Erythema Nodosum Associated with Kerion: A Case Series and Narrative Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Series
2.2. Review of the Literature
3. Results
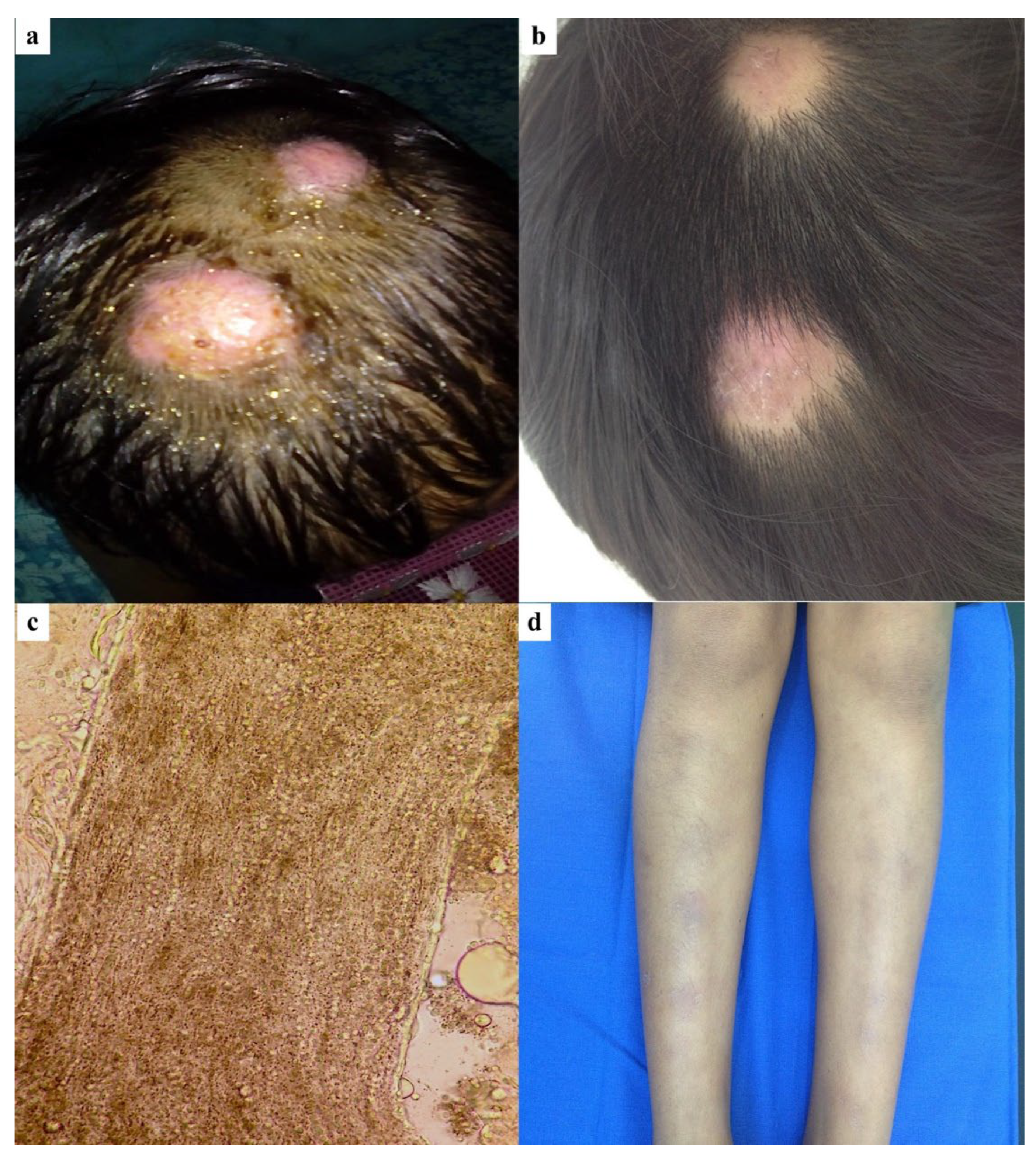
3.1. Case 1
3.2. Case 2
3.3. Case 3
3.4. Literature Review
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaraa, I.; Trojjet, S.; El Guellali, N.; El Euch, D.; Chelly, I.; Mokni, M.; Zitouna, M.; Osman, A.B. Childhood erythema nodosum associated with kerion celsi: A case report and review of literature. Pediatr. Dermatol. 2012, 29, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Gaviria, E.M.; Feci, L.; Fimiani, M. Erythema nodosum complicating kerion of the scalp caused by Trichophyton mentagrophytes. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Ilkit, M.; Durdu, M.; Karakaş, M. Cutaneous id reactions: A comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit. Rev. Microbiol. 2012, 38, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Trapani, S.; Rubino, C.; Lodi, L.; Resti, M.; Indolfi, G. Erythema Nodosum in Children: A Narrative Review and a Practical Approach. Children 2022, 9, 511. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mays, R.R.; Versteeg, S.G.; Piraccini, B.M.; Shear, N.H.; Piguet, V.; Tosti, A.; Friedlander, S.F. Tinea capitis in children: A systematic review of management. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2264–2274. [Google Scholar] [CrossRef]
- Zhan, P.; Li, D.; Wang, C.; Sun, J.; Geng, C.; Xiong, Z.; Seyedmousavi, S.; Liu, W.; de Hoog, G.S. Epidemiological changes in tinea capitis over the sixty years of economic growth in China. Med. Mycol. 2015, 53, 691–698. [Google Scholar] [CrossRef]
- Mohamed, M.; Belkahla, M.; Hammedi, F.; Belhadjali, H.; Zili, J. Erythema nodosum induced by kerion Celsi in a Tunisian child: A case report. Our Dermatol. Online 2016, 7, 351–352. [Google Scholar] [CrossRef]
- Calista, D.; Schianchi, S.; Morri, M. Erythema nodosum induced by kerion celsi of the scalp. Pediatr. Dermatol. 2001, 18, 114–116. [Google Scholar] [CrossRef]
- Herzum, A.; Garibeh, E.; Gariazzo, L.; Occella, C.; Viglizzo, G. Erythema nodosum triggered by kerion celsi in pediatrics: Literature review and case report. An. Bras. Dermatol. 2024, 99, 312–315. [Google Scholar] [CrossRef]
- Ben Salah, N.; Korbi, M.; Soua, Y.; Youssef, M.; Belhadjali, H.; Zili, J. Erythema nodosum in patients with kerion of scalp. Clin. Exp. Dermatol. 2021, 46, 1577–1578. [Google Scholar] [CrossRef]
