MaNrtB, a Putative Nitrate Transporter, Contributes to Stress Tolerance and Virulence in the Entomopathogenic Fungus Metarhizium acridum
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Bioinformatic Analyses
2.3. Fungal Mutant Generation
2.4. Conidial Germination and Conidial Yield Assays
2.5. Stress Tolerance Assays
2.6. Bioassays
2.7. Appressorium Formation Assays
2.8. Data Analyses
3. Results
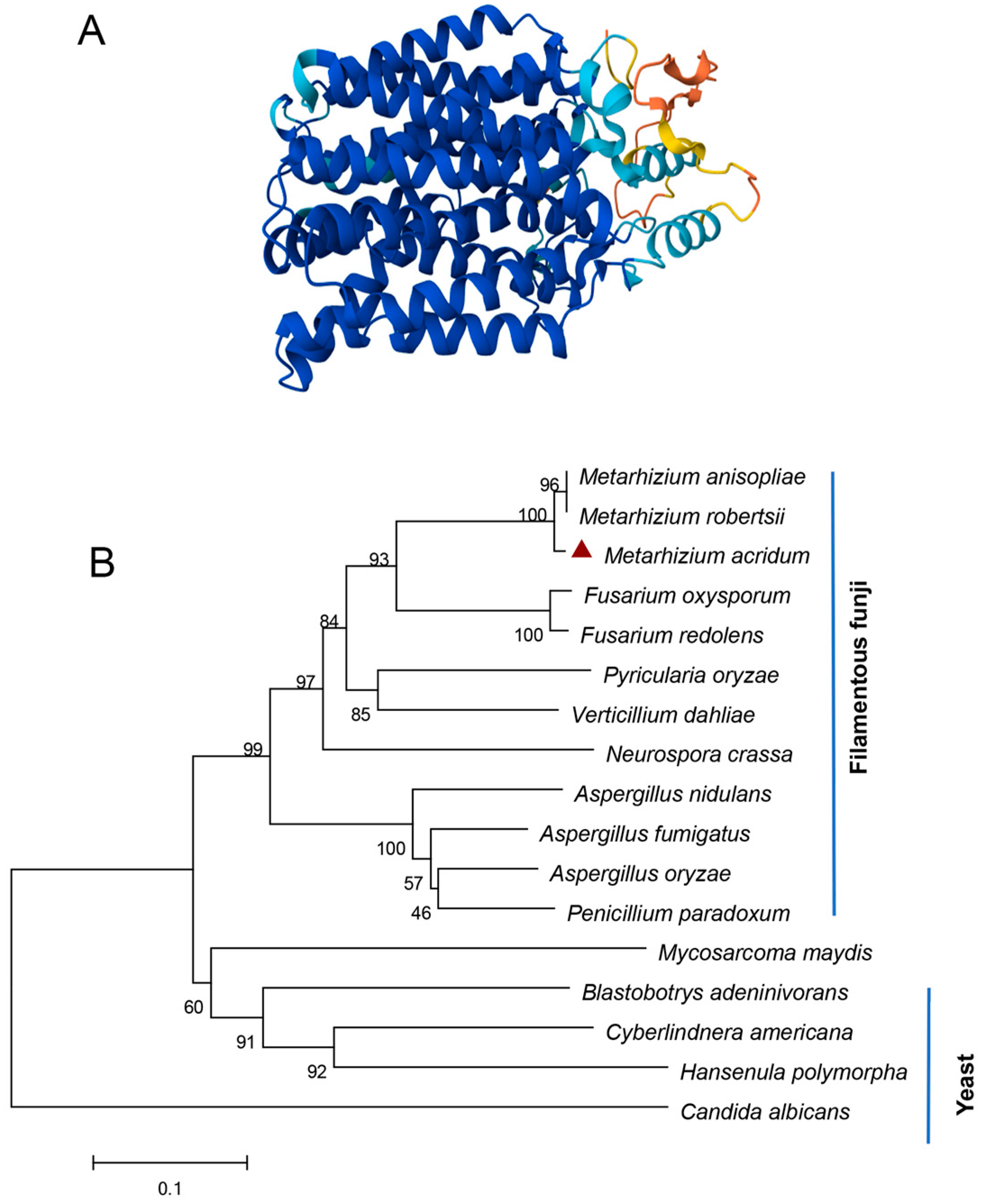
3.1. Features of MaNrtB and Mutant Generation
3.2. Disruption of MaNrtB Impaired Conidial Germination but Not Conidial Yield
3.3. Disruption of MaNrtB Affected Fungal Stress Tolerances
3.4. Disruption of MaNrtB Attenuated Fungal Virulence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic fungi: New insights into host-pathogen interactions. Adv. Genet. 2016, 94, 307–364. [Google Scholar] [PubMed]
- Thomas, M.B.; Read, A.F. Can fungal biopesticides control malaria? Nat. Rev. Microbiol. 2007, 5, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Morschhäuser, J. Nitrogen regulation of morphogenesis and protease secretion in Candida albicans. Int. J. Med. Microbiol. 2011, 301, 390–394. [Google Scholar] [CrossRef]
- Magasanik, B.; Kaiser, C.A. Nitrogen regulation in Saccharomyces cerevisiae. Gene 2002, 290, 1–18. [Google Scholar] [CrossRef]
- Unkles, S.E.; Hawker, K.L.; Grieve, C.; Campbell, E.I.; Montague, P.; Kinghorn, J.R. crnA encodes a nitrate transporter in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 1991, 88, 204–208. [Google Scholar] [CrossRef]
- Zhou, J.J.; Trueman, L.J.; Boorer, K.J.; Theodoulou, F.L.; Forde, B.G.; Miller, A.J. A high affinity fungal nitrate carrier with two transport mechanisms. J. Biol. Chem. 2000, 275, 39894–39899. [Google Scholar] [CrossRef] [PubMed]
- Pellizzaro, A.; Alibert, B.; Planchet, E.; Limami, A.M.; Morère-Le Paven, M.C. Nitrate transporters: An overview in legumes. Planta 2017, 246, 585–595. [Google Scholar] [CrossRef]
- Akhtar, N.; Karabika, E.; Kinghorn, J.R.; Glass, A.D.; Unkles, S.E.; Rouch, D.A. High-affinity nitrate/nitrite transporters NrtA and NrtB of Aspergillus nidulans exhibit high specificity and different inhibitor sensitivity. Microbiology 2015, 161, 1435–1446. [Google Scholar] [CrossRef]
- Machín, F.; Medina, B.; Navarro, F.J.; Pérez, M.D.; Veenhuis, M.; Tejera, P.; Lorenzo, H.; Lancha, A.; Siverio, J.M. The role of Ynt1 in nitrate and nitrite transport in the yeast Hansenula polymorpha. Yeast 2004, 21, 265–276. [Google Scholar] [CrossRef]
- Gomez-Gil, L.; Camara Almiron, J.; Rodriguez Carrillo, P.L.; Olivares Medina, C.N.; Bravo Ruiz, G.; Romo Rodriguez, P.; Corrales Escobosa, A.R.; Gutierrez Corona, F.; Roncero, M.I. Nitrate assimilation pathway (NAP): Role of structural (nit) and transporter (ntr1) genes in Fusarium oxysporum f.sp. lycopersici growth and pathogenicity. Curr. Genet. 2018, 64, 493–507. [Google Scholar]
- Khanal, S.; Schroeder, L.; Nava-Mercado, O.A.; Mendoza, H.; Perlin, M.H. Role for nitrate assimilatory genes in virulence of Ustilago maydis. Fungal Biol. 2021, 125, 764–775. [Google Scholar] [CrossRef]
