Treating Onychomycosis with Efinaconazole: Considerations for Diverse Patient Groups
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Children
3.2. Elderly
3.3. Diabetic Patients
3.4. Racial and Ethnic Groups
3.5. In Vitro Activity of Efinaconazole Against Dermatophytes
3.6. Long-Term and Prophylactic Treatment
3.7. Other Treatment Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, A.K.C.; Lam, J.M.; Leong, K.F.; Hon, K.L.; Barankin, B.; Leung, A.A.M.; Wong, A.H.C. Onychomycosis: An Updated Review. Recent Pat. Inflamm. Allergy Drug Discov. 2020, 14, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Wang, T.; Polla Ravi, S.; Mann, A.; Bamimore, M.A. Global Prevalence of Onychomycosis in General and Special Populations: An Updated Perspective. Mycoses 2024, 67, e13725. [Google Scholar] [CrossRef] [PubMed]
- Benitez, L.L.; Carver, P.L. Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents. Drugs 2019, 79, 833–853. [Google Scholar] [CrossRef] [PubMed]
- Khoza, S.; Moyo, I.; Ncube, D. Comparative Hepatotoxicity of Fluconazole, Ketoconazole, Itraconazole, Terbinafine, and Griseofulvin in Rats. J. Toxicol. 2017, 2017, 6746989. [Google Scholar] [CrossRef]
- Yousefian, F.; Smythe, C.; Han, H.; Elewski, B.E.; Nestor, M. Treatment Options for Onychomycosis: Efficacy, Side Effects, Adherence, Financial Considerations, and Ethics. J. Clin. Aesthet. Dermatol. 2024, 17, 24–33. [Google Scholar]
- U.S. Food and Drug Administration Prescribing Information for JUBLIA (Efinaconazole) Topical Solution. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/203567s010lbl.pdf (accessed on 20 October 2025).
- Alsuhibani, A.A.; Alobaid, N.A.; Alahmadi, M.H.; Alqannas, J.S.; Alfreaj, W.S.; Albadrani, R.F.; Alamer, K.A.; Almogbel, Y.S.; Alhomaidan, A.; Guo, J.J. Antifungal Agents’ Trends of Utilization, Spending, and Prices in the US Medicaid Programs: 2009–2023. Antibiotics 2025, 14, 518. [Google Scholar] [CrossRef]
- Health Canada Product Monograph for JUBLIA (Efinaconazole Topical Solution 10%). Available online: https://pdf.hres.ca/dpd_pm/00054721.PDF (accessed on 13 November 2025).
- Pharmaceuticals and Medical Devices Agency Review Report: Clenafin Topical Solution of Toenails, 10%. Available online: https://www.pmda.go.jp/files/000211073.pdf (accessed on 13 November 2025).
- European Medicines Agency EMA Decision of 13 June 2023 on the Acceptance of a Modification of an Agreed Paediatric Investigation Plan for Efinaconazole. Available online: https://www.ema.europa.eu/en/documents/pip-decision/p-0203-2023-ema-decision-13-june-2023-acceptance-modification-agreed-paediatric-investigation-plan-efinaconazole-emea-001627-pip01-14-m03_en.pdf (accessed on 13 November 2025).
