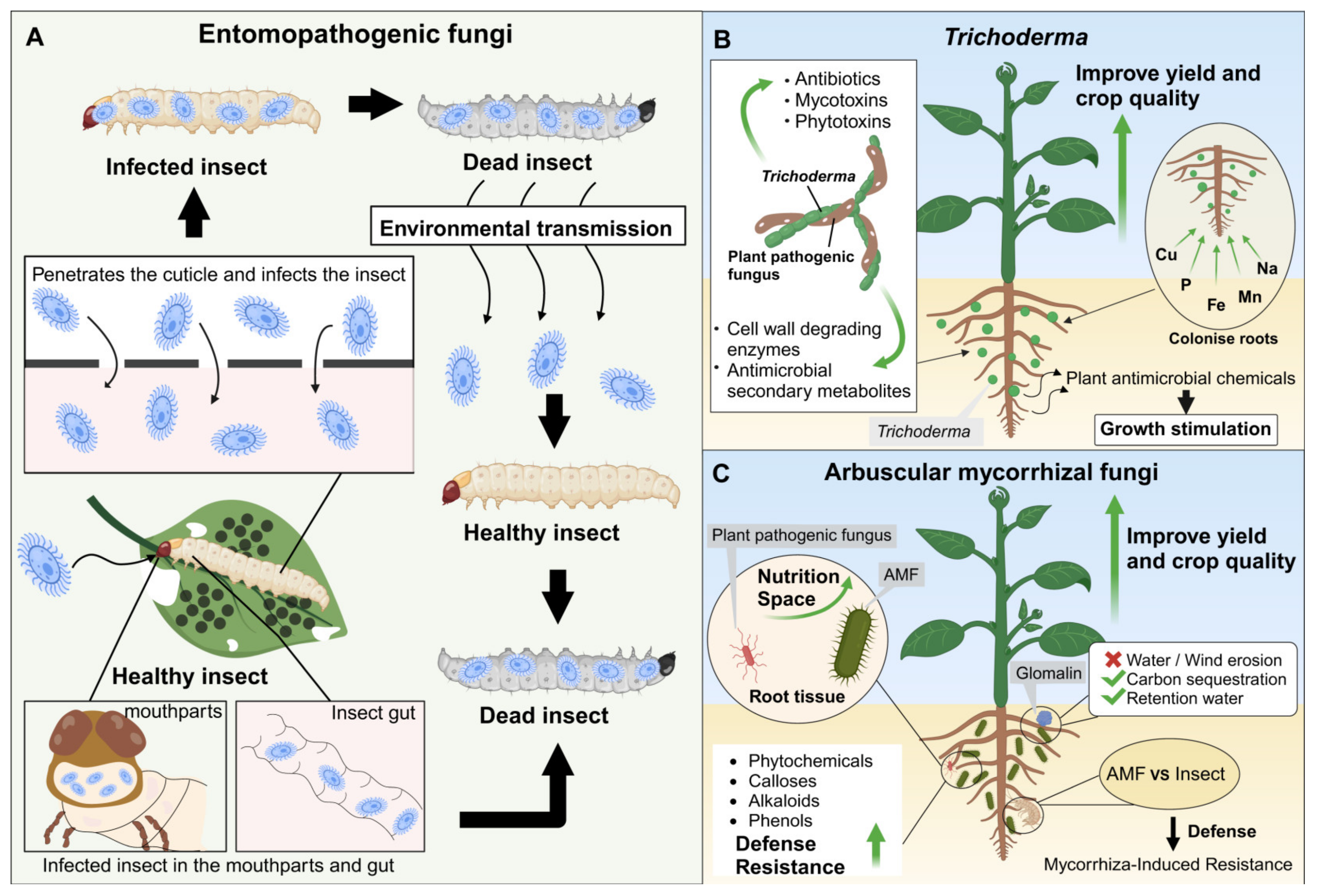
4.1. Species and Mechanisms of EPF with the Potential to Serve as BCAs
4.2. Trichoderma Is a Key Fungal Species That Functions as BCAs and Biofertilisers
4.3. Mechanisms of Mycorrhizal Fungi with the Potential to Serve as BCAs and Biofertilisers
4.4. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCAs | Biocontrol agents |
| WoSCC | Web of Science Core Collection |
| EPF | Entomopathogenic fungi |
| USDA | United States Department of Agriculture |
| AMF | Arbuscular mycorrhizal fungi |
| CWDEs | Cell-wall-degrading enzymes |
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Pirttilä, A.M.; Tabas, H.M.P.; Baruah, N.; Koskimäki, J.J. Biofertilizers and Biocontrol Agents for Agriculture: How to Identify and Develop New Potent Microbial Strains and Traits. Microorganisms 2021, 9, 817. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A Potential Approach for Sustainable Agriculture Development. Environ. Sci. Pollut. Res. 2016, 24, 3315–3335. [Google Scholar] [CrossRef]
- Ammar, E.E.; Rady, H.A.; Khattab, A.M.; Amer, M.H.; Mohamed, S.A.; Elodamy, N.I.; Al-Farga, A.; Aioub, A.A.A. A Comprehensive Overview of Eco-Friendly Bio-Fertilizers Extracted from Living Organisms. Environ. Sci. Pollut. 2023, 30, 113119–113137. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, A.; Chen, Y.; Xu, Z.; Liu, Y.; Yao, Y.; Wang, Y.; Jia, B. Beneficial Microorganisms: Regulating Growth and Defense for Plant Welfare. Plant Biotechnol. J. 2024. Online ahead of print.. [Google Scholar] [CrossRef]
- Shahid, A.; Rao, Q.; Bakhsh, A.; Husnain, T. Entomopathogenic Fungi as Biological Controllers: New Insights into Their Virulence and Pathogenicity. Arch. Biol. Sci. 2012, 64, 21–42. [Google Scholar] [CrossRef]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect Pathogens as Biological Control Agents: Do They Have a Future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. In The Ecology of Fungal Entomopathogens; Roy, H.E., Vega, F.E., Chandler, D., Goettel, M.S., Pell, J., Wajnberg, E., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 55, pp. 113–128. [Google Scholar]
- O’Brien, P.A. Biological Control of Plant Diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Carro-Huerga, G.; Compant, S.; Gorfer, M.; Cardoza, R.E.; Schmoll, M.; Gutiérrez, S.; Casquero, P. Colonization of Vitis Vinifera L. By the Endophyte Trichoderma sp. Strain T154: Biocontrol Activity against Phaeoacremonium Minimum. Front. Plant Sci. 2020, 11, 1170. [Google Scholar] [CrossRef]
- Sallam, N.M.A.; Eraky, A.M.I.; Sallam, A. Effect of Trichoderma spp. On Fusarium Wilt Disease of Tomato. Mol. Biol. Rep. 2019, 46, 4463–4470. [Google Scholar] [CrossRef] [PubMed]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi Vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological Functions of Trichoderma spp. For Agriculture Applications. Ann. Agr. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Malusá, E.; Vassilev, N. A Contribution to Set a Legal Framework for Biofertilisers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, X.; Zheng, G.; Meng, G.; Dong, Z.; Baek, J.H.; Jeon, C.O.; Yao, Y.; Xuan, Y.H.; Zhang, J.; et al. Development of Biofertilizers for Sustainable Agriculture over Four Decades (1980–2022). Geogr. Sustain. 2024, 5, 19–28. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, J.; Arora, N.K. Plant growth-promoting rhizobacteria: Diversity and applications. In Environmental Biotechnology: For Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 129–173. