1. Introduction
Mitogen-activated protein kinase (MAPK) pathways serve as multifaceted regulators of fungal biology, coordinating processes ranging from development and morphogenesis to stress adaptation, pathogenesis, and ecological fitness [
1,
2]. The three conserved MAPK pathways collaboratively orchestrate various aspects of fungal biology, while each also serves as a predominant regulator in response to distinct environmental cues and ecological niches. The Fus3/Kss1 branch governs developmental programs such as mating, filamentation, and appressorium formation, which are essential for host invasion [
3], while the Hog1 and Slt2 cascades mediate osmotic, oxidative, and cell-wall stress conditions by activating glycerol accumulation, antioxidant defenses, and cell-wall remodeling [
4]. In pathogenic fungi, the Pmk1/Fus3-like MAPK is indispensable for virulence, regulating cuticle penetration structures, toxin secretion, and sporulation during host colonization [
5,
6]. Crosstalk among MAPK branches further fine-tunes responses, ensuring coordinated deployment of protective circuits under multifactorial stress conditions [
7,
8]. The complex and interconnected regulation network mediated by MAPK cascades contributes greatly to fungal growth and survival in nature.
Entomopathogenic fungi are indispensable biological control agents in sustainable agriculture due to their specificity, environmental safety, and persistent insecticidal activity against diverse pest species [
9,
10]. Mitogen-activated protein kinase (MAPK) cascades are essential for enabling entomopathogenic fungi to infect host insects and endure adverse environmental conditions by maneuvering cellular responses against osmotic, oxidative, cell-wall, and thermal stress conditions [
11,
12,
13]. In
Beauveria bassiana, the Hog1 MAPK is critical for glycerol accumulation and antioxidant defenses under hyperosmotic and oxidative challenges, with Δ
Bbhog1 mutants exhibiting severe stress sensitivity and attenuated virulence in insect bioassays [
14]. The Slt2 pathway governs cell-wall integrity and heat tolerance by regulating chitin synthases, membrane/cell-wall proteins, and membrane-repair genes, while its disruption leads to compromised conidiation and thermal sensitivity [
13,
15]. Moreover, Fus3/Kss1-like MAPKs orchestrate developmental switches—such as appressorium formation and invasive growth—which are essential for cuticle penetration in both plant and insect pathogens [
5,
12]. Although transcriptome and proteome studies have highlighted MAPK-responsive genes in entomopathogens and model fungi [
12,
16], a significant gap remains in the systematic, time-resolved transcriptomic analyses needed to map the dynamic, MAPK-mediated regulatory networks under multifactorial stress conditions.
Here, the sensitivity of three MAPK mutants under abiotic stress conditions was evaluated with detailed phenotypic profiling, and the regulatory role of those MAPKs in stress response was investigated with time-course RNA-seq (0, 0.5, 12 h) and weighted gene co-expression network analysis (WGCNA) to determine the stress sensitivity of each singular MAPK mutant. This approach uncovers a series of gene modules linked to core cellular processes and reveals both pathway-specific and shared regulons. The mobilization of suites of metabolic pathways by MAPK cascades responding to sensitive stress conditions was observed and verified with sets of fluorescent observations, enzymatic activity tests and qRT-PCR. By elucidating the temporal and network-level architecture of Hog1, Slt2, and Mpk1 signaling under sensitive stress conditions, our study provides a dynamic blueprint for fungal adaptation and virulence. This knowledge not only advances basic understanding of MAPK function in entomopathogenic fungi but also informs the rational design of hyper-resilient biocontrol strains.
2. Materials and Methods
2.1. Fungal Strains and Culture Condition
Wild-type
B. bassiana strain Bb0062 (denoted as WT) isolated from cadavers of
Plutella xylostella larvae was identified and deposited in the China General Microbiological Culture Collection Center (CGMCC, accession number CGMCC 7.34), and MAP-kinase derivative mutant strains Δ
BbHog1, Δ
BbMpk1, and Δ
BbSlt2 were constructed previously [
5,
14,
15]. Those fungal strains were grown on Czapek-dox agar/broth (CZA/B) (Difco Laboratories, Detroit, MI, USA), potato dextrose agar/broth (PDA/PDB) (Difco Laboratories, Detroit, MI, USA), Sabouraud dextrose agar or broth (SDA/SDB) (Difco Laboratories, Detroit, MI, USA), or SDA/SDB (Difco Laboratories, Detroit, MI, USA), supplemented with 1% (
wt/
vol) yeast extract (SDAY/SDBY) as indicated. Unless otherwise indicated, for use as inoculation, conidia were collected from SDAY plates after incubation for 14 days at 26 °C (15 h light: 9 h dark cycle) and subjected to downstream experiments.
2.2. Phenotypic Assays
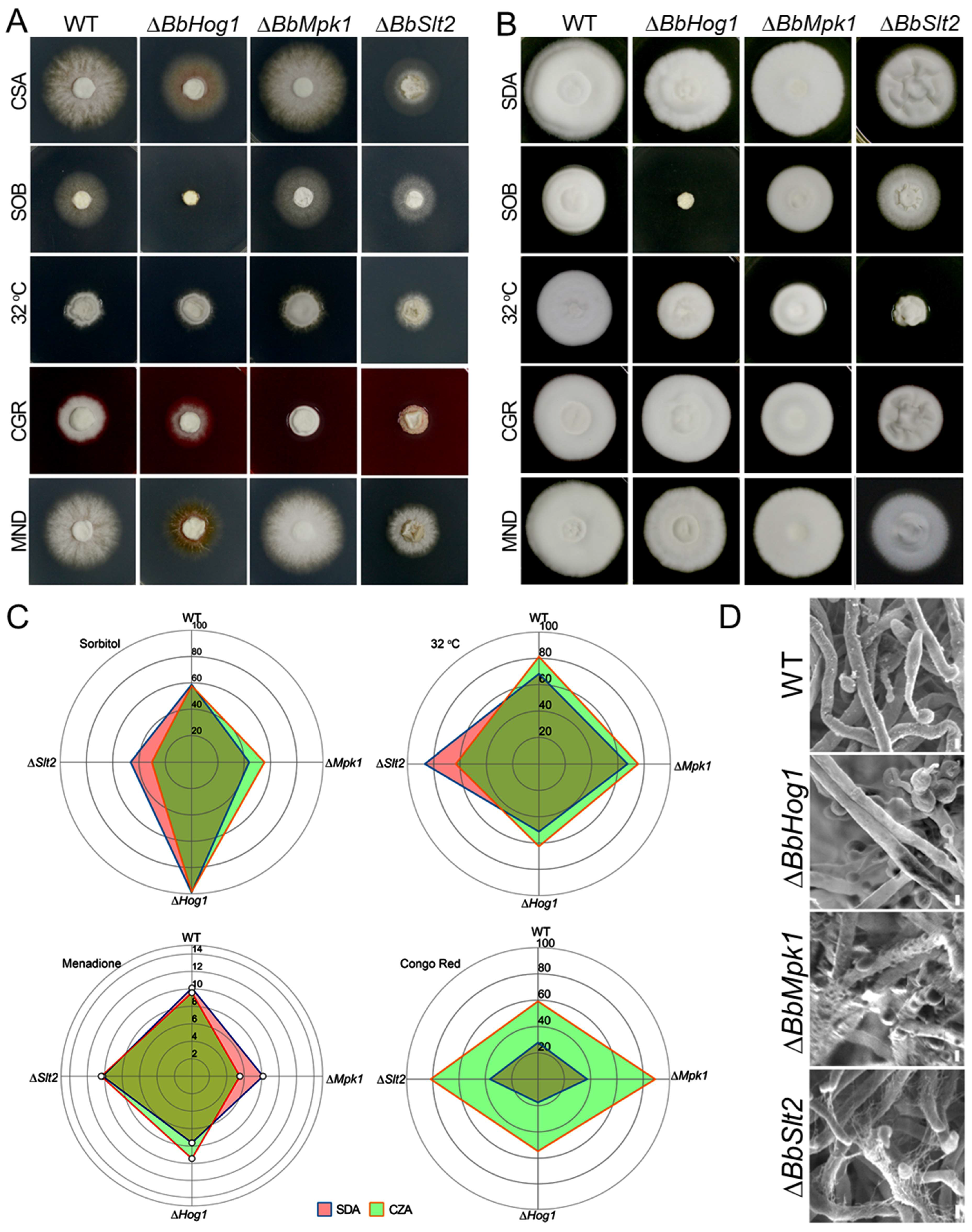