- Eichenfield, L.F.; Elewski, B.; Sugarman, J.L.; Rosen, T.; Vlahovic, T.C.; Gupta, A.K.; Stein Gold, L.; Pillai, R.; Guenin, E. Safety, Pharmacokinetics, and Efficacy of Efinaconazole 10% Topical Solution for Onychomycosis Treatment in Pediatric Patients. J. Drugs Dermatol. 2020, 19, 867–872. [Google Scholar] [CrossRef]
- Lipner, S.R.; Gupta, A.K.; Joseph, W.S.; Elewski, B.; Guenin, E.; Vlahovic, T.C. Efficacy and Safety of Efinaconazole 10% Topical Solution for Treatment of Onychomycosis in Older Adults: A Post Hoc Analysis of Two Phase 3 Randomised Trials. Mycoses 2025, 68, e70069. [Google Scholar] [CrossRef]
- Gupta, A.K.; Elewski, B.E.; Sugarman, J.L.; Ieda, C.; Kawabata, H.; Kang, R.; Pillai, R.; Olin, J.T.; Watanabe, S. The Efficacy and Safety of Efinaconazole 10% Solution for Treatment of Mild to Moderate Onychomycosis: A Pooled Analysis of Two Phase 3 Randomized Trials. J. Drugs Dermatol. 2014, 13, 815–820. [Google Scholar]
- Iozumi, K.; Abe, M.; Ito, Y.; Uesugi, T.; Onoduka, T.; Kato, I.; Kato, F.; Kodama, K.; Takahashi, H.; Takeda, O.; et al. Efficacy of Long-term Treatment with Efinaconazole 10% Solution in Patients with Onychomycosis, Including Severe Cases: A Multicenter, Single-arm Study. J. Dermatol. 2019, 46, 641–651. [Google Scholar] [CrossRef]
- Vlahovic, T.C.; Joseph, W.S. Efinaconazole Topical, 10% for the Treatment of Toenail Onychomycosis in Patients with Diabetes. J. Drugs Dermatol. 2014, 13, 1186–1190. [Google Scholar] [PubMed]
- Shofler, D.; Hamedani, E.; Seun, J.; Navarrete, R.; Thamby, R.; Harkless, L. Efficacy and Safety of Efinaconazole 10% Solution in the Treatment of Onychomycosis in Diabetic Patients. Clin. Podiatr. Med. Surg. 2020, 37, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Cook-Bolden, F.E.; Lin, T. Efinaconazole Solution 10% for Treatment of Toenail Onychomycosis in Latino Patients. Cutis 2017, 99, 286–289. [Google Scholar] [PubMed]
- Tschen, E.H.; Bucko, A.D.; Oizumi, N.; Kawabata, H.; Olin, J.T.; Pillai, R. Efinaconazole Solution in the Treatment of Toenail Onychomycosis: A Phase 2, Multicenter, Randomized, Double-Blind Study. J. Drugs Dermatol. 2013, 12, 186–192. [Google Scholar]
- Markinson, B.; Caldwell, B. Efinaconazole Topical Solution 10% Efficacy in Patients with Onychomycosis and Coexisting Tinea Pedis. J. Am. Podiatr. Med. Assoc. 2015, 105, 407–411. [Google Scholar] [CrossRef]
- Elewski, B.E.; Rich, P.; Pollak, R.; Pariser, D.M.; Watanabe, S.; Senda, H.; Ieda, C.; Smith, K.; Pillai, R.; Ramakrishna, T.; et al. Efinaconazole 10% Solution in the Treatment of Toenail Onychomycosis: Two Phase III Multicenter, Randomized, Double-Blind Studies. J. Am. Acad. Dermatol. 2013, 68, 600–608. [Google Scholar] [CrossRef]
- Gupta, A.K.; Venkataraman, M.; Shear, N.H.; Piguet, V. Onychomycosis in Children—Review on Treatment and Management Strategies. J. Dermatolog. Treat. 2022, 33, 1213–1224. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Friedlander, S.F. Pediatric Onychomycosis: The Emerging Role of Topical Therapy. J. Drugs Dermatol. 2017, 16, 105–109. [Google Scholar]