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial Fungal Communities from Different Habitats and Their Roles in Plant Growth Promotion and Soil Health. Microb. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- Arora, P. Microbial Metabolism of Xenobiotic Compounds; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Ahmad, F.; Saeed, Q.; Shah, S.M.U.; Gondal, M.A.; Mumtaz, S. Environmental sustainability: Challenges and approaches. In Natural Resources Conservation and Advances for Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–270. [Google Scholar]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Guleria, G.; Rana, K.L.; Yadav, N.; Rastegari, A.A. Chapter 18-microbial biotechnology for sustainable agriculture: Current research and future challenges. In New and Future Developments in Microbial Biotechnology and Bioengineering; Rastegari, A.A., Yadav, A.N., Yadav, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–344. [Google Scholar]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-Solubilizing Trichoderma spp. from Amazon Soils Improve Soybean Plant Growth. Sci. Rep. 2020, 10, 2858. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostás, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental Growth Conditions of Trichoderma spp. Affects Indole Acetic Acid Derivatives, Volatile Organic Compounds, and Plant Growth Promotion. Front. Plant Sci. 2017, 8, 102. [Google Scholar] [CrossRef]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile Organic Compounds Emitted by Trichoderma Species Mediate Plant Growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and Its Role in Biological Control of Plant Fungal and Nematode Disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, Q.; Zaman, S.; Anwar, A.; Li, S. The Transcriptomic Analysis Revealed the Molecular Mechanism of Arbuscular Mycorrhizal Fungi (Amf) Inoculation in Watermelon. Sci. Hortic. 2024, 332, 113184. [Google Scholar] [CrossRef]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A.; et al. Arbuscular Mycorrhizal Fungi (Amf) Enhanced the Growth, Yield, Fiber Quality and Phosphorus Regulation in Upland Cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef] [PubMed]
- Leventis, G.; Tsiknia, M.; Feka, M.; Ladikou, E.; Papadakis, I.; Chatzipavlidis, I.; Papadopoulou, K.; Ehaliotis, C. Arbuscular Mycorrhizal Fungi Enhance Growth of Tomato under Normal and Drought Conditions, Via Different Water Regulation Mechanisms. Rhizosphere 2021, 19, 100394. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Enhanced Plant Nutrient Use Efficiency with Pgpr and Amf in an Integrated Nutrient Management System. Can. J. Microbio. 2008, 54, 876–886. [Google Scholar] [CrossRef]
- Caputo, A.; Kargina, M. A User-Friendly Method to Merge Scopus and Web of Science Data During Bibliometric Analysis. J. Mark. Anal. 2022, 10, 82–88. [Google Scholar] [CrossRef]
- Small, H. Paradigms, Citations, and Maps of Science: A Personal History. J. Assoc. Inf. Sci. Technol. 2003, 54, 394–399. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to Conduct a Bibliometric Analysis: An Overview and Guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Visualizing bibliometric networks. In Measuring Scholarly Impact: Methods and Practice; Springer: Berlin/Heidelberg, Germany, 2014; pp. 285–320. [Google Scholar]
- Pu, Z.; Shi, C.; Jeon, C.O.; Fu, J.; Liu, S.; Lan, C.; Yao, Y.; Liu, Y.; Jia, B. Chatgpt and Generative Ai Are Revolutionizing the Scientific Community: A Janus-Faced Conundrum. iMeta 2024, 3, e178. [Google Scholar] [CrossRef]