Colony growth and stress response tests were performed as previously detailed [
17]. Briefly, conidia were collected from SDA plates after 10 d of cultivation, the concentration was adjusted to 10
7 conidia/mL after counting using a hemocytometer, and 100 μL aliquots were uniformly spread on SDAY plates covered with cellophane sheets (90 mm in diameter). Hyphal plugs (diameter = 5 mm) were taken from the SDAY plates after 3 d incubation (26 °C) and placed in the middle of fresh test plates. Test plates included SDA and CZA amended with 1.0 M sorbitol, 16 μM of menadione (MND), or 25 μg/mL Congo red (CR). In indicated cases, a supplement of ergosterol (C
28H
44O, 2.5 mM) was added during MAP-kinase stress response tests to assess their ability to rescue observed phenotypes, particularly in the ∆
BbSlt2 mutant. Unless otherwise indicated, plates were incubated at 26 °C with a 15:9 h light: dark cycle. Colony diameters were measured daily. Relative growth inhibition (RGI) was calculated using the following formula: RGI = (Dc − Ds)/(Ds − d) × 100, where Dc and Ds are the mean diameters of at least 3 fungal colonies in the control and stress treatments, respectively, and d denotes the diameter consistence of the primary plug (5 mm) [
18]. All the RGI data was visualized with a radar plot generated by the ggplot2 R package. All the experiments were performed in triplicate and repeated three times with independent batches of conidia. Aerial hyphae of WT and three MAPK mutants grown on SDAY plates for 3 days were collected and subjected for scanning electron microscopic (SEM) (Hitachi, Tokyo, Japan) observation.
2.3. Transcriptomic Analyses and qRT-PCR Validation
Samples for RNA-seq analyses were prepared as previously described [
18]. Briefly, wild-type
B. bassiana and MAP-kinase mutants were inoculated into SDB broth at an initial conidial concentration of 10
5 conidia/ml. Fungal (hyphal) cultures were collected after 3 days of growth and washed in SDB media containing the indicated stress agent, including (i) 1.0 M sorbitol, (ii) 16 μM of MND, 25 μg/mL of CR, and (iv) SDB incubated at 32 °C. Two time points of induction in the presence of the stress agent—namely, 30 min and 12 h—were adopted for each test condition. Fungal cells were collected by centrifugation and washed with 20 mM PBS (phosphate-buffered saline, pH 7.3), and total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), following the manufacturer’s instructions. Cultures of wild-type and MAPK mutants of
B. bassiana (strain 0062) were grown for each tested condition with three replicates and mixed evenly for RNA extraction. RNA purity and quality were examined via agarose gel electrophoresis and photometric analysis (Pearl nanophotometer, Implen, Munich, Germany). RNA-Seq libraries were generated using a Truseq
TM RNA sample prep Kit (Illumina, San Diego, CA, USA) following the manufacturer’s instructions. Constructed cDNA libraries were subjected to RNA sequencing using an Illumina HiSeq™ 2000 (Majorbio, Shanghai, China) with paired-end sequencing mode for the generation of transcriptomic data bearing an average sequence length of 150 bp. A widely used transcriptomic analysis pipeline [
19] was implemented for the processing of gene expression data through the following steps: RNA-Seq data were initially subjected to FastQC (v 0.11) for sequencing quality control, followed by Trimmomatic (v 0.32) for sequence adaptor trimming and removal of unpaired sequences. Then, Hisat2 (2.2.1) was used for mapping of transcripts to the genome of
Beauveria bassiana strain ARSF 2860. Samtools (v 0.1.18) and Subread (v 2.0.0) were used for transcript assembly and transcript abundance quantification. All analyses were performed on a Tecent cloud server with a Linux system running Ubuntu 18.04.4. The raw RNA-seq data were deposited in the NCBI Gene Expression Ominibus with series accession number PRJNA865046.
A small amount of mycelium (WT/Δ
BbHog1/Δ
BbMpk1) was inoculated into 100 mL of SDAY medium and incubated on a shaker for 4 days. The mycelium was then filtered out, transferred to the corresponding induction medium, and re-filtered to obtain the experimental mycelium. RNA was extracted from the induced mycelium using Biosharp Total RNA Isolation Reagent and an Aidlab FASTeasy Universal Plant & Fungi RNA Kit (according to the manufacturers’ manuals). cDNA was synthesized via reverse transcription (following the Thermo Scientific REvertAid First Strand cDNA Synthesis Kit protocol, Waltham, MA, USA). qPCR was performed using Servicebio
TM 2 × SYBR Green qPCR Master Mix (Wuhan, China) according to the provided instructions. The amplification program consisted of 95 °C for 2 min, followed by 39 cycles of 95 °C for 5 s, 60 °C for 30 s, and a melting curve from 65 °C to 95 °C with a temperature increase of 0.5 °C per 5 s. Actin (GenBank ID: HQ232398) was used as the internal reference gene. A list of the primers used in the study is presented in
Supporting Information Table S1.