- Kelati, A.; Meziane, M.; Soughi, M.; Mernissi, F. Erythema nodosum due to tinea. Arch. Pediatr. 2016, 23, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Castriota, M.; Ricci, F.; Paradisi, A.; Fossati, B.; De Simone, C.; Capizzi, R.; Guerriero, C. Erythema nodosum induced by kerion celsi of the scalp in a child: A case report and mini-review of literature. Mycoses 2013, 56, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Bassi, N.; Kersey, P. Erythema nodosum complicating a case of kerion celsi of the scalp due to Trichophyton mentagrophytes. Clin. Exp. Dermatol. 2009, 34, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Morrone, A.; Calcaterra, R.; Valenzano, M.; Fazio, R.; Franco, G. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses 2011, 54, e237–e239. [Google Scholar]
- Soria, X.; Sanmartín, V.; Martí, R.M.; Baradad, M.; Casanova, J.M. Erythema nodosum associated with inflammatory tinea capitis (kerion celsi). Actas Dermosifiliogr. 2008, 99, 319–321. [Google Scholar] [CrossRef]
- Provini, A.; Cacciaguerra, M.G.; Angelo, C.; Pedicelli, C.; Paradisi, M. Erythema nodosum induced by kerion celsi in a child with hypomelanosis of Ito. Minerva Pediatr. 2003, 55, 621–624. [Google Scholar]
- de las Heras, C.; Borbujo, J.; Pizarro, A.; Casado, M. Erythema nodosum caused by kerion of the scalp. Clin. Exp. Dermatol. 1990, 15, 317–318. [Google Scholar] [CrossRef]
- Martinez-Roig, A.; Llorens-Terol, J.; Torres, J.M. Erythema nodosum and kerion of the scalp. Am. J. Dis. Child. 1982, 136, 440–442. [Google Scholar] [CrossRef]
- Stocker, W.W.; Richtsmeier, A.J.; Rozycki, A.A.; Baughman, R.D. Kerion caused by Trichophyton verrucosum. Pediatrics 1977, 59, 912–915. [Google Scholar] [CrossRef]
- Velasco, J.A.; Martín-Pascual, A.; García-Pérez, A. Trichophyton erythema nodosum. Actas Dermosifiliogr. 1975, 66, 493–496. [Google Scholar]
- Smith, J.F. Erythema nodosum in association with pustular ringworm. Br. Med. J. 1963, 1, 1592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Topaloğlu Demir, F.; Karadag, A.S. Are Dermatophytid Reactions in Patients with Kerion Celsi Much More Common Than Previously Thought? A Prospective Study. Pediatr. Dermatol. 2015, 32, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Koga, T. Immune response in dermatophytosis. Nihon Ishinkin Gakkai Zasshi 2003, 44, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Llorente, L.; Richaud-Patin, Y.; Alvarado, C.; Reyes, E.; Alcocer-Varela, J.; Orozco-Topete, R. Elevated Th1 cytokine mRNA in skin biopsies and peripheral circulation in patients with erythema nodosum. Eur. Cytokine Netw. 1997, 8, 67–71. [Google Scholar] [PubMed]