- Börner, K.; Chen, C.; Boyack, K.W. Visualizing Knowledge Domains. Annu. Rev. Inf. Sci. Technol. 2003, 37, 179–255. [Google Scholar] [CrossRef]
- Carter, J.B. A Survey of Microbial, Insect and Nematode Parasites of Tipulidae (Diptera) Larvae in North-East England. J. Appl. Ecol. 1976, 13, 103–122. [Google Scholar] [CrossRef]
- Buckley, S.A.R.E. A Beauveria Phylogeny Inferred from Nuclear Its and Ef1-a Sequences: Evidence for Cryptic Diversification and Links to Cordyceps Teleomorphs. Mycological 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Gao, Q.; Jin, K.; Ying, S.-H.; Zhang, Y.; Xiao, G.; Shang, Y.; Duan, Z.; Hu, X.; Xie, X.-Q.; Zhou, G.; et al. Genome Sequencing and Comparative Transcriptomics of the Model Entomopathogenic Fungi Metarhizium anisopliae and M. acridum. PLoS Genet. 2011, 7, e1001264. [Google Scholar] [CrossRef] [PubMed]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of Defense Responses in Cucumber Plants (Cucumis sativus L.) by the Biocontrol Agent Trichoderma harzianum. Appl. Environ. Microb. 1999, 65, 1061–1070. [Google Scholar] [CrossRef]
- Callaway, R.M.; Thelen, G.C.; Rodriguez, A.; Holben, W.E. Soil biota and exotic plant invasion. Nature 2004, 427, 731–733. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Roy, H.E. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Chomnunti, P.; Hongsanan, S.; Aguirre-Hudson, B.; Tian, Q.; Peršoh, D.; Dhami, M.K.; Alias, A.S.; Xu, J.; Liu, X.; Stadler, M.; et al. The sooty moulds. Fungal Divers. 2014, 66, 1–36. [Google Scholar] [CrossRef]
- Humber, R.A.; Samson, R.A.; Evans, H.C.; Latge, J.-P. Atlas of Entomopathogenic Fungi. Mycologia 1990, 82, 148–149. [Google Scholar] [CrossRef]
- Peeples, J.L.; Curl, E.A.; Rodriguezjkabana, R. Effect of the Herbicide Eptc on the Biocontrol Activity of Trichoderma Viride against Sclerotium Rolfsii. Plant Dis. Rep. 1977, 60, 1050–1054. [Google Scholar]
- Hu, X.; Prokopy, R.J.; Mason, J. Populations of Predatory and Pest Mites in First-Level and Second-Level Commercial Apple Orchard Blocks in Massachusetts. J. Appl. Entomol. 1996, 120, 47–51. [Google Scholar] [CrossRef]
- Shuqin, J.; Fang, Z. Zero Growth of Chemical Fertilizer and Pesticide Use: China’s Objectives, Progress and Challenges. J. Resour. Ecol. 2018, 9, 50–58. [Google Scholar] [CrossRef]
- Trizelia, T. Keanekaragaman Jenis Cendawan Entomopatogen Endofit Pada Tanaman Kakao (Theobroma cacao). Pros. Semin. Nas. Masy. Biodiversitas Indones. 2016, 2, 277–281. [Google Scholar]
- Meyling, N.V.; Eilenberg, J. Ecology of the Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae in Temperate Agroecosystems: Potential for Conservation Biological Control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on Safety of the Entomopathogenic Fungus Metarhizium anisopliae. Biocontrol Sci. Technol. 2007, 17, 879–920. [Google Scholar] [CrossRef]
- Sánchez-Peña, S.R.; Lara, J.S.-J.; Medina, R.F. Occurrence of Entomopathogenic Fungi from Agricultural and Natural Ecosystems in Saltillo, México, and Their Virulence Towards Thrips and Whiteflies. J. Insect Sci. 2011, 11, 1. [Google Scholar] [CrossRef]
- Rath, A.; Koen, T.; Yip, H. The Influence of Abiotic Factors on the Distribution and Abundance of Metarhizium Anisopliae in Tasmanian Pasture Soils. Mycol. Res. 1992, 96, 378–384. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Navas-Cortés, J.A.; Maranhao, E.A.; Ortiz-Urquiza, A.; Santiago-Álvarez, C. Factors Affecting the Occurrence and Distribution of Entomopathogenic Fungi in Natural and Cultivated Soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Vänninen, I. Distribution and Occurrence of Four Entomopathogenic Fungi in Finland: Effect of Geographical Location, Habitat Type and Soil Type. Mycol. Res. 1996, 100, 93–101. [Google Scholar] [CrossRef]