- Gupta, A.K.; Cooper, E.A. Extended Use of Topical Efinaconazole Remains Safe and Can Provide Continuing Benefits for Dermatophyte Toenail Onychomycosis. J. Fungi 2024, 10, 620. [Google Scholar] [CrossRef]
- Cathcart, S.; Cantrell, W.; Elewski, B.E. Onychomycosis and Diabetes. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1119–1122. [Google Scholar] [CrossRef]
- Choo, Z.-N.; Lipner, S.R. Mendelian Randomization Analysis Supports Causal Effect of Type II Diabetes Mellitus on Onychomycosis. Ski. Appendage Disord. 2024, 10, 220–223. [Google Scholar] [CrossRef]
- Matricciani, L.; Talbot, K.; Jones, S. Safety and Efficacy of Tinea Pedis and Onychomycosis Treatment in People with Diabetes: A Systematic Review. J. Foot Ankle Res. 2011, 4, 26. [Google Scholar] [CrossRef]
- Moseley, I.; Ragi, S.D.; Ouellette, S.; Rao, B. Onychomycosis in Underrepresented Groups: An All of Us Database Analysis. Arch. Dermatol. Res. 2022, 315, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Knusel, K.D.; Ezaldein, H.H.; Scott, J.F.; Bordeaux, J.S. Association of Demographic and Socioeconomic Characteristics With Differences in Use of Outpatient Dermatology Services in the United States. JAMA Dermatol. 2018, 154, 1286. [Google Scholar] [CrossRef]
- Pearlman, R.L.; Brodell, R.T.; Byrd, A.C. Enhancing Access to Rural Dermatological Care. JAMA Dermatol. 2022, 158, 725. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, Y.; Nagashima, M.; Shibanushi, T.; Iwata, A.; Kangawa, Y.; Inui, F.; Siu, W.J.J.; Pillai, R.; Nishiyama, Y. Mechanism of Action of Efinaconazole, a Novel Triazole Antifungal Agent. Antimicrob. Agents Chemother. 2013, 57, 2405–2409. [Google Scholar] [CrossRef] [PubMed]
- Jo Siu, W.J.; Tatsumi, Y.; Senda, H.; Pillai, R.; Nakamura, T.; Sone, D.; Fothergill, A. Comparison of in Vitro Antifungal Activities of Efinaconazole and Currently Available Antifungal Agents against a Variety of Pathogenic Fungi Associated with Onychomycosis. Antimicrob. Agents Chemother. 2013, 57, 1610–1616. [Google Scholar] [CrossRef]
- Iwata, A.; Watanabe, Y.; Kumagai, N.; Katafuchi-Nagashima, M.; Sugiura, K.; Pillai, R.; Tatsumi, Y. In Vitro and In Vivo Assessment of Dermatophyte Acquired Resistance to Efinaconazole, a Novel Triazole Antifungal. Antimicrob. Agents Chemother. 2014, 58, 4920–4922. [Google Scholar] [CrossRef]
- Ishii, M.; Yamada, T.; Ohata, S. An Efficient Gene Targeting System Using Δku80 and Functional Analysis of Cyp51A in Trichophyton Rubrum. AMB Express 2024, 14, 96. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Mann, A.; Piguet, V.; Chowdhary, A.; Bakotic, W.L. Mechanisms of Resistance against Allylamine and Azole Antifungals in Trichophyton: A Renewed Call for Innovative Molecular Diagnostics in Susceptibility Testing. PLOS Pathog. 2025, 21, e1012913. [Google Scholar] [CrossRef]