- López-Bucio, J.; Esparza-Reynoso, S.; Pelagio-Flores, R. Nitrogen availability determines plant growth promotion and the induction of root branching by the probiotic fungus Trichoderma atroviride in Arabidopsis seedlings. Arch. Microbiol. 2022, 204, 380. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhu, X.; Jiao, R.; Xia, Y. The Magas1 gene is involved in pathogenesis by affecting penetration in Metarhizium acridum. J. Microbiol. Biotechnol. 2012, 22, 889–893. [Google Scholar] [CrossRef]
- Jin, K.; Ming, Y.; Xia, Y.X. MaHog1, a Hog1-type mitogen-activated protein kinase gene, contributes to stress tolerance and virulence of the entomopathogenic fungus Metarhizium acridum. Microbiology 2012, 158, 2987–2996. [Google Scholar] [CrossRef]
- Wen, Z.; Tian, H.; Xia, Y.; Jin, K. MaPmt1, a protein O-mannosyltransferase, contributes to virulence through governing the appressorium turgor pressure in Metarhizium acridum. Fungal Genet. Biol. 2020, 145, 103480. [Google Scholar] [CrossRef]
- Du, Y.; Jin, K.; Xia, Y. Involvement of MaSom1, a downstream transcriptional factor of cAMP/PKA pathway, in conidial yield, stress tolerances, and virulence in Metarhizium acridum. Appl. Microbiol. Biotechnol. 2018, 102, 5611–5623. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, J.J.; Xie, J.T.; Jiang, D.H. Functional characterization of BbEaf6 in Beauveria bassiana: Implications for fungal virulence and stress response. Virulence 2024, 15, 2387172. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jiang, H.; Du, Y.; Keyhani, N.O.; Xia, Y.; Jin, K. Members of chitin synthase family in Metarhizium acridum differentially affect fungal growth, stress tolerances, cell wall integrity and virulence. PLoS Pathog. 2019, 15, e1007964. [Google Scholar] [CrossRef]
- Sano, M. Aspergillus oryzae nrtA affects kojic acid production. Biosci. Biotechnol. Biochem. 2016, 80, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.D.; González, C.; Avila, J.; Brito, N.; Siverio, J.M. The YNT1 gene encoding the nitrate transporter in the yeast Hansenula polymorpha is clustered with genes YNI1 and YNR1 encoding nitrite reductase and nitrate reductase, and its disruption causes inability to grow in nitrate. Biochem. J. 1997, 321 Pt 2, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Gao-Rubinelli, F.; Marzluf, G.A. Identification and characterization of a nitrate transporter gene in Neurospora crassa. Biochem. Genet. 2004, 42, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Snoeijers, S.S.; Pérez-García, A.; Joosten, M.H.; De Wit, P.J.G.M. The effect of nitrogen on disease development and gene expression in bacterial and fungal plant pathogens. Eur. J. Plant Pathol. 2000, 106, 493–506. [Google Scholar] [CrossRef]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef] [PubMed]
- Marzluf, G.A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 1997, 61, 17–32. [Google Scholar] [PubMed]
