Nationwide Screening Unveils Endemic Ophidiomyces ophidiicola Presence in Northern Italy, Mainly Affecting Dice Snakes: Evidence from Contemporary and Historical Snake Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.1.1. Wild Snake Sampling
2.1.2. Museum Specimen Sampling
2.2. Molecular Analysis
2.3. Histopathology
2.4. Statistical Analysis
3. Results
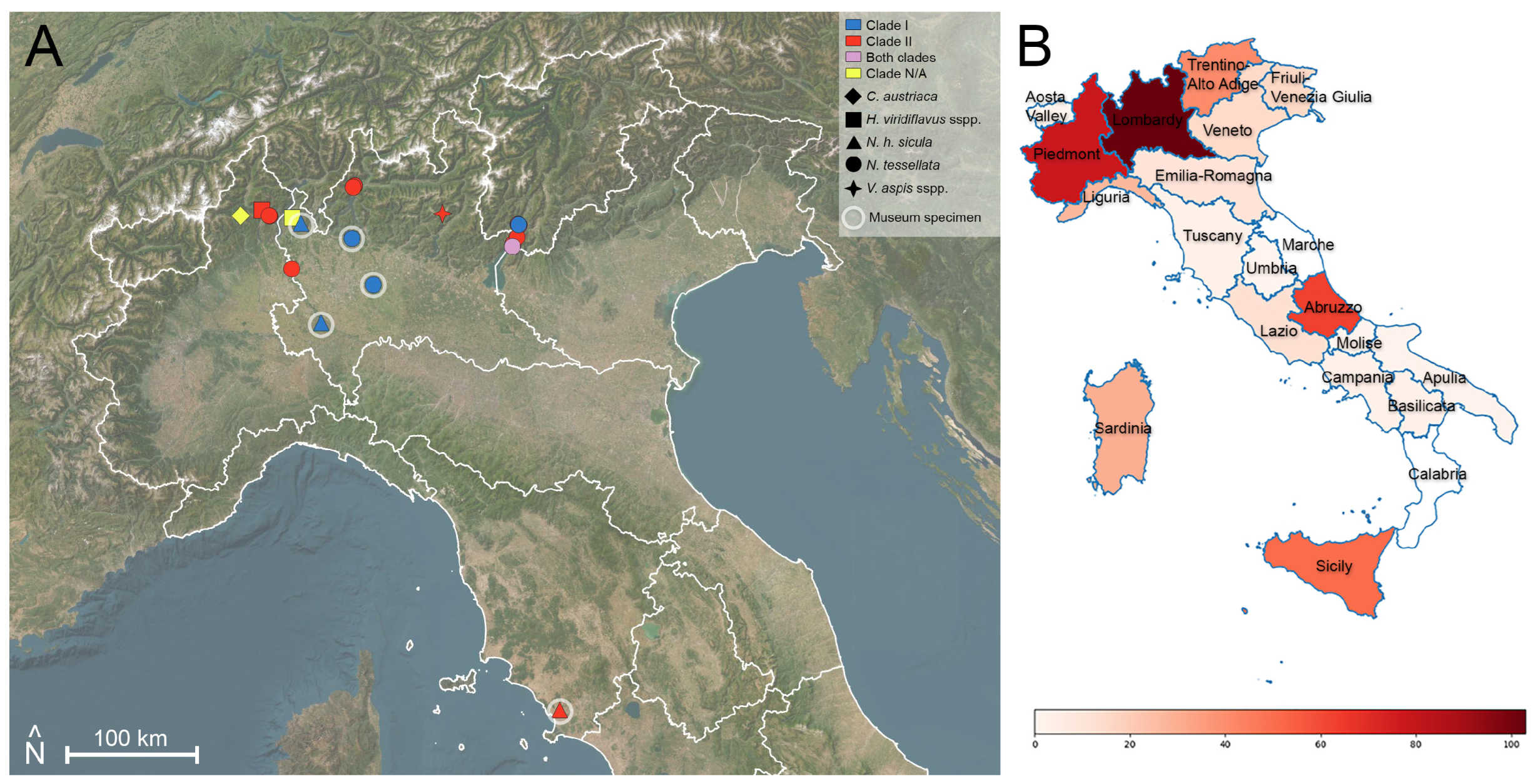
3.1. Sampling and Molecular Analysis
3.2. Clade Determination
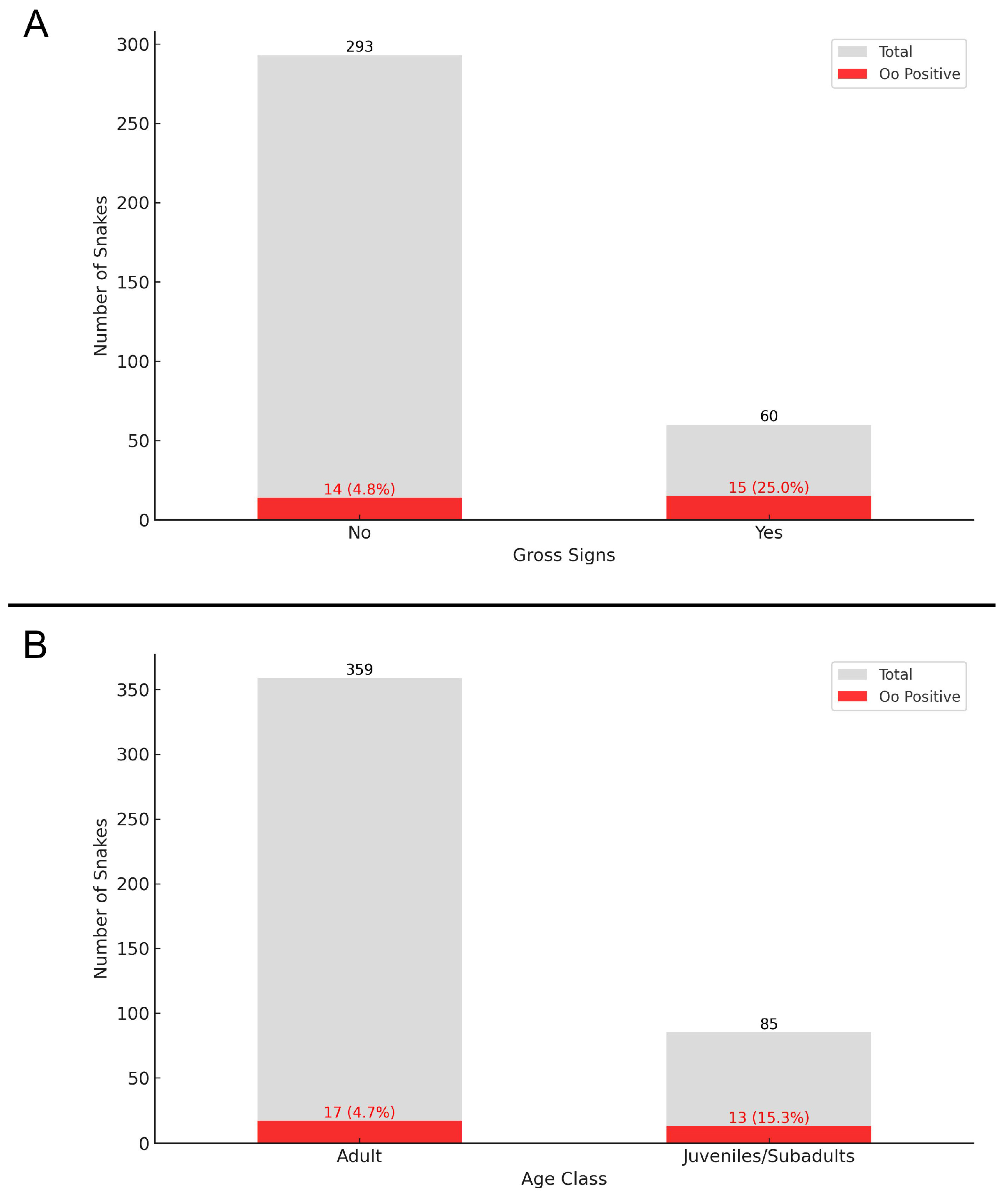
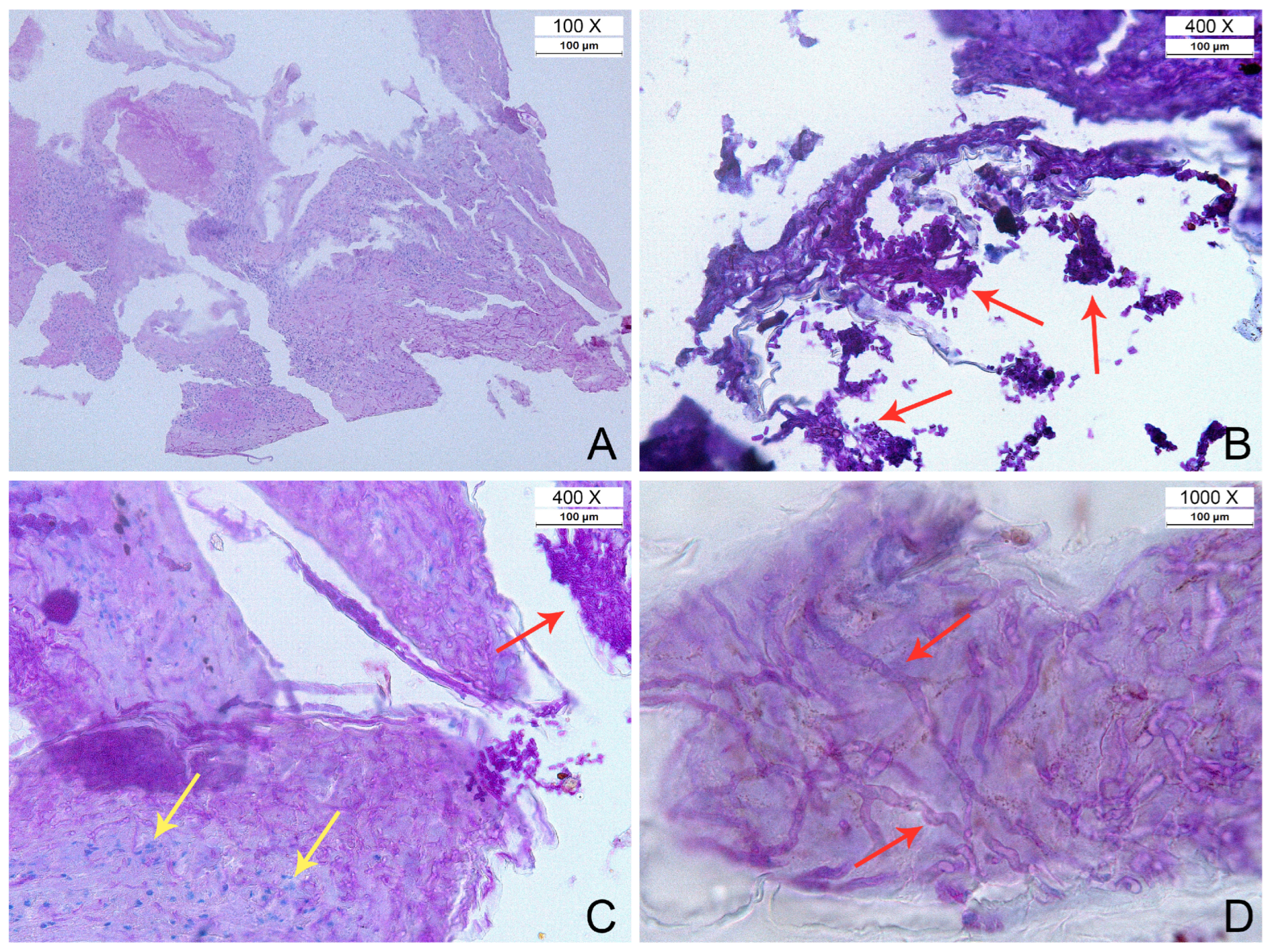
3.3. Histopathology
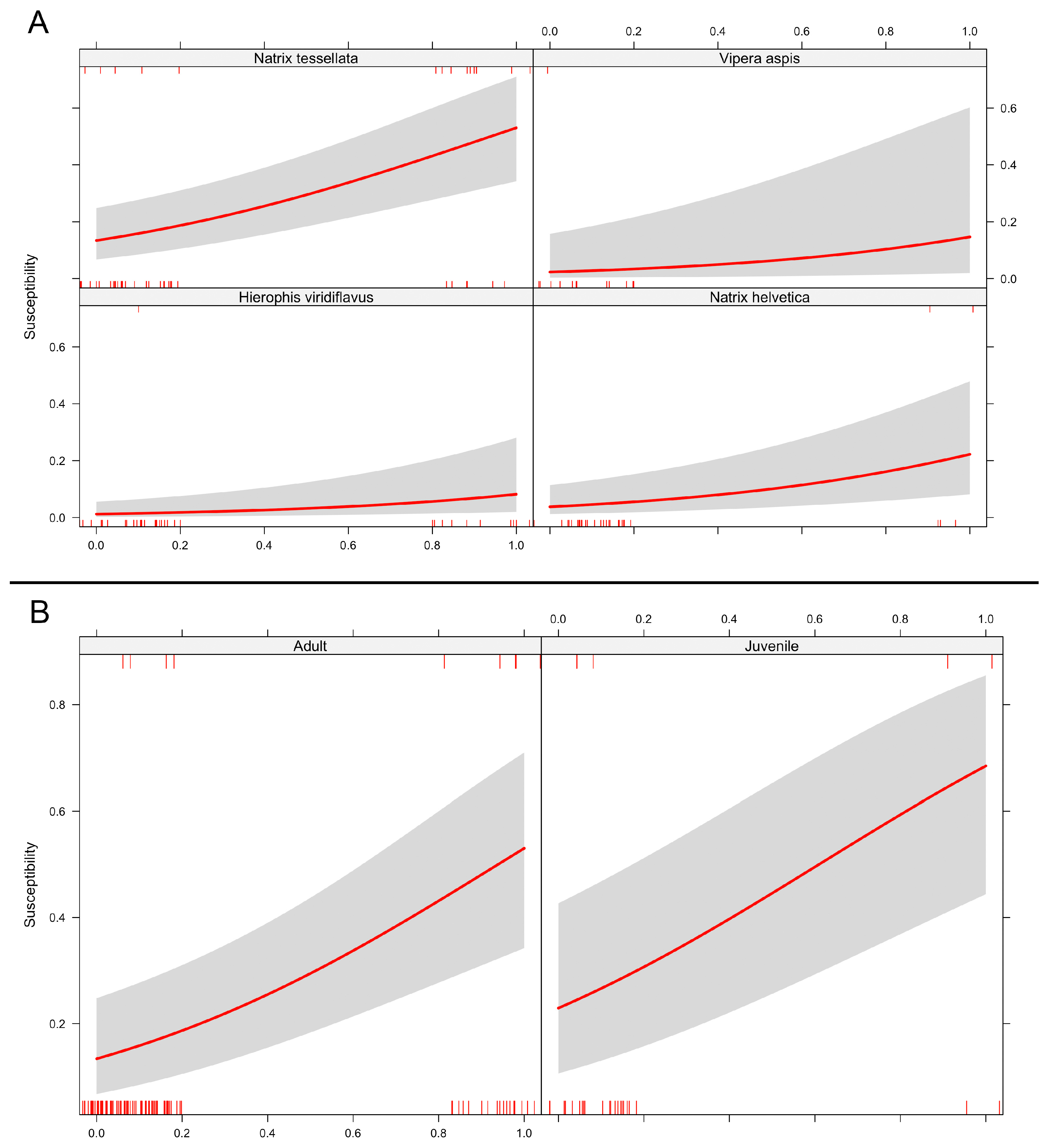
3.4. Statistical Analysis
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Böhm, M.; Collen, B.; Baillie, J.E.M.; Bowles, P.; Chanson, J.; Cox, N.; Hammerson, G.; Hoffmann, M.; Livingstone, S.R.; Ram, M.; et al. The Conservation Status of the World’s Reptiles. Biol. Conserv. 2013, 157, 372–385. [Google Scholar] [CrossRef]
- Cox, N.; Young, B.E.; Bowles, P.; Fernandez, M.; Marin, J.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Hedges, S.B.; Hilton-Taylor, C.; et al. A Global Reptile Assessment Highlights Shared Conservation Needs of Tetrapods. Nature 2022, 605, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Filippi, E.; Luiselli, L. Status of the Italian Snake Fauna and Assessment of Conservation Threats. Biol. Conserv. 2000, 93, 219–225. [Google Scholar] [CrossRef]
- European Red List of Reptiles; Cox, N.A., Temple, H.J., IUCN Red List Programme, IUCN Regional Office for Europe, IUCN Species Survival Commission, IUCN—The World Conservation Union, European Commission, Office for Official Publications of the European Communities, Eds.; IUCN; Office for Official Publications of the European Communities: Gland, Switzerland; Luxembourg, 2009; ISBN 978-92-79-11357-4. [Google Scholar]
- Reading, C.J.; Luiselli, L.M.; Akani, G.C.; Bonnet, X.; Amori, G.; Ballouard, J.M.; Filippi, E.; Naulleau, G.; Pearson, D.; Rugiero, L. Are Snake Populations in Widespread Decline? Biol. Lett. 2010, 6, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Burlakova, L.E.; Karatayev, A.Y.; Karatayev, V.A.; May, M.E.; Bennett, D.L.; Cook, M.J. Endemic Species: Contribution to Community Uniqueness, Effect of Habitat Alteration, and Conservation Priorities. Biol. Conserv. 2011, 144, 155–165. [Google Scholar] [CrossRef]
- Maiorano, L.; Amori, G.; Capula, M.; Falcucci, A.; Masi, M.; Montemaggiori, A.; Pottier, J.; Psomas, A.; Rondinini, C.; Russo, D.; et al. Threats from Climate Change to Terrestrial Vertebrate Hotspots in Europe. PLoS ONE 2013, 8, e74989. [Google Scholar] [CrossRef]
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.-A.; Jenkins, R.; Khan, T.M.; Kiessling, W.; Krause, C.; et al. Endemism Increases Species’ Climate Change Risk in Areas of Global Biodiversity Importance. Biol. Conserv. 2021, 257, 109070. [Google Scholar] [CrossRef]