- Ahmad Nasrollahi, S.; Fattahi, A.; Naeimifar, A.; Lotfali, E.; Firooz, A.; Khamesipoor, A.; Skandari, S.E.; Miramin Mohammadi, A. The in Vitro Effect of Nanoliposomal Amphotericin B against Two Clinically Important Dermatophytes. Int. J. Dermatol. 2022, 61, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Nojo, H.; Hiruma, J.; Noguchi, H.; Shimizu, T.; Hiruma, M.; Harada, K.; Makimura, K.; Kano, R. Terbinafine-Resistant Dermatophytes Isolated in Japan: Investigation of Isolates Collected in 2020, 2022, and 2023. Med. Mycol. J. 2025, 66, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Noguchi, H.; Matsumoto, T.; Kubo, M.; Hayashi, D.; Kashiwada-Nakamura, K.; Yaguchi, T.; Kano, R. Emerging Antifungal-Resistant Onychomycosis in a Dermatology Clinic in Kumamoto, Japan. Med. Mycol. J. 2025, 66, 61–67. [Google Scholar] [CrossRef]
- Rezaei-Matehkolaei, A.; Khodavaisy, S.; Alshahni, M.M.; Tamura, T.; Satoh, K.; Abastabar, M.; Shokoohi, G.R.; Ahmadi, B.; Kord, M.; Taghipour, S.; et al. In Vitro Antifungal Activity of Novel Triazole Efinaconazole and Five Comparators against Dermatophyte Isolates. Antimicrob. Agents Chemother. 2018, 62, e02423-17. [Google Scholar] [CrossRef]
- Sachan, T.; Gupta, P.; Suvirya, S.; Verma, P.; Kalyan, R.K.; Banerjee, G. Evaluation of the Efficacy of Novel Topical Antifungal Agents against Dermatophytes in North India: A Prospective Study. Curr. Med. Mycol. 2024, 10, e2024-345268. [Google Scholar] [PubMed Central]
- Shamsizadeh, F.; Ansari, S.; Zarei Mahmoudabadi, A.; Hubka, V.; Čmoková, A.; Guillot, J.; Rafiei, A.; Zomorodian, K.; Nouripour-Sisakht, S.; Diba, K.; et al. In Vitro Antifungal Susceptibility Patterns of Trichophyton Benhamiae Complex Isolates from Diverse Origin. Mycoses 2021, 64, 1378–1386. [Google Scholar] [CrossRef]
- Shamsizadeh, F.; Zarei Mahmoudabadi, A.; Shariat Nabavi, M.; Guillot, J.; Taghipour, S.; Rezaei-Matehkolaei, A. In Vitro Activities of 8 Antifungal Agents against Geophilic Dermatophyte Isolates. Mycoses 2022, 65, 255–262. [Google Scholar] [CrossRef]
- Tachibana, H.; Kumagai, N.; Tatsumi, Y. Fungicidal Activity in the Presence of Keratin as an Important Factor Contributing to in Vivo Efficacy: A Comparison of Efinaconazole, Tavaborole, and Ciclopirox. J. Fungi 2017, 3, 58. [Google Scholar] [CrossRef]
- Taghipour, S.; Shamsizadeh, F.; Pchelin, I.M.; Rezaei-Matehhkolaei, A.; Mahmoudabadi, A.Z.; Valadan, R.; Ansari, S.; Katiraee, F.; Pakshir, K.; Zomorodian, K.; et al. Emergence of Terbinafine Resistant Trichophyton Mentagrophytes in Iran, Harboring Mutations in the Squalene Epoxidase (SQLE) Gene. Infect. Drug Resist. 2020, 13, 845–850. [Google Scholar] [CrossRef]
- Gamal, A.; Elshaer, M.; Long, L.; McCormick, T.S.; Elewski, B.; Ghannoum, M.A. Antifungal Activity of Efinaconazole Compared with Fluconazole, Itraconazole, and Terbinafine Against Terbinafine- and Itraconazole-Resistant/Susceptible Clinical Isolates of Dermatophytes, Candida, and Molds. J. Am. Podiatr. Med. Assoc. 2024, 114, 1–8. [Google Scholar] [CrossRef]