2.4. Analyses of the Differentially Expressed Genes (DEGs) in MAPK Mutants
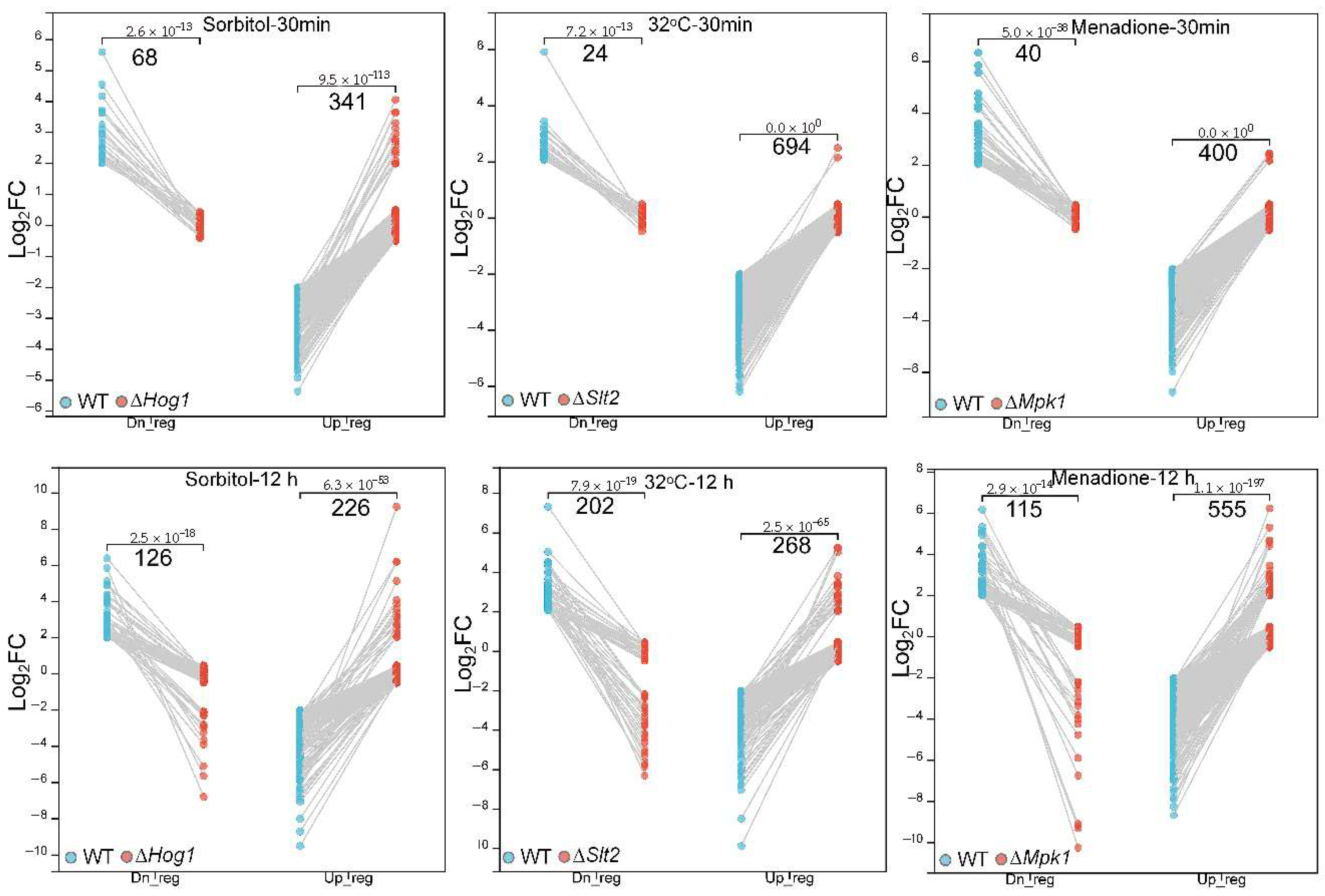
Transcript counts were first filtered by removing genes with 0 counts in the data for the wild-type and mutant strains, then transformed into FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values with R package DESeq2 (v 1.36.0). A value of 1 was added to all FPKM values (FPKM + 1); then, the values were transformed with a log2-based algorithm and quantile normalization to correct data bias and generated DEGs in stress (st) as compared to the normal condition with the following formula: Log2((FPKMst + 1)/(FPKMnor + 1)). A filtration of the expression ratio was performed such that genes with |Log2FC| ≥ 2 were screened as DEGs and those with|Log2FC| ≤ 0.5 were categorized as silent genes. The differential expression genes in MAP-kinase mutants responding to abiotic stress were evaluated according to a curated algorithm to identify upregulated DEGs in MAPK mutants responding to the given stress, i.e., DEGsUp = Log2FC(WTst/WTnor) ≤ −2& Log2FC(ΔMAPKst/ΔMAPKnor) ≥ −0.5), and downregulated DEGs, i.e., DEGsDn = Log2FC(WTst/WTnor) ≥ 2& Log2FC (ΔMAPKst/ΔMAPKnor) ≤ 0.5.
2.5. Global Gene Co-Expression Analysis
A pair of R-based tools—Coseq (1.20.0) [
20] and WGCNA [
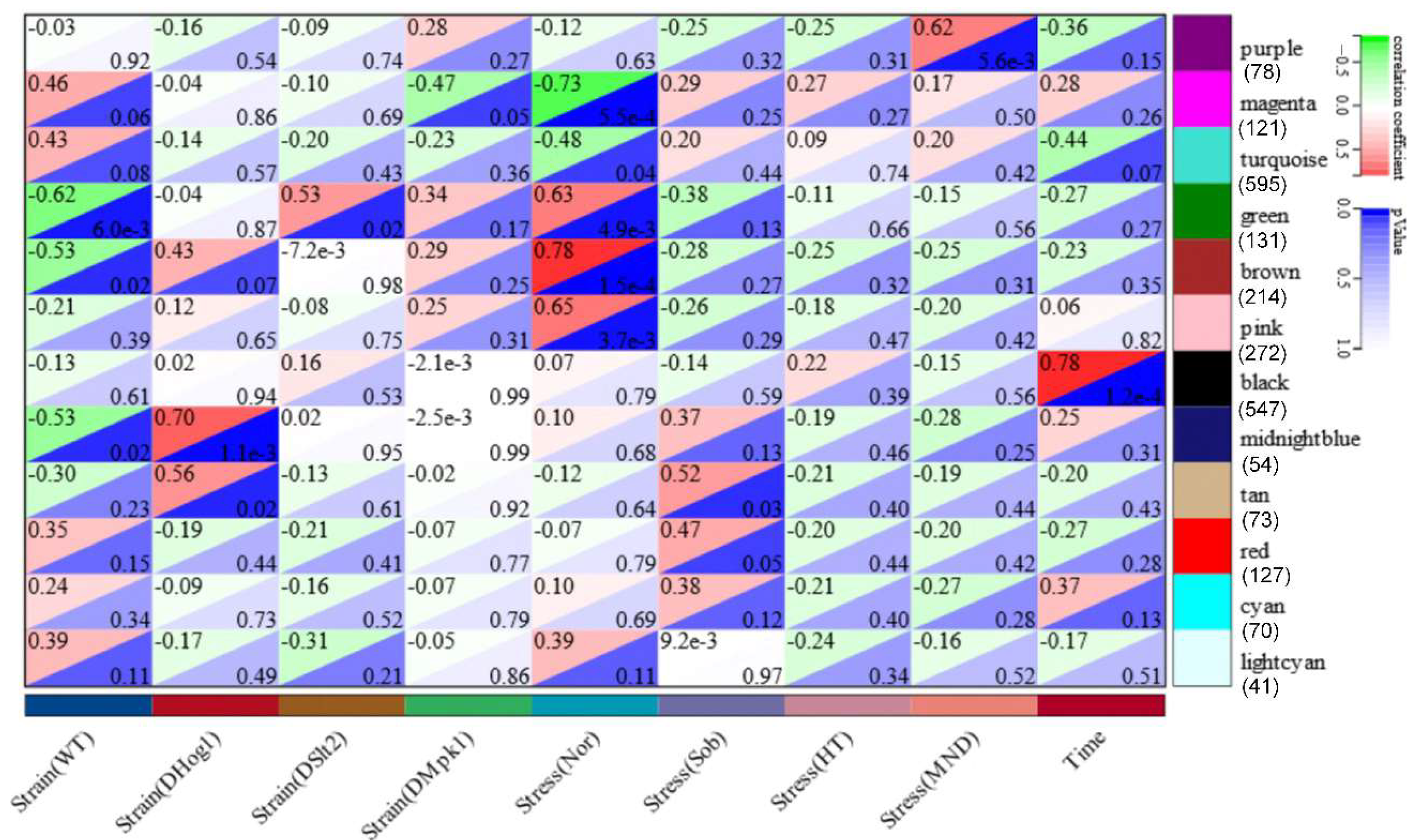
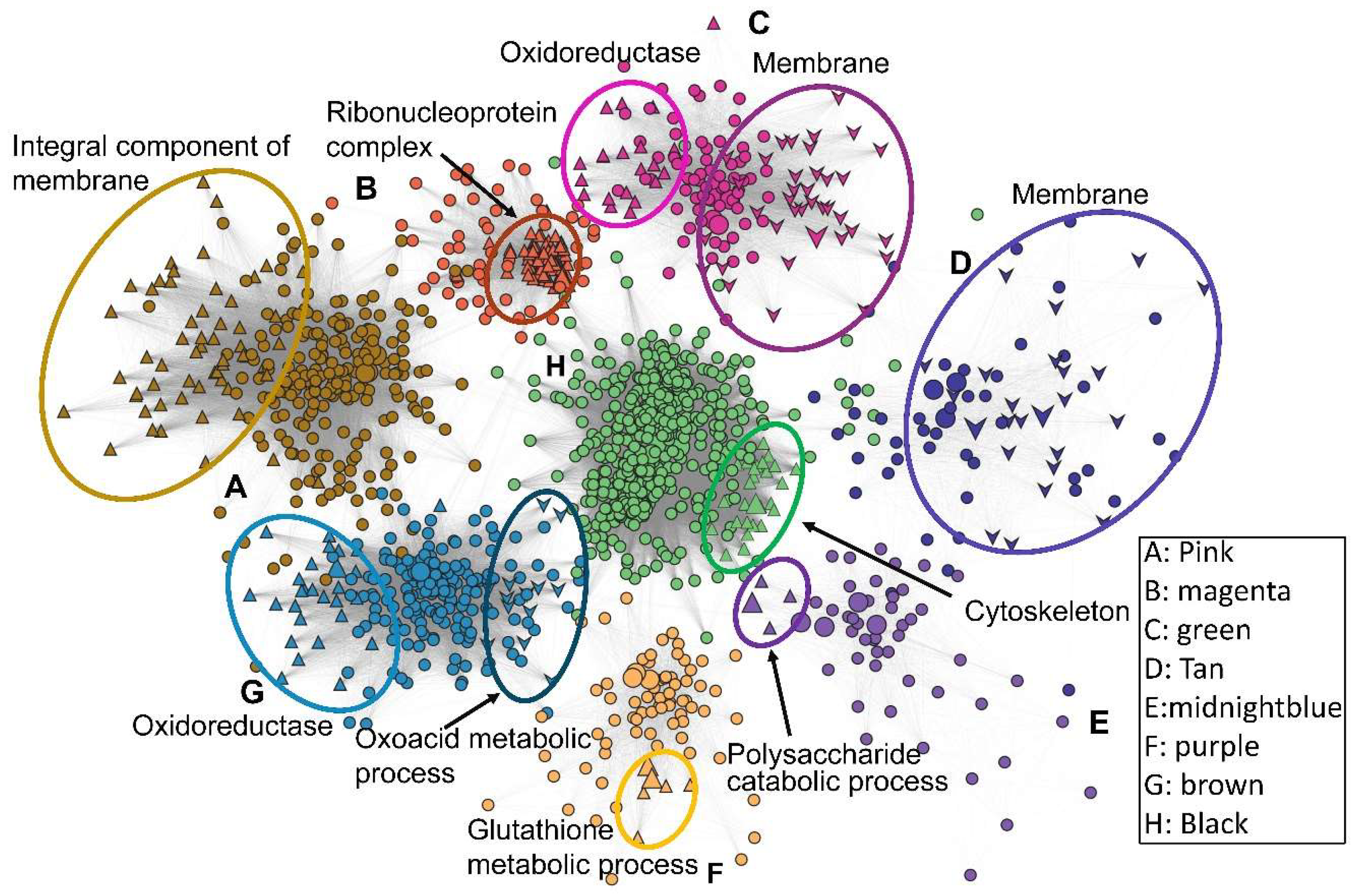
21]—and a hierarchical clustering method (Pearson) were employed for gene co-expression analysis. Briefly, extracted transcript counts from all constructed transcriptomes were subjected to Coseq in R for normalization, with Log-Centered Log Ratio (LogCLR) transformation as an expression index indicating transcript abundance in RNA-seq samples. A co-expression compendium was constructed with the k-means clustering algorithm after TMM normalization of LogCLR-transformed reads. Co-expressed genes across the 18 sets of transcriptomic data were clustered and formed 80 modules. For generation of the co-expression networks, the WGCNA protocol was employed. In brief, FPKM values of all 18 transcriptomes were subjected to the online WGCNA package in R. After sample-type classification, hierarchical clustering was conducted to classify genes with similar expression profiles that generated 12 modules after optimization of the correlation threshold of gene–gene co-expression. All of the 12 modules with co-expression degree and gene information were exported and subjected to Cytoscape (v 35.0) and Gephi (v 0.10.1) for generation of co-expression networks with deliberate decorative editing of modular gene functions.
2.6. Fluorescent Observation and Glycolysis Stress Assay