- Santos, X.; Pleguezuelos, J.M.; Chergui, B.; Geniez, P.; Cheylan, M. Citizen-Science Data Shows Long-Term Decline of Snakes in Southwestern Europe. Biodivers. Conserv. 2022, 31, 1609–1625. [Google Scholar] [CrossRef]
- Li, Q.; Shao, W.; Jiang, Y.; Yan, C.; Liao, W. Assessing Reptile Conservation Status under Global Climate Change. Biology 2024, 13, 436. [Google Scholar] [CrossRef]
- Seigel, R.A.; Mullin, S.J. Snake Conservation, Present and Future. In Snakes: Ecology and Conservation; Cornell University Press: Ithaca, NY, USA, 2009; pp. 281–290. [Google Scholar]
- Marini, D.; Di Nicola, M.R.; Crocchianti, V.; Notomista, T.; Iversen, D.; Coppari, L.; Di Criscio, M.; Brouard, V.; Dorne, J.-L.C.M.; Rüegg, J.; et al. Pilot Survey Reveals Ophidiomycosis in Dice Snakes Natrix tessellata from Lake Garda, Italy. Vet. Res. Commun. 2023, 47, 1707–1719. [Google Scholar] [CrossRef]
- Hagman, M.; Elmberg, J.; Kärvemo, S.; Löwenborg, K. Grass Snakes (Natrix natrix) in Sweden Decline Together with Their Anthropogenic Nesting-Environments. Herpetol. J. 2012, 22, 199–202. [Google Scholar]
- Pomara, L.Y.; LeDee, O.E.; Martin, K.J.; Zuckerberg, B. Demographic Consequences of Climate Change and Land Cover Help Explain a History of Extirpations and Range Contraction in a Declining Snake Species. Glob. Chang. Biol. 2014, 20, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.; Jofré, G. Smooth Snake Population Decline and Its Link with Prey Availability. Amphib.-Reptil. 2020, 41, 43–48. [Google Scholar] [CrossRef]
- Elmberg, J.; Palmheden, L.; Edelstam, C.; Hagman, M.; Kärvemo, S. Climate Change-Induced Shifts in Survival and Size of the Worlds’ Northernmost Oviparous Snake: A 68-Year Study. PLoS ONE 2024, 19, e0300363. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Fisher, M.C. Epidemiological Definitions, Terminology and Classifications with Reference to Fungal Infections of Animals. In Emerging and Epizootic Fungal Infections in Animals; Seyedmousavi, S., de Hoog, G.S., Guillot, J., Verweij, P.E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 17–27. ISBN 978-3-319-72093-7. [Google Scholar]
- Hoberg, E.P.; Brooks, D.R. Evolution in Action: Climate Change, Biodiversity Dynamics and Emerging Infectious Disease. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130553. [Google Scholar] [CrossRef]
- Paré, J.A.; Conley, K.J. Mycotic Diseases of Reptiles [Infectious Diseases and Pathology of Reptiles]. In Infectious Diseases and Pathology of Reptiles; CRC Press: Boca Raton, FL, USA, 2020; pp. 795–858. [Google Scholar]
- Schilliger, L.; Paillusseau, C.; François, C.; Bonwitt, J. Major Emerging Fungal Diseases of Reptiles and Amphibians. Pathogens 2023, 12, 429. [Google Scholar] [CrossRef]
- Allain, S.J.R.; Duffus, A.L.J. Emerging Infectious Disease Threats to European Herpetofauna (Vol 18, Pg 561, 2018). Herpetol. J. 2019, 29, 189–206. [Google Scholar] [CrossRef]
- Allender, M.C.; Raudabaugh, D.B.; Gleason, F.H.; Miller, A.N. The Natural History, Ecology, and Epidemiology of Ophidiomyces ophiodiicola and Its Potential Impact on Free-Ranging Snake Populations. Fungal Ecol. 2015, 17, 187–196. [Google Scholar] [CrossRef]