- Haghani, I.; Shams-Ghahfarokhi, M.; Dalimi Asl, A.; Shokohi, T.; Hedayati, M.T. Molecular Identification and Antifungal Susceptibility of Clinical Fungal Isolates from Onychomycosis (Uncommon and Emerging Species). Mycoses 2019, 62, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Halvaee, S.; Daie-Ghazvini, R.; Hashemi, S.J.; Khodavaisy, S.; Rahimi-Foroushani, A.; Bakhshi, H.; Rafat, Z.; Ardi, P.; Abastabar, M.; Zareei, M.; et al. A Mycological and Molecular Epidemiologic Study on Onychomycosis and Determination In Vitro Susceptibilities of Isolated Fungal Strains to Conventional and New Antifungals. Front. Cell. Infect. Microbiol. 2021, 11, 693522. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, J.; Kimura, U.; Noguchi, H.; Hiruma, M.; Harada, K.; Kano, R. In Vitro Azole Susceptibility Testing of Japanese Isolates of Terbinafine-Resistant Trichophyton Indotineae and Trichophyton Rubrum. Med. Mycol. J. 2023, 64, 23–25. [Google Scholar] [CrossRef]
- Hiruma, J.; Nojyo, H.; Harada, K.; Kano, R. Development of Treatment Strategies by Comparing the Minimum Inhibitory Concentrations and Minimum Fungicidal Concentrations of Azole Drugs in Dermatophytes. J. Dermatol. 2024, 51, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Kubota-Ishida, N.; Takei-Masuda, N.; Kaneda, K.; Nagira, Y.; Chikada, T.; Nomoto, M.; Tabata, Y.; Takahata, S.; Maebashi, K.; Hui, X.; et al. In Vitro Human Onychopharmacokinetic and Pharmacodynamic Analyses of ME1111, a New Topical Agent for Onychomycosis. Antimicrob. Agents Chemother. 2018, 62, e00779-17. [Google Scholar] [CrossRef]
- Lysková, P.; Dobiáš, R.; Čmoková, A.; Kolařík, M.; Hamal, P.; Šmatláková, K.; Hušek, J.; Mencl, K.; Mallátová, N.; Poláčková, Z.; et al. An Outbreak of Trichophyton Quinckeanum Zoonotic Infections in the Czech Republic Transmitted from Cats and Dogs. J. Fungi 2021, 7, 684. [Google Scholar] [CrossRef]
- Nojo, H.; Hiruma, J.; Noguchi, H.; Hiruma, M.; Harada, K.; Makimura, K.; Kano, R. In Vitro Azole Susceptibility Testing of Japanese Isolates of Itraconazole-Resistant Dermatophytes. Med. Mycol. J. 2023, 64, 103–105. [Google Scholar] [CrossRef]
- Gupta, A.K.; Susmita; Nguyen, H.C.; Liddy, A.; Economopoulos, V.; Wang, T. Terbinafine Resistance in Trichophyton Rubrum and Trichophyton Indotineae: A Literature Review. Antibiotics 2025, 14, 472. [Google Scholar] [CrossRef]
- Pathadka, S.; Yan, V.K.C.; Neoh, C.F.; Al-Badriyeh, D.; Kong, D.C.M.; Slavin, M.A.; Cowling, B.J.; Hung, I.F.N.; Wong, I.C.K.; Chan, E.W. Global Consumption Trend of Antifungal Agents in Humans from 2008 to 2018: Data from 65 Middle- and High-Income Countries. Drugs 2022, 82, 1193–1205. [Google Scholar] [CrossRef]
- World Health Organization. Electronic Essential Medicines List. Available online: https://list.essentialmeds.org/ (accessed on 16 October 2025).