For the mitochondrial membrane potential test, a small amount of mycelium (WT/ΔBbHog1/ΔBbMpk1/ΔBbSlt2) was inoculated into 100 mL of SDY medium and incubated on a shaker for 4 days. The mycelium was filtered through a wire mesh, and the conidial suspension was collected. The suspension was concentrated by centrifugation at 4 °C and 4000 rpm using a refrigerated centrifuge, and the supernatant was discarded. The WT and ΔMpk1 mycelium were washed with 50 mL of 16 μM menadione in SDY medium and induced for 30 min. After washing the culture three times, the mycelium was resuspended to prepare a spore suspension. Staining was performed using a Mitochondrial Membrane Potential Assay Kit with JC-1 (Solarbio, Beijing, China) and following the corresponding instructions. Stained cells were visualized under an excitation wavelength of 515 nm and emission wavelength of 529 nm for mitochondrial monomers, and aggregates were observed with an excitation wavelength of 585 nm and emission wavelength of 590 nm with a confocal microscope (Leica, Wetzlar, Germany).
Fungal cell-wall staining was performed with Fluorescein 5-isothiocyanate (FITC)-labeled wheat germ agglutinin (WGA) and Concanavalin A (ConA) (Maokangbio, Shanghai, China). Cell suspensions were prepared same as described above. The collected blastospore suspension was washed by centrifugation and fixed with 4% paraformaldehyde at room temperature for 1 h, followed by two washes with PBS. A 50 µL aliquot of the resuspended sample was mixed with 100 µL of 20 µg/mL FITC-ConA/FITC-WGA and incubated at room temperature for 1 h, then washed and resuspended in PBS. FITC-ConA/FITC-WGA-stained cells were observed with a confocal microscope (Leica, Wetzlar, Germany) (excitation:494 nm; emission: 520 nm).
For glycolysis stress fluorometric assays, cell suspensions were prepared as described above. The collected blastospore suspension was washed by centrifugation and resuspended in PBS. The cell concentration was calculated and balanced to 106 cells/mL. The assays were performed following the protocol of the Glycolysis Stress Fluorometric Assay Kit (Solarbio, Beijing, China). Briefly, measurement wells, 2-DG control wells, and blank wells were set up ahead of time. A volume of 100 µL of the cell suspension was added to each corresponding well of a black, clear-bottom 96-well microplate. The plate was incubated in a non-CO2 environment at 25 °C in the dark for 30 min. The fluorescence microplate reader was set to 25 °C. Then, 100 µL of working assay solution was added to the measurement and blank wells. For the 2-DG control wells, 10 µL of reagent 5 and 90 µL of glucose-free working solution were added. Fluorescence was measured at an excitation wavelength of 490 nm and emission wavelength of 535 nm with a Fluorometric Microplate-Reader (Tacan, Männedorf, Switzerland), and readings were taken every 4 min over 100 min. The extracellular acidification rate (ECAR) was calculated accordingly.