- Wong, K.H.; Hynes, M.J.; Todd, R.B.; Davis, M.A. Transcriptional control of nmrA by the bZIP transcription factor MeaB reveals a new level of nitrogen regulation in Aspergillus nidulans. Mol. Microbiol. 2007, 66, 534–551. [Google Scholar] [CrossRef]
- Ravagnani, A.; Gorfinkiel, L.; Langdon, T.; Diallinas, G.; Adjadj, E.; Demais, S.; Gorton, D.; Arst, H.N.; Scazzocchio, C. Subtle hydrophobic interactions between the seventh residue of the zinc finger loop and the first base of an HGATAR sequence determine promoter-specific recognition by the Aspergillus nidulans GATA factor AreA. EMBO J. 1997, 16, 3974–3986. [Google Scholar] [CrossRef] [PubMed]
- Burger, G.; Tilburn, J.; Scazzocchio, C. Molecular cloning and functional characterization of the pathway-specific regulatory gene nirA, which controls nitrate assimilation in Aspergillus nidulans. Mol. Cell. Biol. 1991, 11, 795–802. [Google Scholar]
- Pan, H.; Feng, B.; Marzluf, G.A. Two distinct protein-protein interactions between the NIT2 and NMR regulatory proteins are required to establish nitrogen metabolite repression in Neurospora crassa. Mol. Microbiol. 1997, 26, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Hubermana, L.B.; Wu, V.W.; Kowbela, D.J.; Lee, J.; Daum, C.; Grigorieva, I.V.; O’Malley, R.C.; Glassa, N.L. DNA affinity purification sequencing and transcriptional profiling reveal new aspects of nitrogen regulation in a filamentous fungus. Proc. Natl. Acad. Sci. USA 2021, 118, e2009501118. [Google Scholar] [CrossRef]
- Berger, H.; Basheer, A.; Bock, S.; Reyes-Dominguez, Y.; Dalik, T.; Altmann, F.; Strauss, J. Dissecting individual steps of nitrogen transcription factor cooperation in the Aspergillus nidulans nitrate cluster. Mol. Microbiol. 2008, 69, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Kotaka, M.; Johnson, C.; Lamb, H.K.; Hawkins, A.R.; Ren, J.; Stammers, D.K. Structural analysis of the recognition of the negative regulator NmrA and DNA by the zinc finger from the GATA-type transcription factor AreA. J. Mol. Biol. 2008, 381, 373–382. [Google Scholar] [CrossRef]
- Wang, Z.L.; Jin, K.; Xia, Y.X. Transcriptional analysis of the conidiation pattern shift of the entomopathogenic fungus Metarhizium acridum in response to different nutrients. BMC Genom. 2016, 17, 586. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Xu, D.X.; Hu, M.W.; Zhang, Q.P.; Xia, Y.X.; Jin, K. MaNCP1, a C2H2 zinc finger protein, governs the conidiation pattern shift through regulating the reductive pathway for nitric oxide synthesis in the filamentous fungus Metarhizium acridum. Microbiol. Spectr. 2022, 10, e00538-22. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Xia, Y.X.; Jin, K. The C2H2 zinc finger protein MaNCP1 contributes to conidiation through governing the nitrate assimilation pathway in the entomopathogenic fungus Metarhizium acridum. J. Fungi 2022, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Lim, J.Y.; Xu, J.Y.; Yu, J.H.; Zheng, W. Nitric oxide as a developmental and metabolic signal in filamentous fungi. Mol. Microbiol. 2020, 113, 872–882. [Google Scholar] [CrossRef]