- Lorch, J.M.; Knowles, S.; Lankton, J.S.; Michell, K.; Edwards, J.L.; Kapfer, J.M.; Staffen, R.A.; Wild, E.R.; Schmidt, K.Z.; Ballmann, A.E.; et al. Snake Fungal Disease: An Emerging Threat to Wild Snakes. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150457. [Google Scholar] [CrossRef]
- Davy, C.M.; Shirose, L.; Campbell, D.; Dillon, R.; McKenzie, C.; Nemeth, N.; Braithwaite, T.; Cai, H.; Degazio, T.; Dobbie, T.; et al. Revisiting Ophidiomycosis (Snake Fungal Disease) After a Decade of Targeted Research. Front. Vet. Sci. 2021, 8, 665805. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.R.; Coppari, L.; Notomista, T.; Marini, D. Ophidiomyces Ophidiicola Detection and Infection: A Global Review on a Potential Threat to the World’s Snake Populations. Eur. J. Wildl. Res. 2022, 68, 64. [Google Scholar] [CrossRef]
- McKenzie, J.M.; Price, S.J.; Fleckenstein, J.L.; Drayer, A.N.; Connette, G.M.; Bohuski, E.; Lorch, J.M. Field Diagnostics and Seasonality of Ophidiomyces ophiodiicola in Wild Snake Populations. EcoHealth 2019, 16, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Haynes, E.; Stanford, K.; Gramhofer, M.; Vivirito, K.; Durante, K.; Wright, A.; Varga, C.; Allender, M.C. Epidemiology Of Ophidiomycosis In Lake Erie Watersnakes (Nerodia sipedon insularum). J. Wildl. Dis. 2021, 58, 100–113. [Google Scholar] [CrossRef]
- Kendall, M.W.; Wright, A.D.; Adamovicz, L.A.; Durante, K.; Andersson, K.E.; Frederickson, K.; Vivirito, K.; Ospina, E.A.; Delaney, M.A.; Allender, M.C. Environmental Temperature Influences Ophidiomycosis Progression and Survival in Experimentally Challenged Prairie Rattlesnakes (Crotalus viridis). PLoS ONE 2023, 18, e0289641. [Google Scholar] [CrossRef]
- Dillon, R.M.; Paterson, J.E.; Manorome, P.; Ritchie, K.; Shirose, L.; Slavik, E.; Davy, C.M. Effects of Ophidiomycosis on Movement, Survival, and Reproduction of Eastern Foxsnakes (Pantherophis vulpinus). Sci. Rep. 2024, 14, 4948. [Google Scholar] [CrossRef]
- Friedeman, N.; Carter, E.; Kingsbury, B.A.; Ravesi, M.J.; Josimovich, J.M.; Matthews, M.; Jordan, M.A. Environmental Associations of Ophidiomyces ophidiicola, the Causative Agent of Ophidiomycosis in Snakes. PLoS ONE 2024, 19, e0310954. [Google Scholar] [CrossRef]
- Meier, G.; Notomista, T.; Marini, D.; Ferri, V. First Case of Snake Fungal Disease Affecting a Free-Ranging Natrix natrix (Linnaeus, 1758) in Ticino Canton, Switzerland. Herpetol. Notes 2018, 11, 885–891. [Google Scholar]
- Baker, S.J.; Haynes, E.; Gramhofer, M.; Stanford, K.; Bailey, S.; Christman, M.; Conley, K.; Frasca, S.; Ossiboff, R.J.; Lobato, D. Case Definition and Diagnostic Testing for Snake Fungal Disease. Herpetol. Rev. 2019, 50, 279–285. [Google Scholar]