2.7. Data Analysis and Visualization
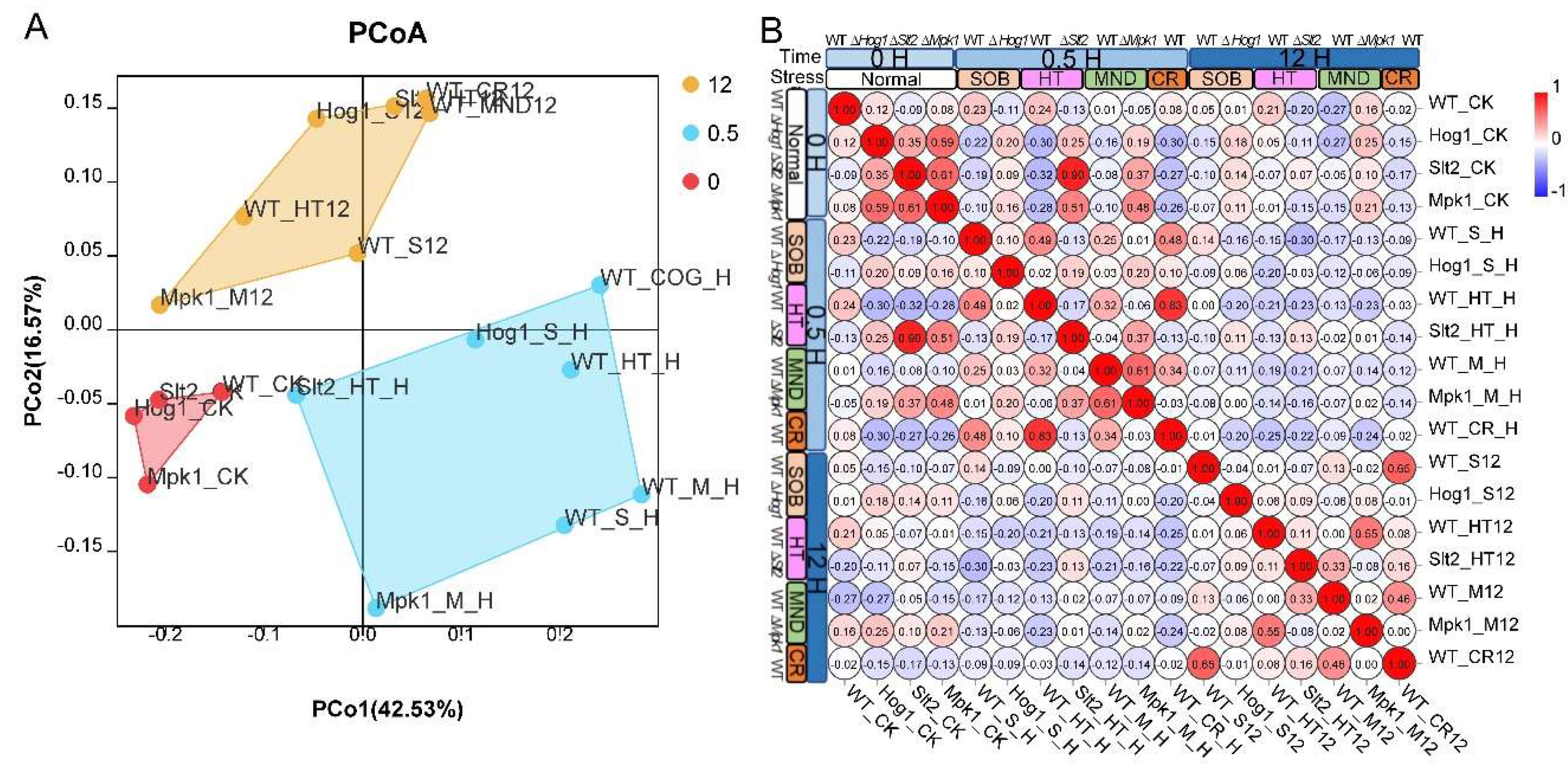
For transcriptomic data similarity analysis, transcript reads from all 18 samples were collected and subjected to R package “ape” (v 5.6) for generation of a PCoA plot, and the hierarchical clustering visualization tool in the Omicshare platform was used for RNA-seq sample data clustering. DEG overlap datasets comparing stress conditions were analyzed by collecting DEG groups under 6 stress conditions and subjected to the nVenn tool for generation of a Venn plot [
22]. Gene ontology and KEGG analyses were used for functional classification of given subsets of DEGs or co-expressed gene modules, with genes subjected to GO/KEGG classification tools in the Omicshare platform (
www.omicshare.com) with Uniprot-annotated GO terms and KEGG BlastKOALA-mapped terms of the
B. bassiana genome as references. Functional enriched pathways were visualized with a dot plot or heat map (R package ggplot2). Enriched pathways were also regenerated and visualized manually based on KEGG pathway collections. For other gene expression visualization plots, a either violin plot (R package ggplot2) or bar plot in Sangerbox 3.0 was employed. For all experimental data, at least three replicates were analyzed, and the mean value, standard deviation. (
t-test) were collected for data visualization.
4. Discussion
MAPK cascades convergently evolved as part of the fungus kingdom and are essential for fungal development, stress response, and host–microbe interactions, and the roles of three MAP kinases in stress responses were unveiled in insect pathogenic fungi, with specialized roles in pathogenesis and stress resistance [
5,
12,
14,
15,
27]. However, the regulation networks of MAP kinases responding to specialized stress conditions and the crosstalk between MAP kinases had not been fully investigated in entomopathogenic fungi. Our study revealed the regulatory network mediated by Hog1, Slt2, and Mpk1 MAP kinases as highly specialized stress response commanders in
Beauveria bassiana: Hog1 is indispensable for osmotic adaptation, Slt2 for thermal and cell-wall integrity, and Mpk1 for oxidative stress resilience. Time-course RNA-seq and WGCNA identified 12 co-expression modules (547 genes), highlighting core processes—cell-cycle, glycolysis, cytoskeletal dynamics, and MAPK signaling pathways—regulated by a singular MAP kinase under specialized stress (acute/sustained) treatment. Comparative transcriptomic analysis uncovered the fact the main metabolic and signal pathways mobilized by MAP kinases echo specialized stress environments. These insights deepen our theoretical understanding of fungal stress–response networks and pinpoint MAP kinase-regulated nodes for the engineering of superior biocontrol strains and the dissection of pathogen–host interactions.