- Souza, B.; Vázquez, L.; Marucci, R. Natural Enemies of Insect Pests in Neotropical Agroecosystems Biological Control and Functional Biodiversity: Biological Control and Functional Biodiversity; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Nyasani, J.O.; Subramanian, S.; Poehling, H.-M.; Maniania, N.K.; Ekesi, S.; Meyhöfer, R. Optimizing Western Flower Thrips Management on French Beans by Combined Use of Beneficials and Imidacloprid. Insects 2015, 6, 279–296. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Eliopoulos, P.A. Endophytic Entomopathogenic Fungi: A Valuable Biological Control Tool against Plant Pests. Appl. Sci. 2020, 10, 360. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the Surface: Entomopathogenic Fungi Versus the Insect Cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef]
- Bitencourt, R.d.O.B.; Mallet, J.R.d.S.; Mesquita, E.; Gôlo, P.S.; Fiorotti, J.; Bittencourt, V.R.E.P.; Pontes, E.G.; Angelo, I.d.C. Larvicidal Activity, Route of Interaction and Ultrastructural Changes in Aedes Aegypti Exposed to Entomopathogenic Fungi. Acta Trop. 2021, 213, 105732. [Google Scholar] [CrossRef]
- Reddy, S.G.E.; Sahotra, S. Multiplication of Entomopathogenic Fungus (Lecanicillium lecanii) on Apple Pomace and Its Toxicity against Aphid (Aphis craccivora). Toxin Rev. 2018, 39, 252–257. [Google Scholar] [CrossRef]
- Wraight, S.; Lopes, R.; Faria, M. Microbial Control of Mite and Insect Pests of Greenhouse Crops. In Microbial Control of Insect and Mite Pests; Academic Press: Cambridge, MA, USA, 2017; pp. 237–252. [Google Scholar]
- Perdikis, D.; Kapaxidi, E.; Papadoulis, G. Biological Control of Insect and Mite Pests in Greenhouse Solanaceous Crops. Eur. J. Plant Sci. Biotechnol. 2008, 2, 125–144. [Google Scholar]
- Vosátka, M.; Látr, A.; Gianinazzi, S.; Albrechtová, J. Development of Arbuscular Mycorrhizal Biotechnology and Industry: Current Achievements and Bottlenecks. Symbiosis 2013, 58, 29–37. [Google Scholar] [CrossRef]
- Herlinda, S.; Hartono, H.; Irsan, C. Efikasi bioinsektisida formulasi cair berbahan aktif Beauveria bassiana (Bals.) Vuzzill. dan Metarhizium sp. pada wereng punggung putih (Sogatella furcifera Horv.). In Proceedings of the Seminar Nasional dan Kongres PATPI, Palembang, Indonesia, 14–16 October 2008. [Google Scholar]
- Wowiling, S.S.; Pelealu, J.; Maramis, R.T. Pemanfaatan Cendawan Beauveria bassiana Dalam Mengendalikan Hama Paraeucosmetus sp. Pada Tanaman Padi Sawah Di Kabupaten Minahasa Selatan (the Use of Endophytic Fungi Beauveria bassiana to Control Paraeucosmetus sp. on Rice Plant in South Minahasa Dist. J. Bios Logos 2015, 5, 55–62. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-Fungal-Interactions: A Detailed Review on Entomopathogenic Fungi Pathogenicity to Combat Insect Pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Ilan, D.; Hazir, S.; Glazer, I. Chapter 6-basic and applied research: Entomopathogenic nematodes. In Microbial Control of Insect and Mite Pests; Lacey, L.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 91–105. [Google Scholar]
- Litwin, A.; Nowak, M.; Różalska, S. Entomopathogenic Fungi: Unconventional Applications. Rev. Environ. Sci. Bio/Technol. 2020, 19, 23–42. [Google Scholar] [CrossRef]