- Lorch, J.M.; Lankton, J.; Werner, K.; Falendysz, E.A.; McCurley, K.; Blehert, D.S. Experimental Infection of Snakes with Ophidiomyces ophiodiicola Causes Pathological Changes That Typify Snake Fungal Disease. mBio 2015, 6, e01534-15. [Google Scholar] [CrossRef]
- Lind, C.M.; Meyers, R.A.; Moore, I.T.; Agugliaro, J.; McPherson, S.; Farrell, T.M. Ophidiomycosis Is Associated with Alterations in the Acute Glycemic and Glucocorticoid Stress Response in a Free-Living Snake Species. Gen. Comp. Endocrinol. 2023, 339, 114295. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.M.; Oesterle, P.T.; Stevens, B.; Shirose, L.; Mastromonaco, G.F.; Lillie, B.N.; Davy, C.M.; Jardine, C.M.; Nemeth, N.M. Ophidiomycosis in Red Cornsnakes (Pantherophis guttatus): Potential Roles of Brumation and Temperature on Pathogenesis and Transmission. Vet. Pathol. 2020, 57, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Britton, M.; Allender, M.C.; Hsiao, S.-H.; Baker, S.J. Postnatal Mortality in Neonate Rattlesnakes Associated with Ophidiomyces ophiodiicola. J. Zoo Wildl. Med. 2019, 50, 672. [Google Scholar] [CrossRef] [PubMed]
- Lind, C.M.; Lorch, J.M.; Moore, I.T.; Vernasco, B.J.; Farrell, T.M. Seasonal Sex Steroids Indicate Reproductive Costs Associated with Snake Fungal Disease. J. Zool. 2019, 307, 104–110. [Google Scholar] [CrossRef]
- McKenzie, J.M.; Price, S.J.; Connette, G.M.; Bonner, S.J.; Lorch, J.M. Effects of Snake Fungal Disease on Short-Term Survival, Behavior, and Movement in Free-Ranging Snakes. Ecol. Appl. 2021, 31, e02251. [Google Scholar] [CrossRef]
- Tetzlaff, S.J.; Ravesi, M.J.; Allender, M.C.; Carter, E.T.; DeGregorio, B.A.; Josimovich, J.M.; Kingsbury, B.A. Snake Fungal Disease Affects Behavior of Free-Ranging Massasauga Rattlesnakes (Sistrurus catenatus). Herpetol. Conserv. Biol. 2017, 12, 624–634. [Google Scholar]
- Sigler, L.; Hambleton, S.; Paré, J.A. Molecular Characterization of Reptile Pathogens Currently Known as Members of the Chrysosporium anamorph of Nannizziopsis vriesii Complex and Relationship with Some Human-Associated Isolates. J. Clin. Microbiol. 2013, 51, 3338–3357. [Google Scholar] [CrossRef]
- Franklinos, L.H.V.; Lorch, J.M.; Bohuski, E.; Rodriguez-Ramos Fernandez, J.; Wright, O.N.; Fitzpatrick, L.; Petrovan, S.; Durrant, C.; Linton, C.; Baláž, V.; et al. Emerging Fungal Pathogen Ophidiomyces ophiodiicola in Wild European Snakes. Sci. Rep. 2017, 7, 3844. [Google Scholar] [CrossRef]
- Sun, P.-L.; Yang, C.-K.; Li, W.-T.; Lai, W.-Y.; Fan, Y.-C.; Huang, H.-C.; Yu, P.-H. Infection with Nannizziopsis guarroi and Ophidiomyces ophiodiicola in Reptiles in Taiwan. Transbound. Emerg. Dis. 2022, 69, 764–775. [Google Scholar] [CrossRef]
- Takami, Y.; Nam, K.-O.; Takaki, Y.; Kadekaru, S.; Hemmi, C.; Hosoya, T.; Une, Y. First Report of Ophidiomycosis in Asia Caused by Ophidiomyces ophiodiicola in Captive Snakes in Japan. J. Vet. Med. Sci. 2021, 83, 1234–1239. [Google Scholar] [CrossRef]