- Nakamura, T.; Nishibu, A.; Yasoshima, M.; Tanoue, C.; Yoshida, N.; Hatta, J.; Miyamoto, T.; Nishii, M.; Yanagibashi, T.; Nagai, Y.; et al. Analysis of Trichophyton antigen-induced contact hypersensitivity in mouse. J. Dermatol. Sci. 2012, 66, 144–153. [Google Scholar] [CrossRef]
- Elewski, B.E.; El Charif, M.; Cooper, K.D.; Ghannoum, M.; Birnbaum, J.E. Reactivity to trichophytin antigen in patients with onychomycosis: Effect of terbinafine. J. Am. Acad. Dermatol. 2002, 46, 371–375. [Google Scholar] [CrossRef]
- Hussain, I.; Muzaffar, F.; Rashid, T.; Ahmad, T.J.; Jahangir, M.; Haroon, T.S. A randomized, comparative trial of treatment of kerion celsi with griseofulvin plus oral prednisolone vs. griseofulvin alone. Med. Mycol. 1999, 37, 97–99. [Google Scholar] [CrossRef]


| Number of Patients (n = 23) | |
|---|---|
| Gender, n (%) | |
| 16 (69.57%) |
| 7 (30.43%) |
| Age, years ± IQR | 8 (6.5–9) |
| Onset of kerion, days ± IQR | 14.5 (13.75–28.5) |
| Location of kerion, n (%) | |
| 8 (34.78%) |
| 6 (26.08%) |
| 4 (17.39%) |
| 2 (8.7%) |
| 2 (8.7%) |
| 1 (4.35%) |
| Lymphadenopathy, n (%) | 6 (26.08%) |
| History of contact animals, n (%) | 7 (30.43%) |
| Culture of pathogen, n (%) | |
| 18 (78.25%) |
| 1 (4.35%) |
| 1 (4.35%) |
| 1 (4.35%) |
| 1 (4.35%) |
| 1 (4.35%) |
| EN location, n (%) | |
| 10 (43.48%) |
| 8 (34.78%) |
| 3 (13.04%) |
| 1 (4.35%) |
| 1 (4.35%) |
| |
| 21 (91.3%) |
| 2 (8.7%) |
| EN develop before receiving antifungal treatment | 10 (43.49%) |
| EN developed after initiating antifungal treatment | 12 (52.17%) |
| Time from treatment to onset EN, days ± SD | 11.58 (7.3%) |
| Treatment, n (%) | |
| 20 (86.95%) |
| 1 (4.35%) |
| 2 (8.7%) |
| Healing time of EN, days ± SD | 8.31 (4.15) |
| Treatment duration for kerion, weeks ± IQR | 6 (6–8) |
| Authors/Year | Country | Gender/Age (Years) | Onset of Kerion (Days) | Kerion Location | History of Contact Animals | Culture | EN Location/Other Id Reaction | Time in Days from Treatment to Onset EN | Treatment | Healing Time of EN (Days) | Treatment Duration for Kerion (Weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Our series | Mexico | M/8 | 7 | Occipital scalp + cervical lymphadenopathy | No | Trichophyton mentagrophytes ^ | Shins | Before treatment | Griseofulvin 25 MKD | 7 | 4 |
| F/8 | 15 | Occipitoparietal scalp | No | Trichophyton mentagrophytes ^ | Shins | 7 | Itraconazole 5 MKD + prednisolone 1 MKD | 7 | 6 | ||