- Leger, R.J.S.; Wang, C. Genetic Engineering of Fungal Biocontrol Agents to Achieve Greater Efficacy against Insect Pests. Appl. Microbiol. Biotechnol. 2010, 85, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Mejía, L.C.; Rojas, E.I.; Maynard, Z.; Van Bael, S.; Arnold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic Fungi as Biocontrol Agents of Theobroma Cacao Pathogens. Biol. Control 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Baverstock, J.; Roy, H.E.; Pell, J.K. Entomopathogenic Fungi and Insect Behaviour: From Unsuspecting Hosts to Targeted Vectors. BioControl 2009, 55, 89–102. [Google Scholar] [CrossRef]
- Hajek, E.A.; Leger, R.J.S. Interactions between Fungal Pathogens and Insect Hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Lu, H.L.; Leger, R.J.S. Chapter seven-insect immunity to entomopathogenic fungi. In Advances in Genetics; Lovett, B., Leger, R.J.S., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 94, pp. 251–285. [Google Scholar]
- Mondal, S.; Baksi, S.; Koris, A.; Vatai, G. Journey of Enzymes in Entomopathogenic Fungi. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 85–99. [Google Scholar] [CrossRef]
- Mannino, M.C.; Huarte-Bonnet, C.; Davyt-Colo, B.; Pedrini, N. Is the Insect Cuticle the Only Entry Gate for Fungal Infection? Insights into Alternative Modes of Action of Entomopathogenic Fungi. J. Fungi 2019, 5, 33. [Google Scholar] [CrossRef]
- Veen, K.H. Oral Infection of Second-Instar Nymphs of Schistocerca Gregaria by Metarrhizium Anisopliae. J. Invertebr. Pathol. 1966, 8, 254–256. [Google Scholar] [CrossRef]
- Dillon, R.J.; Charnley, A.K. Invasion of the Pathogenic Fungus Metarhizium Anisopliae through the Guts of Germfree Desert Locusts, Schistocerca Gregaria. Mycopathologia 1986, 96, 59–66. [Google Scholar] [CrossRef]
- Dillon, R.J.; Charnley, A.K. Inhibition of Metarhizium Anisopliae by the Gut Bacterial Flora of the Desert Locust, Schistocerca Gregaria: Evidence for an Antifungal Toxin. J. Invertebr. Pathol. 1986, 47, 350–360. [Google Scholar] [CrossRef]
- Schabel, H.G. Oral Infection of Hylobius Pales by Metarrhizium Anisopliae. J. Invertebr. Pathol. 1976, 27, 377–383. [Google Scholar] [CrossRef]
- Batta, Y.A. Efficacy of Two Species of Entomopathogenic Fungi against the Stored-Grain Pest, Sitophilus granarius L. (Curculionidae: Coleoptera), Via Oral Ingestion. Egypt. J. Biol. Pest Control 2018, 28, 44. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, B.; Verma, S.; Pal, J.; Mukesh; Akanksha; Chauhan, P. Unveiling the Biocontrol Potential of Trichoderma. Eur. J. Plant Pathol. 2023, 167, 569–591. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Z.; Yang, X.; Ran, W.; Shen, Q. Trichoderma Harzianum T-E5 Significantly Affects Cucumber Root Exudates and Fungal Community in the Cucumber Rhizosphere. Appl. Soil Ecol. 2013, 72, 41–48. [Google Scholar] [CrossRef]
- Cai, F.; Chen, W.; Wei, Z.; Pang, G.; Li, R.; Ran, W.; Shen, Q. Colonization of Trichoderma harzianum Strain Sqr-T037 on Tomato Roots and Its Relationship to Plant Growth, Nutrient Availability and Soil Microflora. Plant Soil 2015, 388, 337–350. [Google Scholar] [CrossRef]
- Senger, M.; Urrea-Valencia, S.; Nazari, M.T.; Vey, R.T.; Piccin, J.S.; Martin, T.N. Evaluation of Trichoderma Asperelloides-Based Inoculant as Growth Promoter of Soybean (Glycine Max (L.) Merr.): A Field-Scale Study in Brazil. J. Crop Sci. Biotechnol. 2022, 26, 255–263. [Google Scholar] [CrossRef]