- Gupta, A.K.; Venkataraman, M.; Renaud, H.J.; Summerbell, R.; Shear, N.H.; Piguet, V. A Paradigm Shift in the Treatment and Management of Onychomycosis. Ski. Appendage Disord. 2021, 7, 351–358. [Google Scholar] [CrossRef]
- Tosti, A.; Elewski, B.E. Onychomycosis: Practical Approaches to Minimize Relapse and Recurrence. Ski. Appendage Disord. 2016, 2, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Cooper, E.A. Safety and Efficacy of a 48-Month Efinaconazole 10% Solution Treatment/Maintenance Regimen: 24-Month Daily Use Followed by 24-Month Intermittent Use. Infect. Dis. Rep. 2025, 17, 7. [Google Scholar] [CrossRef]
- Vlahovic, T.C.; Coronado, D.; Chanda, S.; Merchant, T.; Zane, L.T. Evaluation of the Appearance of Nail Polish Following Daily Treatment of Ex Vivo Human Fingernails With Topical Solutions of Tavaborole or Efinaconazole. J. Drugs Dermatol. 2016, 15, 89–94. [Google Scholar]
- Zeichner, J.A.; Stein Gold, L.; Korotzer, A. Penetration of ((14)C)-Efinaconazole Topical Solution, 10%, Does Not Appear to Be Influenced by Nail Polish. J. Clin. Aesthet. Dermatol. 2014, 7, 34–36. [Google Scholar] [PubMed]
- Canavan, T.N.; Bevans, S.L.; Cantrell, W.C.; Wang, C.; Elewski, B.E. Single-Center, Prospective, Blinded Study Comparing the Efficacy and Compatibility of Efinaconazole 10% Solution in Treating Onychomycosis with and without Concurrent Nail Polish Use. Ski. Appendage Disord. 2018, 5, 9–12. [Google Scholar] [CrossRef] [PubMed]
| Study ID | Randomization (Y/N) | Blinding | Diagnosis | Pathogen | No. Patients | Severity |
|---|---|---|---|---|---|---|
| Children (<18 years) | ||||||
| Eichenfield 2020 [11] | N | N/A | DLSO | Dermatophyte | 62 | ≥20% toenail area affected No matrix (lunula) involvement or dermatophytomas |
| Elderly (≥65 years) | ||||||
| Lipner 2025 [12] Gupta 2014 [13] | Y | Double | DLSO | Dermatophyte * | 218 | 36–37% toenail area affected No matrix (lunula) involvement or dermatophytomas |
| Iozumi 2019 [14] | N | N/A | DLSO SWO | T. rubrum TMTISC Trichophyton spp. | 118 | ≥20% toenail area affected No proximal nail fold involvement |
| Diabetic Patients | ||||||
| Vlahovic 2014 [15] | Y | Double | DLSO | Dermatophyte * | 112 | 36.4% toenail area affected No matrix (lunula) involvement or dermatophytomas |
| Shofler 2020 [16] | N | N/A | NR | T. rubrum TMTISC | 40 | ≥20% toenail area affected |
| Hispanic/Latino Patients | ||||||
| Cook-Bolden 2017 [17] | Y | Double | DLSO | Dermatophyte * | 270 | 20–50% toenail area affected No matrix (lunula) involvement or dermatophytomas |