- Lorch, J.M.; Price, S.J.; Lankton, J.S.; Drayer, A.N. Confirmed Cases of Ophidiomycosis in Museum Specimens from as Early as 1945, United States. Emerg. Infect. Dis. 2021, 27, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Origgi, F.C.; Pisano, S.R.R.; Glaizot, O.; Hertwig, S.T.; Schmitz, A.; Ursenbacher, S. Ophiodimyces ophiodiicola, Etiologic Agent of Snake Fungal Disease, in Europe since Late 1950s. Emerg. Infect Dis. 2022, 28, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Ladner, J.T.; Palmer, J.M.; Ettinger, C.L.; Stajich, J.E.; Farrell, T.M.; Glorioso, B.M.; Lawson, B.; Price, S.J.; Stengle, A.G.; Grear, D.A.; et al. The Population Genetics of the Causative Agent of Snake Fungal Disease Indicate Recent Introductions to the USA. PLoS Biol. 2022, 20, e3001676. [Google Scholar] [CrossRef] [PubMed]
- Blanvillain, G.; Lorch, J.M.; Joudrier, N.; Bury, S.; Cuenot, T.; Franzen, M.; Martínez-Freiría, F.; Guiller, G.; Halpern, B.; Kolanek, A.; et al. Contribution of Host Species and Pathogen Clade to Snake Fungal Disease Hotspots in Europe. Commun. Biol. 2024, 7, 440. [Google Scholar] [CrossRef]
- Joudrier, N.; Blanvillain, G.; Meier, G.; Hoyt, J.; Chèvre, M.; Dubey, S.; Origgi, F.C.; Ursenbacher, S. Unravelling the Disease Ecology of Snake Fungal Disease: High Genetic Variability and Ecological Features of Ophidiomyces ophidiicola in Switzerland. Amphib.-Reptil. 2024, 45, 85–98. [Google Scholar] [CrossRef]
- Schüler, L.; Lenz, S.; Marschang, E.R. Ophidiomyces ophidiicola bei wildlebenden Würfelnattern (Natrix tessellata) in Deutschland im Jahre 2021. Tierarztl Prax Ausg K Kleintiere Heimtiere 2022, 50, 151. [Google Scholar]
- Schüler, L.; Lenz, S.; Mittenzwei, F.; Gletscher, I.; Müller, E.; Heckers, K.; Marschang, R.E. Ophidiomycosis in Wild Dice Snakes (Natrix Tessellata) in Germany. J. Herpetol. Med. Surg. 2024, 34, 11–15. [Google Scholar] [CrossRef]
- Marini, D.; Di Nicola, M.R.; Crocchianti, V.; Di Criscio, M.; Rüegg, J.; Marenzoni, M.L. Ophidiomyces ophidiicola from Lake Garda (Italy) Belongs to Clade I. In Proceedings of the 5th International Conference of the European College of Veterinary Microbiology, Bled, Slovenia, 21–23 September 2023. [Google Scholar]
- Přibyl, M.; Kabelka, R.; Hanzlík, P.M.; Mikulíček, P.; Folk, N.; Piaček, V.; Pikula, J.; Baláž1, V. Ophidiomyces ophidiicola in Free-Ranging and Captive Snakes in the Czech and Slovak Republics. J. Vertebr. Biol. 2023, 72, 23050. [Google Scholar] [CrossRef]
- Allain, S.J.R.; Leech, D.I.; Hopkins, K.; Seilern-Moy, K.; Rodriguez-Ramos Fernandez, J.; Griffiths, R.A.; Lawson, B. Characterisation, Prevalence and Severity of Skin Lesions Caused by Ophidiomycosis in a Population of Wild Snakes. Sci. Rep. 2024, 14, 5162. [Google Scholar] [CrossRef]
- Stark, T.; Beukema, W.; Gilbert, M.J.; Goverse, E.; Spitzen-van der Sluijs, A.; Struijk, R.; Verbrugghe, E.; Pasmans, F.; Martel, A. Detection of Ophidiomyces ophidiicola in Wild Barred Grass Snakes (Natrix helvetica) in the Netherlands. Vlaams Diergeneeskd. Tijdschr. 2024, 93, 79–84. [Google Scholar] [CrossRef]