- Silva, L.G.; Camargo, R.C.; Mascarin, G.M.; Nunes, P.S.d.O.; Dunlap, C.; Bettiol, W. Dual Functionality of Trichoderma: Biocontrol of Sclerotinia Sclerotiorum and Biostimulant of Cotton Plants. Front. Plant Sci. 2022, 13, 983127. [Google Scholar] [CrossRef]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Servaz Tubaña, B.; de Castro, S.G.Q.; De Oliveira, E.F.; Hungria, M. Trichoderma Asperellum Inoculation as a Tool for Attenuating Drought Stress in Sugarcane. Front. Plant Sci. 2021, 12, 645542. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Beltrán, C.; Narvaez, Y.Z.; Chaparro, M.; Gómez Alvarez, M.; Barrera, F.M. Seed Coating as a Delivery System for the Endophyte Trichoderma koningiopsis Th003 in Rice (Oryza sativa). Appl. Microbiol. Biotechnol. 2021, 105, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Baiyee, B.; Pornsuriya, C.; Ito, S.-I.; Sunpapao, A. Trichoderma Spirale T76-1 Displays Biocontrol Activity against Leaf Spot on Lettuce (Lactuca sativa L.) Caused by Corynespora Cassiicola or Curvularia Aeria. Biol. Control 2018, 129, 195–200. [Google Scholar] [CrossRef]
- Saldana, S.; Chávez, M.L.; Gonzalez, C. Protocol for the Production of Trichoderma Spores for Use as a Biological Control Agent through the Revalorization of Agro-Industrial Waste. In Food Waste Conversion; Springer: Dordrecht, The Netherlands, 2023; pp. 169–176. [Google Scholar]
- Druzhinina, I.; Shelest, S.E.; Kubicek, C.P. Novel Traits of Trichoderma Predicted through the Analysis of Its Secretome. FEMS Microbiol. Lett. 2012, 337, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elamathi, E.; Malathi, P.; Viswanathan, R.; Ramesh Sundar, A. Expression Analysis on Mycoparasitism Related Genes During Antagonism of Trichoderma with Colletotrichum Falcatum Causing Red Rot in Sugarcane. J. Plant Biochem. Biot. 2018, 27, 351–361. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Contreras-Cornejo, H.A.; Adame-Garnica, S.G.; Del-Val, E.; Larsen, J. The Interactions of Trichoderma at Multiple Trophic Levels: Inter-Kingdom Communication. Microbiol. Res. 2020, 240, 126552. [Google Scholar] [CrossRef]
- Dou, K.; Lu, Z.; Wu, Q.; Ni, M.; Yu, C.; Wang, M.; Li, Y.; Wang, X.; Xie, H.; Chen, J.; et al. Mist: A Multilocus Identification System for Trichoderma. Appl. Environ. Microb. 2020, 86, e01532-01520. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic Properties of Species-Groups of Trichoderma: I. Production of Non-Volatile Antibiotics. Trans. Br. Mycol. Soc. 1971, 57, 25–30. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Monfil, V.O.; Casas-Flores, S. Chapter 3-molecular mechanisms of biocontrol in Trichoderma spp. and their applications in agriculture. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 429–453. [Google Scholar]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Species, M.L. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Harman, G.E. Trichoderma—Not Just for Biocontrol Anymore. Phytoparasitica 2011, 39, 103–108. [Google Scholar] [CrossRef]
- Zamioudis, C.; Pieterse, C.M.J. Modulation of Host Immunity by Beneficial Microbes. Mol. Plant Microbe Interact. 2012, 25, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Benami, M.; Isack, Y.; Grotsky, D.; Levy, D.; Kofman, Y. The economic potential of arbuscular mycorrhizal fungi in agriculture. In Grand Challenges in Fungal Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 239–279. [Google Scholar]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2010, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Berg, G. Plant–Microbe Interactions Promoting Plant Growth and Health: Perspectives for Controlled Use of Microorganisms in Agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Allsup, C.M.; Lankau, R.A.; Paige, K.N. Herbivory and Soil Water Availability Induce Changes in Arbuscular Mycorrhizal Fungal Abundance and Composition. Microb. Ecol. 2022, 84, 141–152. [Google Scholar] [CrossRef]