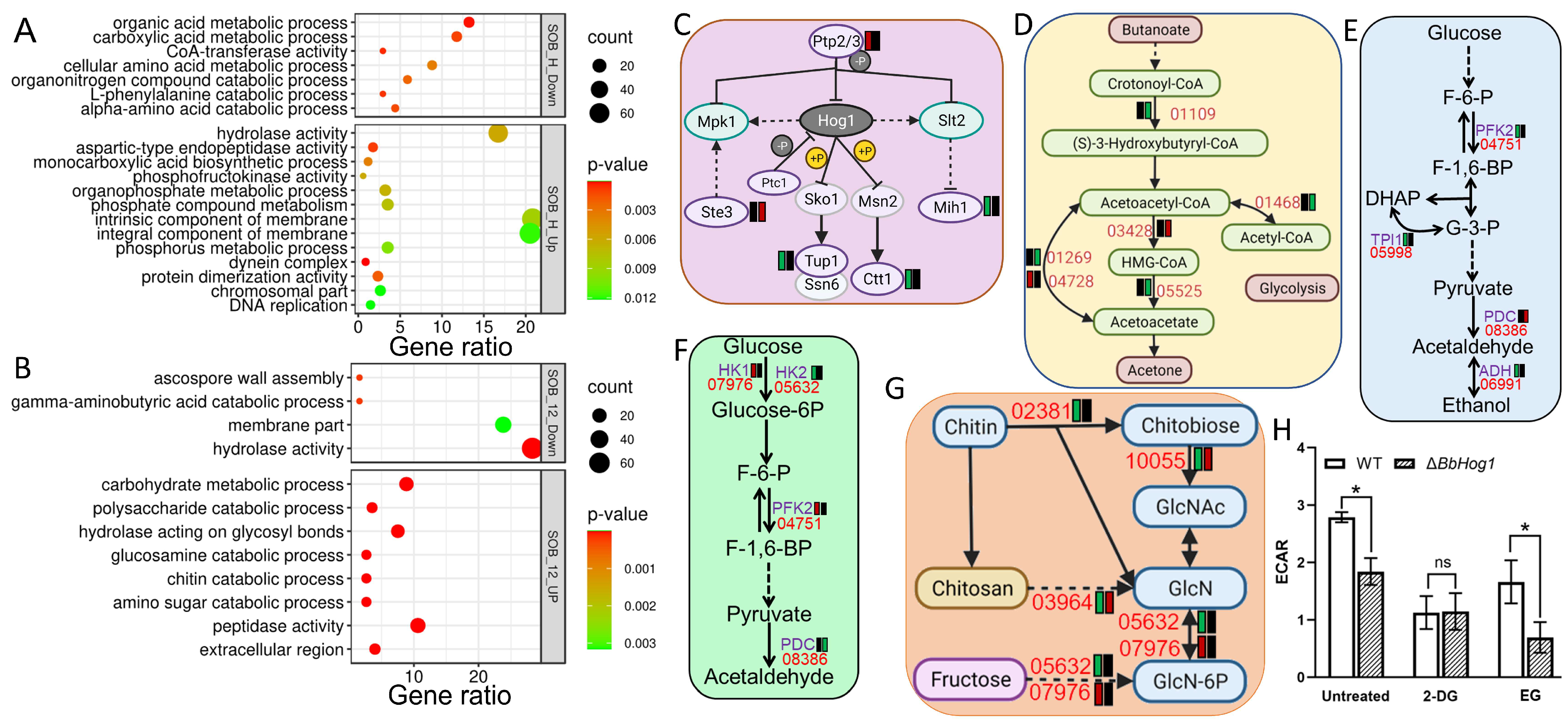
Consistent with previous reports in model fungi and entomopathogens, the Hog1 cascade in
B. bassiana was found to be indispensable for osmotic stress tolerance [
14,
27]. Our analysis showed that the deletion of Hog1 in
B. bassiana greatly altered cell morphology in cell-surface and cell-wall carbohydrates and attenuated fungal growth under osmotic stress, indicating an essential role of the Hog1 cascade in fungal development and osmotic stress tolerance. The Δ
Bbhog1 mutant exhibits markedly reduced levels of erythritol and arabinitol under osmotic stress, which are crucial for maintaining cellular osmotic balance (14). Our data also indicated that the ablation of BbHog1 downregulated three key genes in glycolysis, and the glycolysis stress assay showed decreased glycolysis activity of Δ
Bbhog1 in response to acute osmotic stress. Therefore, the defected glycolysis metabolism in Δ
Bbhog1 may negatively disrupt the biosynthesis of erythritol and arabinitol in the mutant. ConA and WGA antigens labeled carbohydrates on the cell wall and the expression of cell wall-associated processes, including cell-wall integrity, polysaccharide metabolism, and chitosan biosynthesis, were also decreased in ∆
BbHog1 as compared to the WT strain under osmotic stress, indicating a potential domino effect of MAP kinase Hog1 on the Slt2 cascade in regulating cell-wall integrity. Suites of lipid metabolic pathways, including glycerophospholipid metabolism, lipid metabolism, and ketone-body metabolism, were also disrupted with the loss of Hog1, indicating that an alternative lipid-mediated cell turgor generation pathway may exist to facilitate fungal resistance to osmotic stress [
16]. The core MAP-kinase signal pathway was also disrupted, with two downstream regulators (Tup1/Ctt1) of Hog1 downregulated and the expression of three other components of the MAP-kinase pathway abnormally elevated in Δ
Bbhog1 in response to acute osmotic stress. Comparative transcriptomic analysis revealed that the metabolic processes of suites of Hog1 regulating new metabolic pathways, including amino acids (tryptophan and valine/leucine/isoleucine), downregulated, while members of cell skeleton dynamics and suites of CYP450s were abnormally upregulated in ∆
BbHog1 under acute/sustained osmotic stress treatment. These results indicate that the Hog1 cascade mobilized diverse pathways and generated complex regulatory networks, enabling the fungus to maintain homeostasis under osmotic stress conditions.