- Unkles, S.E.; Zhuo, D.; Siddiqi, M.Y.; Kinghorn, J.R.; Glass, A.D.M. Apparent genetic redundancy facilitates ecological plasticity for nitrate transport. EMBO J. 2001, 22, 6246–6255. [Google Scholar] [CrossRef]
- Li, C.C.; Xia, Y.X.; Jin, K. N-terminal zinc fingers of MaNCP1 contribute to growth, stress tolerance, and virulence in Metarhizium acridum. Int. J. Biol. Macromol. 2022, 216, 426–436. [Google Scholar] [CrossRef]
- Li, C.C.; Zhang, Q.P.; Xia, Y.X.; Jin, K. MaNmrA, a negative transcription regulator in nitrogen catabolite repression pathway, contributes to nutrient utilization, stress resistance and virulence in entomopathogenic fungus Metarhizium acridum. Biology 2021, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.Q.; Li, F.; Ying, S.H.; Feng, M.G. Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana. PLoS ONE 2012, 7, e30298. [Google Scholar] [CrossRef]
- Ibe, C.; Munro, C.A. Fungal cell wall proteins and signaling pathways form a cytoprotective network to combat stresses. J. Fungi 2021, 7, 739. [Google Scholar] [CrossRef]
- Perez-Nadales, E.; Di Pietro, A. The transmembrane protein Sho1 cooperates with the mucin Msb2 to regulate invasive growth and plant infection in Fusarium oxysporum. Mol. Plant Pathol. 2015, 16, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Ram, A.F.; Klis, F.M. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef]
- Landi, S.; Esposito, S. Nitrate Uptake Affects Cell wall synthesis and modeling. Front. Plant Sci. 2017, 8, 1376. [Google Scholar] [CrossRef] [PubMed]
- Pfannmüller, A.; Boysen, J.M.; Tudzynski, B. Nitrate assimilation in Fusarium fujikuroi is controlled by multiple levels of regulation. Front. Microbiol. 2017, 8, 381. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Kazan, K.; Manners, J.M. Nutrient profiling revealspotent inducers of trichothecene biosynthesis in Fusarium graminearum. Fungal Genet. Biol. 2009, 46, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.X.; Chen, F.X.; Li, Z.Z. Effects of carbon sources and nitrogen sources on the biological characteristics and antifungal activity of Beauveria bassiana. Acta Laser Biol. Sin. 2011, 20, 38–44. [Google Scholar]
- Liu, Y.; Li, Y.X.; Tong, S.; Wang, J.Y.; Zhu, S.A.; Zhang, L.Y.; Fan, Y.H. Effects of NirA1 gene on growth, stress resistance and virulence of Beauveria bassiana. Acta Microbiol. Sin. 2021, 61, 2469–2480. [Google Scholar]
- Brown, N.A.; Urban, M.; Van de Meene, A.M.L.; Hammond-Kosack, K.E. The infection biology of Fusarium graminearum: Defining the pathways of spikelet to spikelet colonisation in wheat ears. Fungal Biol. 2010, 114, 555–571. [Google Scholar] [CrossRef]
- Horowitz, S.; Freeman, S.; Zveibil, A.; Yarden, O. A defect in nir1, a nirA-like transcription factor, confers morphological abnormalities and loss of pathogenicity in Colletotrichum acutatum. Mol. Plant Pathol. 2006, 7, 341–354. [Google Scholar] [CrossRef]