| F/5 | 7 | Occipital scalp | No | Trichophyton mentagrophytes ^ | Shins/Erythmatous scaly plaques on trunk and face | Before treatment | Itraconazole 5 MKD + prednisolone 1 MKD | 7 | 6 | ||
| Herzum et al. 2024 [9] | Italy | M/7 | 30 | Scalp + occipital lymphadinopathy | No | Microsporum canis | Legs | Before treatment | Griseofulvin 20 MKD | NA | 8 |
| Salah et al. 2021 [10] | Tunisia | M/14 | NA | Vertex | NA | Trichophyton mentagrophytes | Legs | 14 | Griseofulvin | 1 | NA |
| M/9 | NA | Vertex | NA | Trichophyton mentagrophytes | Legs | 7 | |||||
| M/4 | NA | Vertex | NA | Trichophyton mentagrophytes | Legs | 20 | |||||
| Romano et al. 2016 [2] | Italy | F/4 | 13 | Scalp + cervical lymphadenopathy | Cat | Trichophyton mentagrophytes ^ | Lower legs/Erythematous papular lesions on ears, abdomen and back | 2 | Griseofulvin 20 MKD + oral betamethasone | 9 | 8 |
| Kelati et al. 2016 [11] | Morroco | M/9 | 15 | Vertex | Dogs | Trichophyton mentagrophytes | Legs, forearms * | Before treatment | Griseofulvin 25 MKD | NA | 6 |
| Mohamed et al. 2016 [7] | Tunisia | M/7 | 14 | Temporal scalp | Rabbit | Trichophyton mentagrophytes | Legs * | Before treatment | Griseofulvin 20 MKD + topical econazole + mefenamic acid 500 mg/day | 7 | 6 |
| Castriota et al. 2013 [12] | Italy | F/9 | 48 | Occipital scalp + Retronuchal lymphadenopathy | Rabbit, dogs | Trichophyton mentagrophytes ^ | Legs * | 14 | Griseofulvin 25 MKD + topical mupirocin and tioconazole + prednisone 1 MKD | 10 | 6 |
| Zaraa et al. 2012 [1] | Tunisia | M/7 | 60 | Occipitopariental scalp + cervical lymphadenopathy | No | Mega sporic parasitism ^ | Shins, thighs */Erythematous patches on trunk | 18 | Griseofulvin 25 MKD + ciclopirox olamine cream | 7 | 12 |
| Bassi et al. 2009 [13] | UK | F/8 | 8 | Vertex + cervical lymphadenopathy | Pet rats | Trichophyton mentagrophytes | Shins, thighs | 1 | Griseofulvin 10 MKD | 10 | 6 |
| Morrone et al. 2011 [14] | Italy (Philippine nationality) | F/35 | 21 | Vertex ^ | No | Trichophyton mentagrophytes ^ | Pretibial area * | Before treatment | Terbinafine 250 mg/day + naproxen 1 g/day | 15 | 8 |
| Soria et al. 2008 [15] | Spain | M/11 | NA | Parietal scalp | NA | Trichophyton mentagrophytes | Lower legs | 26 | Griseofulvin + Ibuprofen | 7 | NA |
| M/9 | NA | Scalp | NA | Trichophyton mentagrophytes | Lower legs | 16 | Griseofulvin | 7 | NA | ||