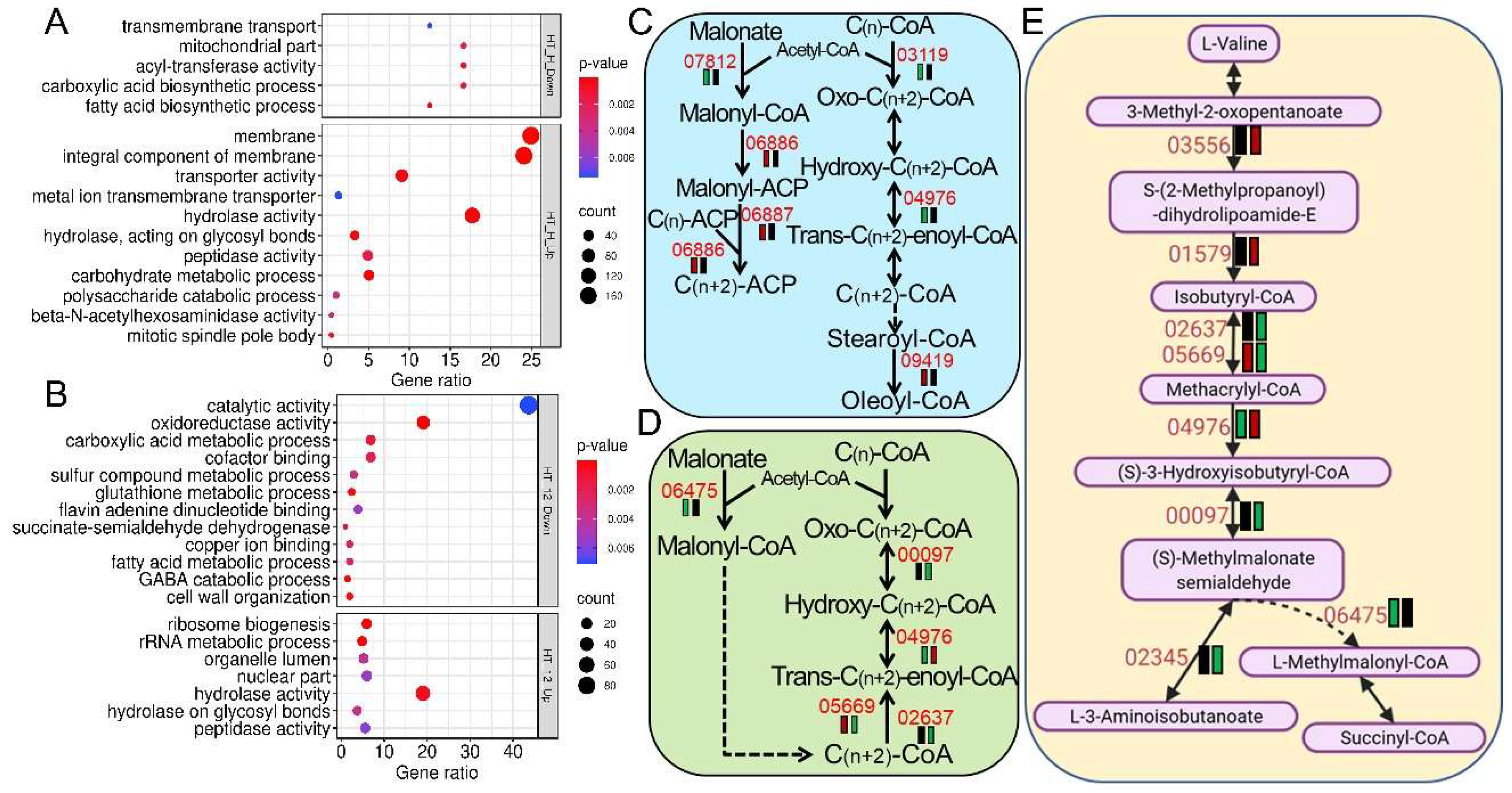
The Slt2 cascade was found to be crucial for the regulation of cell-wall integrity (CWI) maintenance and repair in response to cell wall-perturbing stress agents (Congo red and calcofluor white) and thermal stress in both yeast and filamentous fungi [
8,
28,
29]. Deletion of Slt2 in
B. bassiana yielded a weakened cell wall, decreased content or activity of cell wall-associated glycoproteins and enzymes, and deteriorated fungal growth under cell wall-perturbing stress [
15,
18,
30]. The abnormal filiform textured hyphae of ∆
BbSlt2 and decreased ConA and WGA antigens labeled types of carbohydrates under heat stress also indicated an essential role of BbSlt2 in fungal cell-wall integrity. ∆
BbSlt2 showed severe colony collapse compared to the WT strain and other two MAPK mutants on SDA plates at 32 °C. The deletion of Slt2 also paralyzed fungal response to acute heat stress while greatly changing the global expression profile under sustained heat stress as compared to the WT parent, highlighting a master role of Slt2 in heat resistance other than cell-wall stress. Under acute thermal stress, ∆
BbSlt2 yielded 718 DEGs, with a striking 98% of those being heat-responsive in the WT strain but transcriptionally silent in ∆
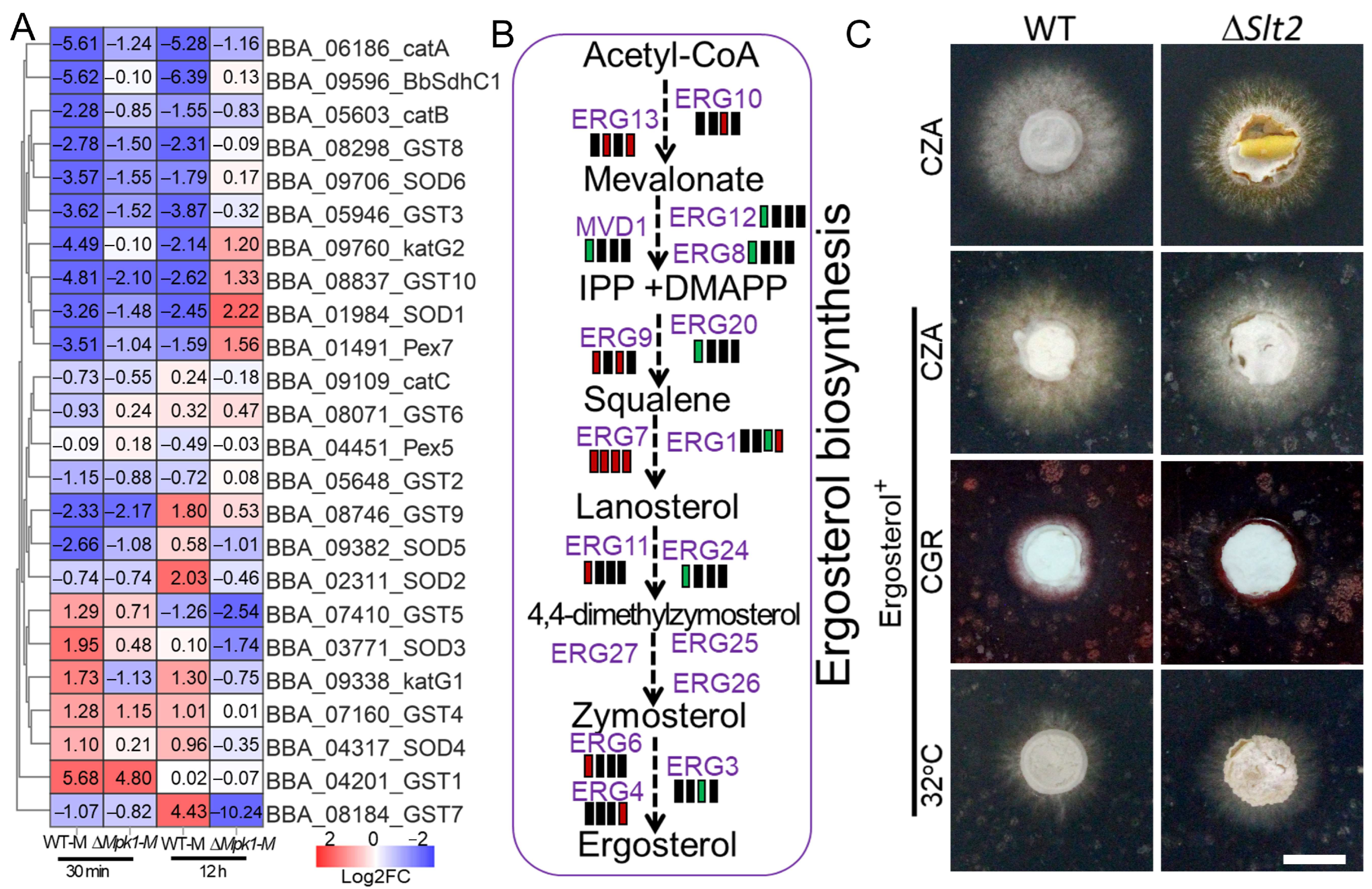
BbSlt2. Those dysregulated pathways were enriched in membrane proteins, hydrolases, transporters, lipid metabolism, oxidoreductases, and cell-wall remodeling, indicating that Slt2 not only regulates the CWI pathway but also governs multifaceted stress-responsive networks that are essential for fungal survival. Ergosterol is essential for cellular membrane integrity and plays vital role in environmental stress response, especially the response to thermal stress [
31,
32,
33,
34]. Ergosterol metabolism was found to be severely impaired in ∆
BbSlt2, leading to possible membrane instability, and ergosterol supplementation partially rescued ∆
BbSlt2 growth under heat and cell-wall stress, indicating that Slt2 positively regulates membrane sterol balance, a phenomenon also observed in lipid-deficient strains of
B. bassiana [
17]. These results indicate a robust role of Slt2 in regulating metabolic adaptability and redox defense under thermal pressure, offering a new molecular explanation for fungal heat-stress response and ecological adaptation to host insects in entomopathogenic fungi.