- Miransari, M. Interactions between Arbuscular Mycorrhizal Fungi and Soil Bacteria. Appl. Microbiol. Biotechnol. 2011, 89, 917–930. [Google Scholar] [CrossRef]
- Hijri, M. Analysis of a Large Dataset of Mycorrhiza Inoculation Field Trials on Potato Shows Highly Significant Increases in Yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef]
- Sabia, E.; Claps, S.; Morone, G.; Bruno, A.; Sepe, L.; Aleandri, R. Field Inoculation of Arbuscular Mycorrhiza on Maize (Zea mays L.) under Low Inputs: Preliminary Study on Quantitative and Qualitative Aspects. Ital. J. Agron. 2015, 10, 30–33. [Google Scholar] [CrossRef]
- Lu, F.-C.; Lee, C.-Y.; Wang, C.-L. The Influence of Arbuscular Mycorrhizal Fungi Inoculation on Yam (Dioscorea spp.) Tuber Weights and Secondary Metabolite Content. PeerJ 2015, 3, e2166. [Google Scholar] [CrossRef]
- Aamir, M.; Rai, K.K.; Zehra, A.; Dubey, M.K.; Kumar, S.; Shukla, V.; Upadhyay, R.S. Microbial bioformulation-based plant biostimulants: A plausible approach toward next generation of sustainable agriculture. In Microbial Endophytes; Woodhead Publishing: Cambridge, UK, 2020; pp. 195–225. [Google Scholar]
- Wu, Q.-S.; Xia, R.-X.; Zou, Y.-N. Improved Soil Structure and Citrus Growth after Inoculation with Three Arbuscular Mycorrhizal Fungi under Drought Stress. Eur. J. Soil Biol. 2008, 44, 122–128. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Huang, Y.-M.; Li, Y.; He, X.H. Contribution of Arbuscular Mycorrhizas to Glomalin-Related Soil Protein, Soil Organic Carbon and Aggregate Stability in Citrus Rhizosphere. Int. J. Agri. Biol. 2014, 16, 207–212. [Google Scholar]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of Arbuscular Mycorrhizae on Tomato Yield, Nutrient Uptake, Water Relations, and Soil Carbon Dynamics under Deficit Irrigation in Field Conditions. Sci. Total Environ. 2016, 566–567, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Arbuscular Mycorrhizae, Glomalin, and Soil Aggregation. Can. J. Soil. Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular Mycorrhizal Fungi Trigger Transcriptional Expression of Flavonoid and Chlorogenic Acid Biosynthetic Pathways Genes in Tomato against Tomato Mosaic Virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef]
- Balestrini, R.; Brunetti, C.; Chitarra, W.; Nerva, L. Photosynthetic Traits and Nitrogen Uptake in Crops: Which Is the Role of Arbuscular Mycorrhizal Fungi? Plants 2020, 9, 1105. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Streitwolf-Engel, R.; Riedl, R.; Siegrist, S.; Neudecker, A.; Ineichen, K.; Boller, T.; Wiemken, A.; Sanders, I.R. The Mycorrhizal Contribution to Plant Productivity, Plant Nutrition and Soil Structure in Experimental Grassland. New Phytol. 2006, 172, 739–752. [Google Scholar] [CrossRef]
- Schouteden, N.; De Waele, D.; Panis, B.; Vos, C.M. Arbuscular Mycorrhizal Fungi for the Biocontrol of Plant-Parasitic Nematodes: A Review of the Mechanisms Involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef]
- Tahat, M. Ultrastructural Changes of Tomatoes (Lycopersicon esculentum) Root Colonized by Glomus Mosseae and Ralstonia Solanacearum. Afr. J. Biotechnol. 2012, 11, 6681–6686. [Google Scholar]
- Li, Y.; Yanagi, A.; Miyawaki, Y.; Okada, T.; Matsubara, Y.-I. Disease Tolerance and Changes in Antioxidative Abilities in Mycorrhizal Strawberry Plants. J. Jpn. Soc. Hort. Sci. 2010, 79, 174–178. [Google Scholar] [CrossRef][Green Version]