- Joudrier, N.; Blanvillain, G.; Ursenbacher, S. First Detection of Apparent Ophidiomycosis in the Vipera Genus in Europe: Findings on Two Asp Vipers, Vipera aspis (Linnaeus, 1758), in Switzerland. Herpetol. Notes 2024, 17, 311–314. [Google Scholar]
- Martinez-Silvestre, A.; Blanvillain, G.; Gonzalez, J.; Ribo, J. First Record of Ophidiomycosis in a Wild Aesculapian Snake, Zamenis longissimus (Laurenti, 1768), in Spain. Herpetol. Notes 2024, 17, 423–426. [Google Scholar]
- Marini, D.; Szczygieł, P.; Kurek, K.; Di Nicola, M.R.; Dorne, J.-L.C.M.; Marenzoni, M.L.; Rüegg, J.; Bury, S.; Kiraga, Ł. Retrospective Detection of Ophidiomyces ophidiicola from Snake Moults Collected in Bieszczady Mountains, Poland. Microorganisms 2024, 12, 1467. [Google Scholar] [CrossRef] [PubMed]
- Martínez Silvestre, A.; Ferrer, J.R.; González, J.; Loras, F. Diagnòstic Dels Primers Casos de La Malaltia Fúngica de Les Serps (Ofidiomicosi) a Catalunya. Butlletí Soc. Catalana D’Herpetologia 2024, 31, 17–26. [Google Scholar]
- Di Nicola, M.R.; Faraone, F.P.; Zabbia, T. An Updated Dichotomous Key to the Snakes of Europe. Basic Appl. Herpetol. 2022, 36, 47–64. [Google Scholar] [CrossRef]
- Nania, D.; Lumbierres, M.; Ficetola, G.F.; Falaschi, M.; Pacifici, M.; Rondinini, C. Maps of Area of Habitat for Italian Amphibians and Reptiles. Nat. Conserv. 2022, 49, 117–129. [Google Scholar] [CrossRef]
- Marini, D.; Filippi, E.; Montinaro, G.; Origgi, F.C. Screening of Ophidiomyces ophidiicola in the Free-Ranging Snake Community Annually Harvested for the Popular Ritual of San Domenico e Dei Serpari (Cocullo, AQ, Italy). Acta Herpetol. 2023, 18, 45–52. [Google Scholar] [CrossRef]
- Pecoraro, E. La Festa Dei Serpari a Cocullo Tra Antropologia, Arte, Religione e Mito. Quad. Villa Sandra 2018, XXIX, 29–32. [Google Scholar]
- Cianfarani, V. Reliquie Viventi Del Dramma Sacro in Abruzzo a Pretoro Tra Lupi e Serpi. Lares 1950, 16, 75–81. [Google Scholar]
- Hyatt, A.; Boyle, D.; Olsen, V.; Boyle, D.; Berger, L.; Obendorf, D.; Dalton, A.; Kriger, K.; Hero, M.; Hines, H.; et al. Diagnostic Assays and Sampling Protocols for the Detection of Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2007, 73, 175–192. [Google Scholar] [CrossRef]
- Allender, M.C.; Bunick, D.; Dzhaman, E.; Burrus, L.; Maddox, C. Development and Use of a Real-Time Polymerase Chain Reaction Assay for the Detection of Ophidiomyces ophiodiicola in Snakes. J. Vet. Diagn. Investig. 2015, 27, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Dibadj, B.; Haynes, E.; Vivirito, K.; Wright, A.; Stanford, K.; Allender, M.C. Investigating Agreement between Snake Sheds and Skin Swabs in Detection of Ophidiomyces ophidiicola. J. Herpetol. Med. Surg. 2021, 31, 119–123. [Google Scholar] [CrossRef]