- Wilson, R.A.; Jenkinson, J.M.; Gibson, R.P.; Littlechild, J.A.; Wang, Z.Y.; Talbot, N.J. Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. EMBO J. 2007, 26, 3673–3685. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Gibson, R.P.; Quispe, C.F.; Littlechild, J.A.; Talbot, N.J. An NADPH-dependent genetic switch regulates plant infection by the rice blast fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 21902–21907. [Google Scholar] [CrossRef] [PubMed]
- Marroquin-Guzman, M.; Wilson, R.A. GATA-dependent glutaminolysis drives appressorium formation in Magnaporthe oryzae by suppressing TOR inhibition of cAMP/PKA signaling. PLoS Pathog. 2015, 11, e1004851. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gong, Z.; Dulal, N.; Marroquin-Guzman, M.; Rocha, R.O.; Richter, M.; Wilson, R.A. A protein kinase coordinates cycles of autophagy and glutaminolysis in invasive hyphae of the fungus Magnaporthe oryzae within rice cells. Nat. Commun. 2023, 14, 4146. [Google Scholar] [CrossRef]
- Li, C.C.; Zhang, Q.P.; Xia, Y.X.; Jin, K. MaAreB, a GATA transcription factor, is involved in nitrogen source utilization, stress tolerances and virulence in Metarhizium acridum. J. Fungi 2021, 7, 512. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Romani, L.; Netea, M.G.; Latgé, J.P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, S.; Chen, L.; Zhan, T.; Zhang, X.; Liang, H.; Chen, B.; Peng, Y. Gut microbial diversity reveals differences in pathogenicity between Metarhizium rileyi and Beauveria bassiana during the early stage of infection in Spodoptera litura larvae. Microorganisms 2024, 12, 1129. [Google Scholar] [CrossRef]
- Jin, K.; Peng, G.X.; Liu, Y.C.; Xia, Y.X. The acid trehalase, ATM1, contributes to the in vivo growth and virulence of the entomopathogenic fungus, Metarhizium acridum. Fungal Genet. Biol. 2015, 77, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; St Leger, R.J. A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 6647–6652. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zou, Y.; Xia, Y.; Jin, K. MaNrtB, a Putative Nitrate Transporter, Contributes to Stress Tolerance and Virulence in the Entomopathogenic Fungus Metarhizium acridum. J. Fungi 2025, 11, 111. https://doi.org/10.3390/jof11020111
Wang J, Zou Y, Xia Y, Jin K. MaNrtB, a Putative Nitrate Transporter, Contributes to Stress Tolerance and Virulence in the Entomopathogenic Fungus Metarhizium acridum. Journal of Fungi. 2025; 11(2):111. https://doi.org/10.3390/jof11020111
Chicago/Turabian StyleWang, Jia, Yuneng Zou, Yuxian Xia, and Kai Jin. 2025. "MaNrtB, a Putative Nitrate Transporter, Contributes to Stress Tolerance and Virulence in the Entomopathogenic Fungus Metarhizium acridum" Journal of Fungi 11, no. 2: 111. https://doi.org/10.3390/jof11020111
APA StyleWang, J., Zou, Y., Xia, Y., & Jin, K. (2025). MaNrtB, a Putative Nitrate Transporter, Contributes to Stress Tolerance and Virulence in the Entomopathogenic Fungus Metarhizium acridum. Journal of Fungi, 11(2), 111. https://doi.org/10.3390/jof11020111