- Chandanie, W.A.; Kubota, M.; Hyakumachi, M. Interactions between the Arbuscular Mycorrhizal Fungus Glomus Mosseae and Plant Growth-Promoting Fungi and Their Significance for Enhancing Plant Growth and Suppressing Damping-Off of Cucumber (Cucumis sativus L.). Appl. Soil Ecol. 2009, 41, 336–341. [Google Scholar] [CrossRef]
- Jia, B.; Jia, X.; Kim, K.H.; Pu, Z.J.; Kang, M.-S.; Jeon, C.O. Computational, and Biochemical Studies of the Salicylaldehyde Dehydrogenases in the Naphthalene Degradation Pathway. Sci. Rep. 2017, 7, 43489. [Google Scholar] [CrossRef]
- Pozo, M.J.; Verhage, A.; García-Andrade, J.; García, J.M.; Azcón-Aguilar, C. Priming Plant Defence against Pathogens by Arbuscular Mycorrhizal Fungi; Springer: Berlin/Heidelberg, Germany, 2009; pp. 123–135. [Google Scholar]
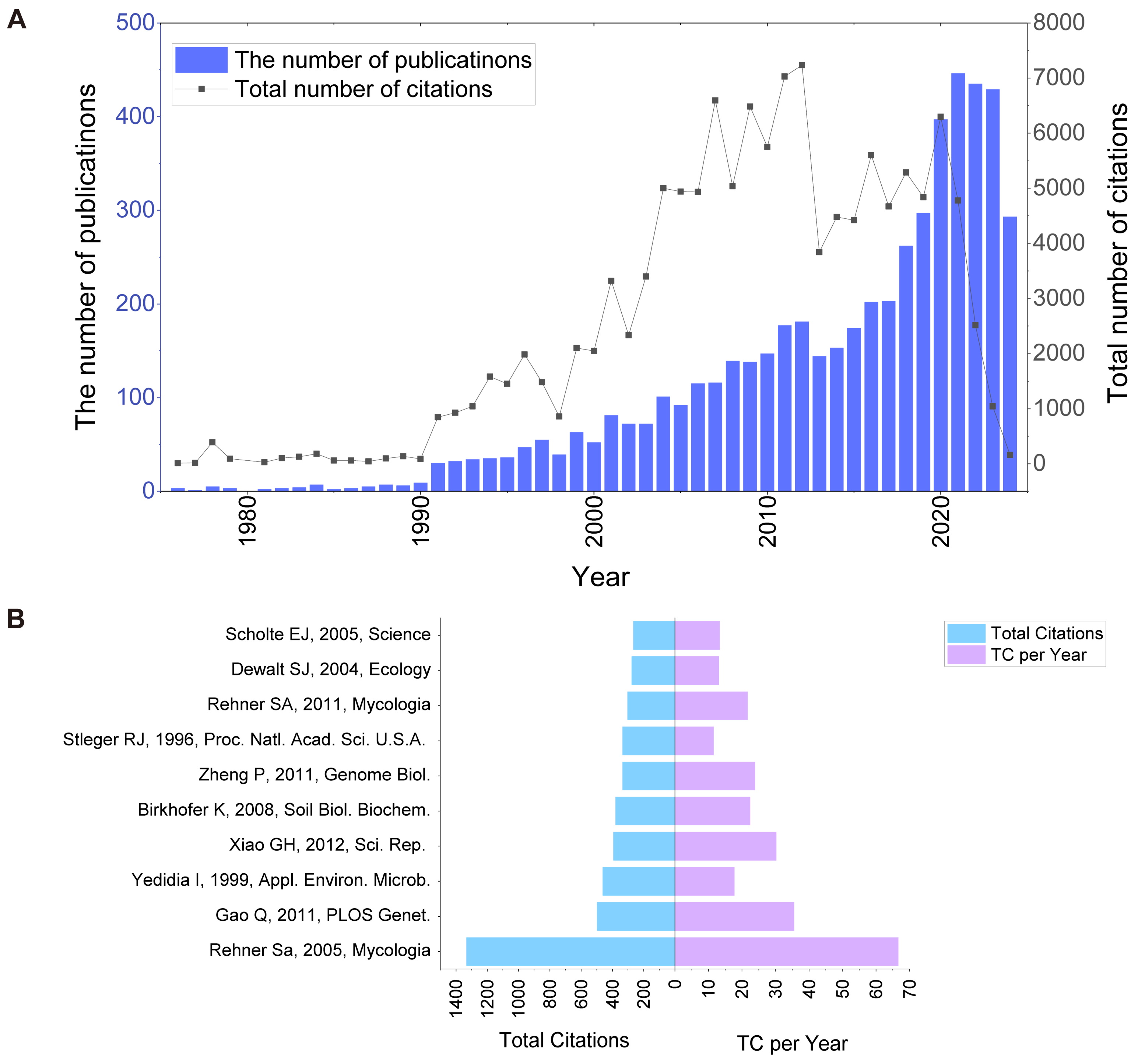

| No. | Title | TC | TC/Y | Journal | Year | Country | DOI |
|---|---|---|---|---|---|---|---|
| 1 | A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs | 1332 | 66.60 | Mycologia | 2005 | USA | https://doi.org/10.3852/mycologia.97.1.84 |
| 2 | Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland | 898 | 59.87 | Basic Appl. Ecol. | 2010 | The Netherlands | https://doi.org/10.1016/j.baae.2009.12.001 |
| 3 | Natural products in crop protection | 840 | 52.50 | Bioorg. Med. Chem. | 2009 | USA | https://doi.org/10.1016/j.bmc.2009.01.046 |
| 4 | Soil biota and exotic plant invasion | 712 | 33.90 | Nature | 2004 | USA | https://doi.org/10.1038/nature02322 |
| 5 | Trichoderma: The genomics of opportunistic success | 617 | 44.07 | Nat. Rev. Microbiol. | 2011 | Austria | https://doi.org/10.1038/nrmicro2637 |
| 6 | Mycorrhiza-induced resistance and priming of plant defenses | 599 | 46.08 | J. Chem Ecol. | 2012 | Spain | https://doi.org/10.1007/s10886-012-0134-6 |
| 7 | Soil biota, ecosystem services and land productivity | 574 | 31.89 | Ecol. Econ. | 2007 | Colombia | https://doi.org/10.1016/j.ecolecon.2007.03.004 |
| 8 | Twenty years of postharvest biocontrol research: Is it time for a new paradigm? | 545 | 34.06 | Postharvest Biol. Technol. | 2008 | USA | https://doi.org/10.1016/j.postharvbio.2008.11.009 |
| 9 | Fungal entomopathogens: new insights on their ecology | 540 | 33.75 | Fungal Ecol. | 2009 | USA | https://doi.org/10.1016/j.funeco.2009.05.001 |
| 10 | The sooty moulds | 517 | 47.00 | Fungal Divers. | 2014 | China | https://doi.org/10.1007/s13225-014-0278-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Shen, Q.; Yu, K.; Liu, Y.; Zheng, H.; Yao, Y.; Jia, B. Half-Century Scientometric Analysis: Unveiling the Excellence of Fungi as Biocontrol Agents and Biofertilisers. J. Fungi 2025, 11, 117. https://doi.org/10.3390/jof11020117
Yuan Z, Shen Q, Yu K, Liu Y, Zheng H, Yao Y, Jia B. Half-Century Scientometric Analysis: Unveiling the Excellence of Fungi as Biocontrol Agents and Biofertilisers. Journal of Fungi. 2025; 11(2):117. https://doi.org/10.3390/jof11020117
Chicago/Turabian StyleYuan, Ziqi, Qi Shen, Kefei Yu, Yan Liu, Huabao Zheng, Yanlai Yao, and Baolei Jia. 2025. "Half-Century Scientometric Analysis: Unveiling the Excellence of Fungi as Biocontrol Agents and Biofertilisers" Journal of Fungi 11, no. 2: 117. https://doi.org/10.3390/jof11020117
APA StyleYuan, Z., Shen, Q., Yu, K., Liu, Y., Zheng, H., Yao, Y., & Jia, B. (2025). Half-Century Scientometric Analysis: Unveiling the Excellence of Fungi as Biocontrol Agents and Biofertilisers. Journal of Fungi, 11(2), 117. https://doi.org/10.3390/jof11020117