| Tschen 2013 [18] | Y | Double | DLSO | Dermatophyte Candida | 135 | 40% toenail area affected No matrix (lunula) involvement or dermatophytomas |
| Tinea Pedis Patients | ||||||
| Markinson 2015 [19] | Y | Double | DLSO | Dermatophyte * | 352 | 20–50% toenail area affected No matrix (lunula) involvement or dermatophytomas |
| Dermatophytes (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC90 (µg/mL) | ≤0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ≥32 |
| Amorolfine (N = 668) | 38.8 | 0.0 | 0.0 | 4.5 | 4.5 | 6.6 | 41.3 | 4.3 | 0.0 | 0.0 | 0.0 |
| Ciclopirox (N = 717) | 0.0 | 0.0 | 1.4 | 42.1 | 32.8 | 21.5 | 2.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Efinaconazole (N = 2912) | 78.4 | 12.2 | 6.7 | 0.0 | 1.5 | 0.1 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Fluconazole (N = 442) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 9.7 | 4.1 | 0.0 | 66.5 | 19.7 |
| Griseofulvin (N = 577) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 47.1 | 45.1 | 7.8 | 0.0 | 0.0 | 0.0 |
| Itraconazole (N = 1051) | 1.5 | 18.6 | 19.8 | 8.6 | 12.0 | 35.6 | 2.9 | 0.0 | 0.1 | 0.2 | 0.8 |
| Ketoconazole (N = 208) | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Luliconazole (N = 974) | 99.9 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ravuconazole (N = 264) | 14.4 | 49.6 | 32.6 | 0.8 | 1.5 | 0.0 | 0.0 | 0.8 | 0.0 | 0.4 | 0.0 |
| Tavaborole (N = 512) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 39.8 | 10.5 | 49.6 | 0.0 | 0.0 |
| Terbinafine Susceptible Dermatophytes (MIC90: ≤0.03 µg/mL; %) | |||||||||||
| MIC90 (µg/mL) | ≤0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ≥32 |
| Amorolfine (N = 270) | 95.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 3.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ciclopirox (N = 270) | 0.0 | 0.0 | 3.7 | 95.9 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Efinaconazole (N = 417) | 93.0 | 2.6 | 4.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Fluconazole (N = 137) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 48.2 | 51.8 |
| Itraconazole (N = 417) | 0.2 | 31.2 | 48.2 | 16.1 | 2.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.7 |
| Luliconazole (N = 158) | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Terbinafine Non-Susceptible Dermatophytes (MIC90: ≥0.25 µg/mL; %) | |||||||||||
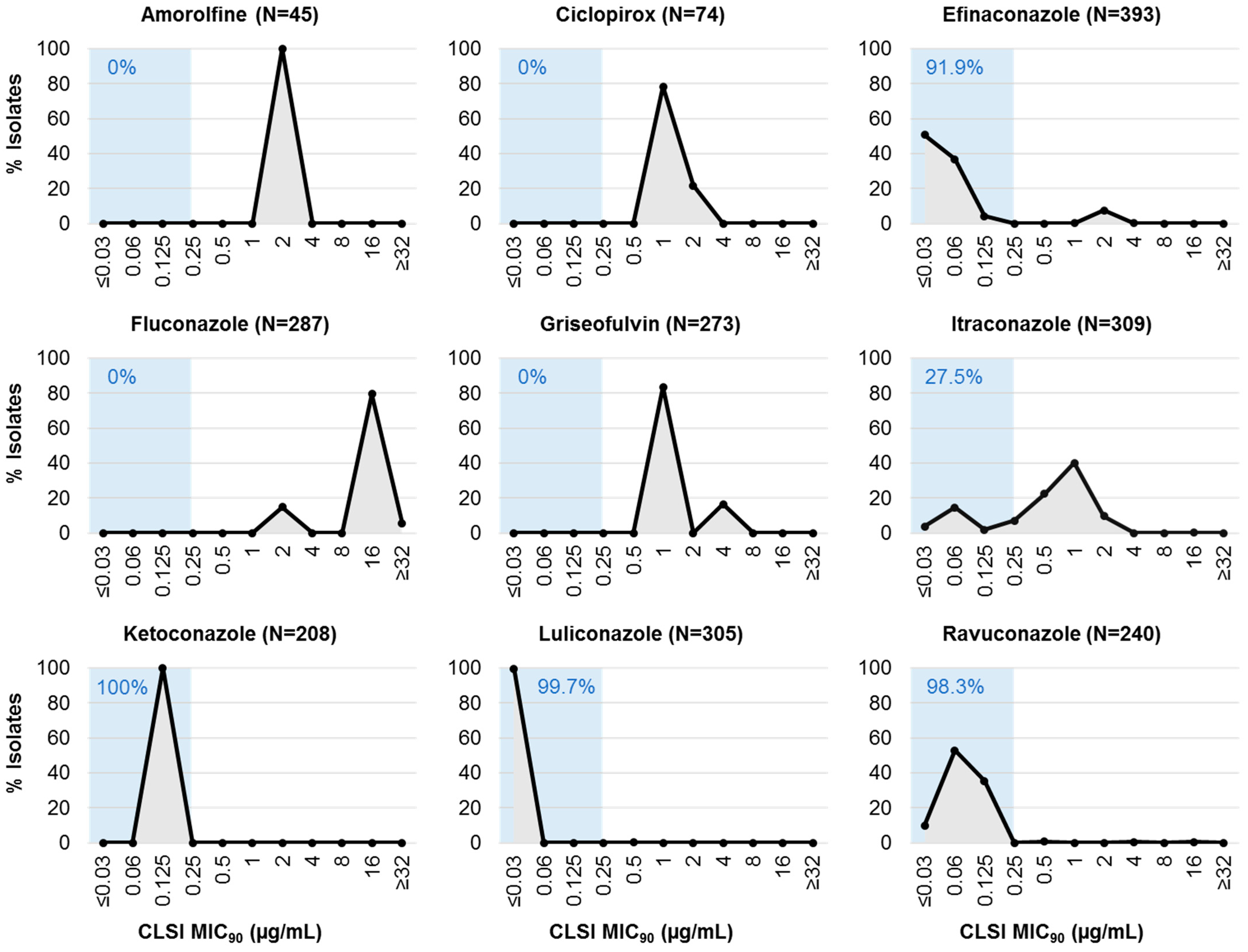
| MIC90 (µg/mL) | ≤0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ≥32 |