- Bohuski, E.; Lorch, J.M.; Griffin, K.M.; Blehert, D.S. TaqMan Real-Time Polymerase Chain Reaction for Detection of Ophidiomyces ophiodiicola, the Fungus Associated with Snake Fungal Disease. BMC Vet. Res. 2015, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Lorch, J.M.; Winzeler, M.E.; Lankton, J.S.; Raverty, S.; Snyman, H.N.; Schwantje, H.; Thacker, C.; Knowles, S.; Cai, H.Y.; Grear, D.A. Paranannizziopsis Spp. Infections in Wild Snakes and a qPCR Assay for Detection of the Fungus. Front. Microbiol. 2023, 14, 1302586. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Breheny, P.; Burchett, W. Visualization of Regression Models Using Visreg. R J. 2017, 9, 56. [Google Scholar] [CrossRef]
- Harding, S.F.; Becker, C.G.; Yates, J.R.; Crump, P.; Forstner, M.R.J.; Mullin, S.J.; Rodriguez, D. Comparative Host-Pathogen Associations of Snake Fungal Disease in Sympatric Species of Water Snakes (Nerodia). Sci. Rep. 2022, 12, 12303. [Google Scholar] [CrossRef]
- Shine, R. Reproductive Strategies in Snakes. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 995–1004. [Google Scholar] [CrossRef]
- Mebert, K.; Ott, T. Mating Aggregations in Natrix tessellata. Mertensiella 2011, 18, 437–439. [Google Scholar]
- Burger, J.; Jeitner, C.; Zappalorti, R.T.; Bunnell, J.F.; Ng, K.; DeVito, E.; Schneider, D.; Gochfeld, M. Snake Fungal Disease in Free-Ranging Northern Pine Snakes (Pituophis melanoleucus melanoleucus) in New Jersey: Lesions, Severity of Sores and Investigator’s Perceptions. J. Fungi Basel Switz. 2024, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.J.; Burger, J.; Zappalorti, R.T.; Bunnell, J.F.; Winzeler, M.E.; Taylor, D.R.; Lorch, J.M. Soil Reservoir Dynamics of Ophidiomyces ophidiicola, the Causative Agent of Snake Fungal Disease. J. Fungi Basel Switz. 2021, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.R.; Crevani, M.; Avella, I.; Cerullo, A.; Dorne, J.-L.C.M.; Paolino, G.; Zattera, C. A Guide to the Clinical Management of Vipera Snakebite in Italy. Toxins 2024, 16, 255. [Google Scholar] [CrossRef] [PubMed]
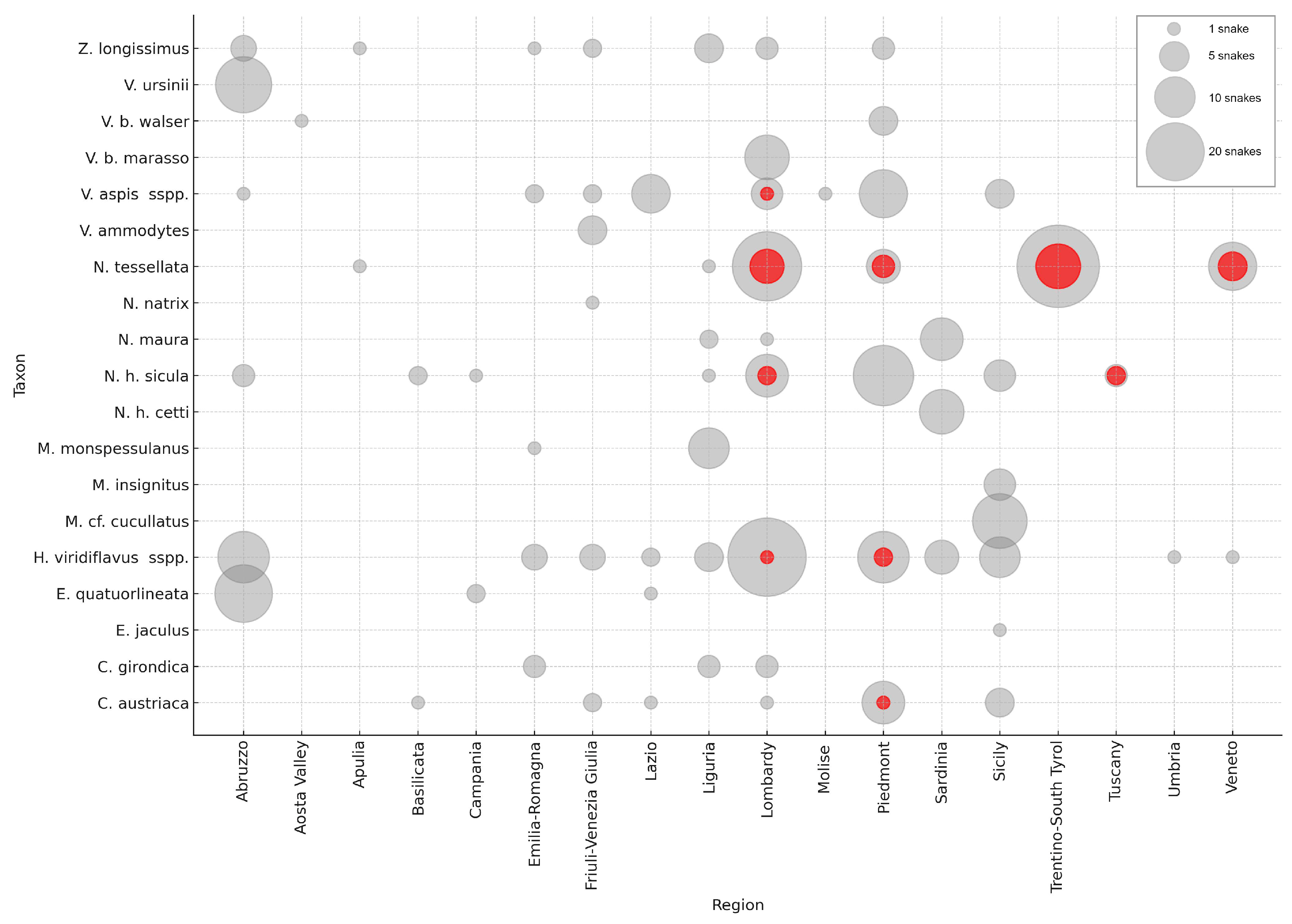


| Present Study | Overall Results | |||
| Snake Taxon | N° of Snakes | % Oo Positives | N° of Snakes | % Oo Positives |
| Coronella austriaca | 21 | 4.8% | 21 | 4.8% |
| Coronella girondica | 6 | 0% | 9 | 0% |
| Elaphe quatuorlineata | 10 | 0% | 23 | 0% |
| Eryx jaculus | 1 | 0% | 1 | 0% |
| Hierophis viridiflavus sspp. | 90 | 3.3% | 103 | 2.9% |
| Macroprotodon cf. cucullatus | 18 | 0% | 18 | 0% |
| Malpolon insignitus | 6 | 0% | 6 | 0% |
| Malpolon monspessulanus | 11 | 0% | 11 | 0% |
| Natrix helvetica cetti | 11 | 0% | 12 | 0% |
| Natrix helvetica sicula | 37|9 | 0%|44.4% | 40|9 | 0%|44.4% |
| Natrix maura | 14 | 0% | 14 | 0% |
| Natrix natrix vulgaris | 1 | 0% | 1 | 0% |
| Natrix tessellata | 81|8 | 25.9%|25% | 85|8 | 29.4%|25% |
| Vipera ammodytes | 5 | 0% | 5 | 0% |
| Vipera aspis sspp. | 40 | 2.5% | 40 | 2.5% |
| Vipera berus marasso | 12 | 0% | 12 | 0% |
| Vipera berus walser | 6 | 0% | 6 | 0% |
| Vipera ursinii | 19 | 0% | 19 | 0% |
| Zamenis longissimus | 16 | 0% | 19 | 0% |
| Unlabelled sample | 1 | 0% | 1 | 0% |
| Total | 406|17 | 6.4%|35.3% | 446|17 | 6.7%|35.3% |