| Provini et al. 2003 [16] | Italy | M/3 | 56 | Scalp | NA | Epidermophyton floccosum | Lower legs | Before treatment | Griseolfuvin+ topical eosine and myconazole | NA | NA |
| Calista et al. 2001 [8] | Italy | F/5 | 14 | Right parietal scalp | No | Trichophyton mentagrophytes | Shins, thighs | Before treatment | Griseofulvin 18 MKD + topical crystal violet | 10 | 6 |
| De las Heras et al. 1991 [17] | Spain | M/9 | 7 | Scalp | Rabbitsdogs | Trichophyton mentagrophytes | Shins * | Before treatment | Griseofulvin 10 MKD + topical tioconazole | 12 | 6 |
| Martinez-Roig et al. 1982 [18] | Spain | M/8 | NA | Occipital scalp | NA | Trichophyton mentagrophytes | Shins | 7 | Griseofulvin + topical potassium permanganate solution | 12 | NA |
| M/6 | NA | Occipital scalp | NA | Trichophyton mentagrophytes | Shins | 7 | 12 | NA | |||
| M/3 | NA | Occipital scalp | NA | Trichophyton mentagrophytes | Shins | 7 | 12 | NA | |||
| Stocker et al. 1977 [19] | USA | F/12 | NA | Scalp | NA | Trichophyton verrucosum | Shins | Before treatment | Griseofulvin | NA | NA |
| Velasco et al. 1975 [20] | Spain | M/7 | NA | Scalp | NA | Trichophyton mentagrophytes | Legs | NA | Griseofulvin | NA | NA |
| Smith et al. 1963 [21] | UK | M/7 | 14 | Occipitopariental scalp + cervical adenitis | White mouse | Trichophyton mentagrophytes | Lower legs | Before treatment | Griseofulvin + topical tioconazole | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rattananukrom, T.; Uh-Sánchez, I.; Atoche-Dieguez, C.; Eljure, N.; Garcia-Rementeria, C.; Arenas, R. Erythema Nodosum Associated with Kerion: A Case Series and Narrative Review of the Literature. J. Fungi 2025, 11, 103. https://doi.org/10.3390/jof11020103
Rattananukrom T, Uh-Sánchez I, Atoche-Dieguez C, Eljure N, Garcia-Rementeria C, Arenas R. Erythema Nodosum Associated with Kerion: A Case Series and Narrative Review of the Literature. Journal of Fungi. 2025; 11(2):103. https://doi.org/10.3390/jof11020103
Chicago/Turabian StyleRattananukrom, Teerapong, Isaías Uh-Sánchez, Carlos Atoche-Dieguez, Nixma Eljure, Carlos Garcia-Rementeria, and Roberto Arenas. 2025. "Erythema Nodosum Associated with Kerion: A Case Series and Narrative Review of the Literature" Journal of Fungi 11, no. 2: 103. https://doi.org/10.3390/jof11020103
APA StyleRattananukrom, T., Uh-Sánchez, I., Atoche-Dieguez, C., Eljure, N., Garcia-Rementeria, C., & Arenas, R. (2025). Erythema Nodosum Associated with Kerion: A Case Series and Narrative Review of the Literature. Journal of Fungi, 11(2), 103. https://doi.org/10.3390/jof11020103