| Amorolfine (N = 45) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ciclopirox (N = 74) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 78.4 | 21.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| Efinaconazole (N = 393) | 50.6 | 36.9 | 4.3 | 0.0 | 0.0 | 0.3 | 7.6 | 0.3 | 0.0 | 0.0 | 0.0 |
| Fluconazole (N = 287) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 15.0 | 0.0 | 0.0 | 79.4 | 5.6 |
| Griseofulvin (N = 273) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 83.5 | 0.0 | 16.5 | 0.0 | 0.0 | 0.0 |
| Itraconazole (N = 309) | 3.9 | 14.6 | 1.9 | 7.1 | 22.3 | 40.1 | 9.7 | 0.0 | 0.0 | 0.3 | 0.0 |
| Ketoconazole (N = 208) | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Luliconazole (N = 305) | 99.7 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ravuconazole (N = 240) | 10.0 | 52.9 | 35.4 | 0.0 | 0.8 | 0.0 | 0.0 | 0.4 | 0.0 | 0.4 | 0.0 |
| Parameter | Considerations for Special Populations | Recommendations |
|---|---|---|
| Diagnosis | Vulnerable populations often are predisposed to nail thickening and discoloration secondary to co-morbidities, trauma and inflammation, which may mimic onychomycosis. |
|
| Oral vs. Topical? | Vulnerable populations may have comorbidity risks for oral drug use, and those using polypharmacy may be at higher risk of oral drug interactions. |
|
| When is it cured? | Vulnerable populations may be at high risk for reinfection where immunocompromised; nail dystrophy may not resolve, preventing visual confirmation of infection clearance |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.K.; Taylor, D.; Dennis, D.; Wang, T.; Cooper, E.A. Treating Onychomycosis with Efinaconazole: Considerations for Diverse Patient Groups. J. Fungi 2025, 11, 843. https://doi.org/10.3390/jof11120843
Gupta AK, Taylor D, Dennis D, Wang T, Cooper EA. Treating Onychomycosis with Efinaconazole: Considerations for Diverse Patient Groups. Journal of Fungi. 2025; 11(12):843. https://doi.org/10.3390/jof11120843
Chicago/Turabian StyleGupta, Aditya K., Daniel Taylor, Daniel Dennis, Tong Wang, and Elizabeth A. Cooper. 2025. "Treating Onychomycosis with Efinaconazole: Considerations for Diverse Patient Groups" Journal of Fungi 11, no. 12: 843. https://doi.org/10.3390/jof11120843
APA StyleGupta, A. K., Taylor, D., Dennis, D., Wang, T., & Cooper, E. A. (2025). Treating Onychomycosis with Efinaconazole: Considerations for Diverse Patient Groups. Journal of Fungi, 11(12), 843. https://doi.org/10.3390/jof11120843