| Present Study | Overall Results | |||
| Region | N° of Snakes | % Oo Positives | N° of Snakes | % Oo Positives |
| Abruzzo | 40 | 0% | 63 | 0% |
| Aosta Valley | 1 | 0% | 1 | 0% |
| Apulia | 2 | 0% | 2 | 0% |
| Basilicata | 3 | 0% | 3 | 0% |
| Campania | 3 | 0% | 3 | 0% |
| Emilia-Romagna | 9 | 0% | 11 | 0% |
| Friuli-Venezia Giulia | 16 | 0% | 16 | 0% |
| Lazio | 12 | 0% | 13 | 0% |
| Liguria | 26 | 0% | 27 | 0% |
| Lombardy | 84|13 | 8.3%|30.8% | 90|13 | 7.8%|30.8% |
| Molise | 1 | 0% | 1 | 0% |
| Piedmont | 77 | 7.8% | 78 | 7.7% |
| Sardinia | 29 | 0% | 30 | 0% |
| Sicily | 51 | 0% | 51 | 0% |
| Trentino-Alto Adige/Südtirol | 37 | 21.6% | 41 | 29.3% |
| Tuscany | 0|3 | 0|66.7% | 0|3 | 0|66.7% |
| Umbria | 0 | 0% | 1 | 0% |
| Veneto | 14|1 | 35.7%|0% | 14|1 | 35.7%|0% |
| Unlabelled sample | 1 | 0% | 1 | 0% |
| Total | 406|17 | 8.3%|30.8% | 446|17 | 6.7%|35.3% |
| Case Classification | Gross Signs | Molecular Detection | Hyphae (Histology) | Arthroconidia (Histology) | Present Study Results | Overall Results |
| Oo present | N or N/A | Y | N or N/A | N or N/A | 13 | 14 |
| Apparent ophidiomycosis | Y | Y | N or N/A | N or N/A | 14 | 14 |
| Ophidiomycosis | Y or N/A | Y | Y | N | 3 | 4 |
| Ophidiomycosis and Oo shedder | Y or N/A | Y | Y | Y | 2 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nicola, M.R.; Mulder, K.P.; Verbrugghe, E.; Storniolo, F.; Terriere, N.; Colla, L.; Sacchi, R.; Vanzo, G.; Zanfei, G.; Marini, D.; et al. Nationwide Screening Unveils Endemic Ophidiomyces ophidiicola Presence in Northern Italy, Mainly Affecting Dice Snakes: Evidence from Contemporary and Historical Snake Samples. J. Fungi 2025, 11, 118. https://doi.org/10.3390/jof11020118
Di Nicola MR, Mulder KP, Verbrugghe E, Storniolo F, Terriere N, Colla L, Sacchi R, Vanzo G, Zanfei G, Marini D, et al. Nationwide Screening Unveils Endemic Ophidiomyces ophidiicola Presence in Northern Italy, Mainly Affecting Dice Snakes: Evidence from Contemporary and Historical Snake Samples. Journal of Fungi. 2025; 11(2):118. https://doi.org/10.3390/jof11020118
Chicago/Turabian StyleDi Nicola, Matteo Riccardo, Kevin P. Mulder, Elin Verbrugghe, Federico Storniolo, Naomi Terriere, Luca Colla, Roberto Sacchi, Giacomo Vanzo, Giovanni Zanfei, Daniele Marini, and et al. 2025. "Nationwide Screening Unveils Endemic Ophidiomyces ophidiicola Presence in Northern Italy, Mainly Affecting Dice Snakes: Evidence from Contemporary and Historical Snake Samples" Journal of Fungi 11, no. 2: 118. https://doi.org/10.3390/jof11020118
APA StyleDi Nicola, M. R., Mulder, K. P., Verbrugghe, E., Storniolo, F., Terriere, N., Colla, L., Sacchi, R., Vanzo, G., Zanfei, G., Marini, D., Pasmans, F., & Martel, A. (2025). Nationwide Screening Unveils Endemic Ophidiomyces ophidiicola Presence in Northern Italy, Mainly Affecting Dice Snakes: Evidence from Contemporary and Historical Snake Samples. Journal of Fungi, 11(2), 118. https://doi.org/10.3390/jof11020118