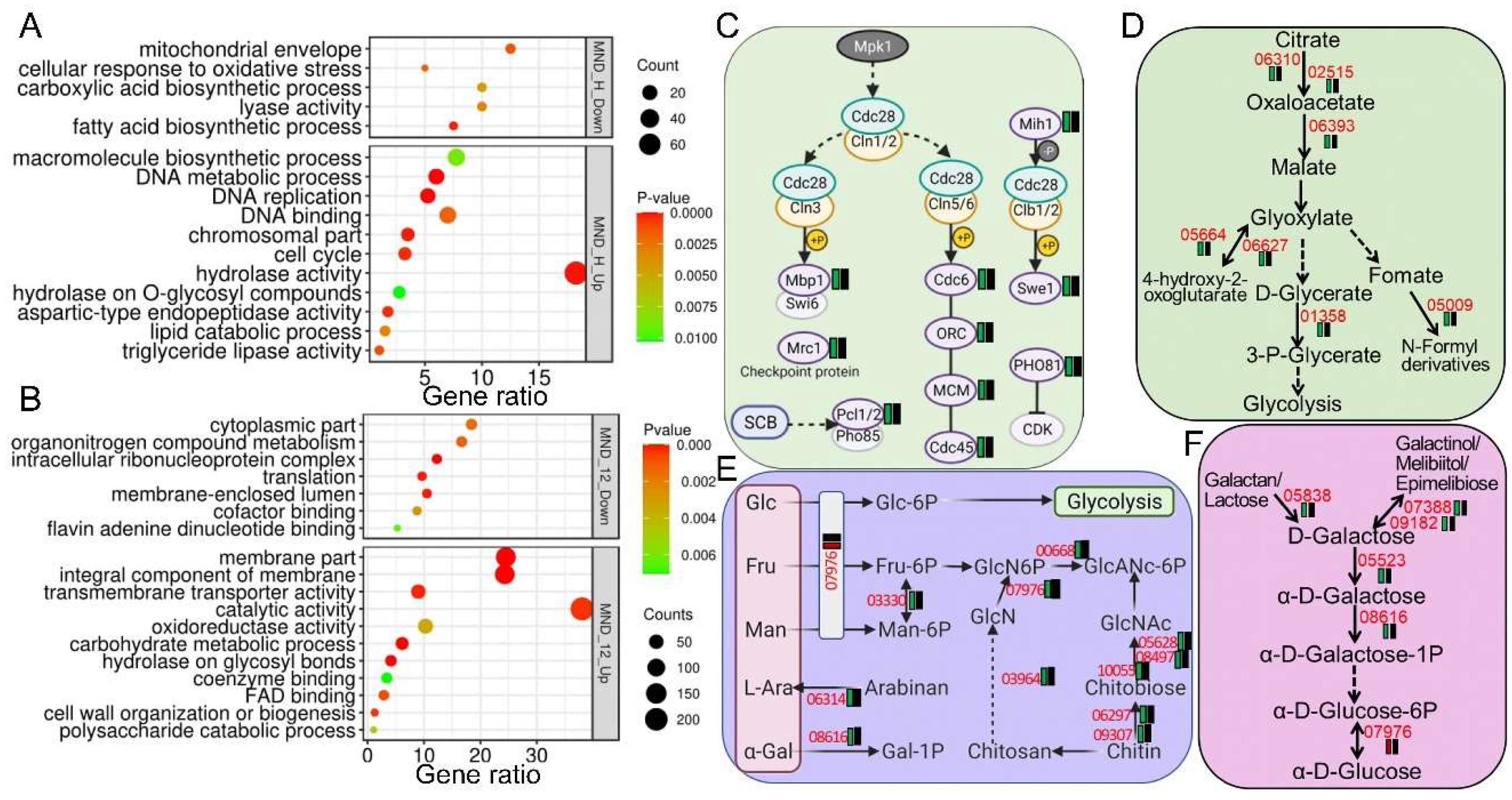
The Fus3 (Mpk1) MAP-kinase cascade is known to regulate fungal sexual/asexual development and filamentous growth in yeast and filamentous fungi [
13]. Pathogenic fungi adopt Mpk1 (Fus3) for the regulation of host penetration and appressorium formation [
5,
12,
35]. In addition to its roles in development and pathogenesis, Fus3 (Mpk1) has emerged as a key mediator of fungal stress responses, orchestrating gene expression and signaling networks that enable adaptation to diverse environmental challenges. The results of our phenotypic test coincide with those of previous studies that found that the deletion of BbMpk1 paralyzed host cuticle penetration and increased tolerance to oxidative stress in
B. bassiana [
5,
23]. The abnormal protrusions on the cell surface and increased WGA antigen-tagged N-acetylglucosamine signal on the cell surface of ∆
BbMpk1 indicated an increase in cell-wall ingredients after loss of BbMpk1, which may somehow contribute to oxidative stress resistance by shielding cells from exogenous oxidative radical damage via an enhanced cell wall [
7,
36]. Transcription factors BbOsrR2 and BbOsrR3 were found to mediate fungal oxidative stress response by regulating the Fus3-MAP kinase pathway in
B. bassiana [
37]. Transcriptomic analysis revealed that the BbMpk1 MAP kinase plays a pivotal role in mediating
Beauveria bassiana’s response to oxidative stress by orchestrating transcriptional reprogramming of multiple protective and metabolic pathways. Upon acute oxidative challenge (0.5 h MND), the ∆
BbMpk1 mutant displayed a severely blunted transcriptional response, with only 5.9% of differentially expressed genes (DEGs) upregulated, while the wild-type (WT) strain exhibited robust regulation of 414 DEGs, including significant repression of DNA metabolism, hydrolase activity, and lipid catabolism, alongside the induction of mitochondrial envelope- and monocarboxylic acid biosynthesis-related genes. The increased accumulation of MT membranal monomers in ∆
BbMpk1 also indicated decreased membrane potential with the loss of BbMpk1. These results suggest that BbMpk1 is essential for activating mitochondrial functions and metabolic suppression under stress, likely to limit ROS generation and preserve cellular integrity [
11,
25]. Furthermore, the failure of ∆
BbMpk1 to downregulate cell-cycle genes indicates an inability to arrest proliferation under stress, potentially exacerbating ROS damage, consistent with the conserved role of Mpk1 in cell-cycle regulation under stress in fungi [
8]. Under prolonged oxidative stress (12 h), the ∆
BbMpk1 mutant activated alternative detoxification pathways, such as the overexpression of MFS transporters, oxidoreductases, and cytochrome P450s, reflecting a compensatory but possibly less efficient ROS mitigation mechanism. Notably, BbSdhC1, a key mitochondrial component linked to ROS homeostasis [
24], was sharply downregulated in the WT strain but unaltered in ∆
BbMpk1, implicating BbMpk1 in suppressing mitochondrial respiration to reduce ROS load. Similarly, canonical antioxidant genes, including catalases, GSTs, and SODs, were transcriptionally repressed in the WT strain under stress, a response that was either alleviated or reversed in ∆
BbMpk1, indicating disrupted fine-tuning of ROS scavenging systems. These findings highlight the fact that BbMpk1 confers oxidative stress resistance by integrating transcriptional networks involving respiration, metabolism, and detoxification, reinforcing its potential as a genetic target for improved fungal fitness and biocontrol efficacy in hostile environments.