MaCsbD Mediates Thermotolerance and UV-B Resistance in Metarhizium acridum by Regulating DNA Repair, Antioxidant Defense, and Protective Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Bioinformatic Analyses
2.3. Gene Deletion and Complementation
2.4. Conidial Germination and Conidiation Capacity Analysis
2.5. Stress Resistance Analysis
2.6. Trehalose Extraction and Quantification
2.7. Determination of Melanin Content
2.8. ROS Scavenging Capacity Analysis
2.9. DNA Damage Repair Assessment
2.10. Virulence Analysis
2.11. qRT-PCR
2.12. Data Analyses
3. Results
3.1. Phylogenetic and Structural Analysis Reveals MaCsbD as a Conserved Fungal Protein
3.2. MaCsbD Is Required for Efficient Conidial Germination and Production
3.3. MaCsbD Is Essential for Tolerance to UV-B Irradiation and Heat Shock
3.4. MaCsbD Confers Resistance to Osmotic and Cell Wall Stressors
3.5. MaCsbD Positively Regulates Melanin Biosynthesis
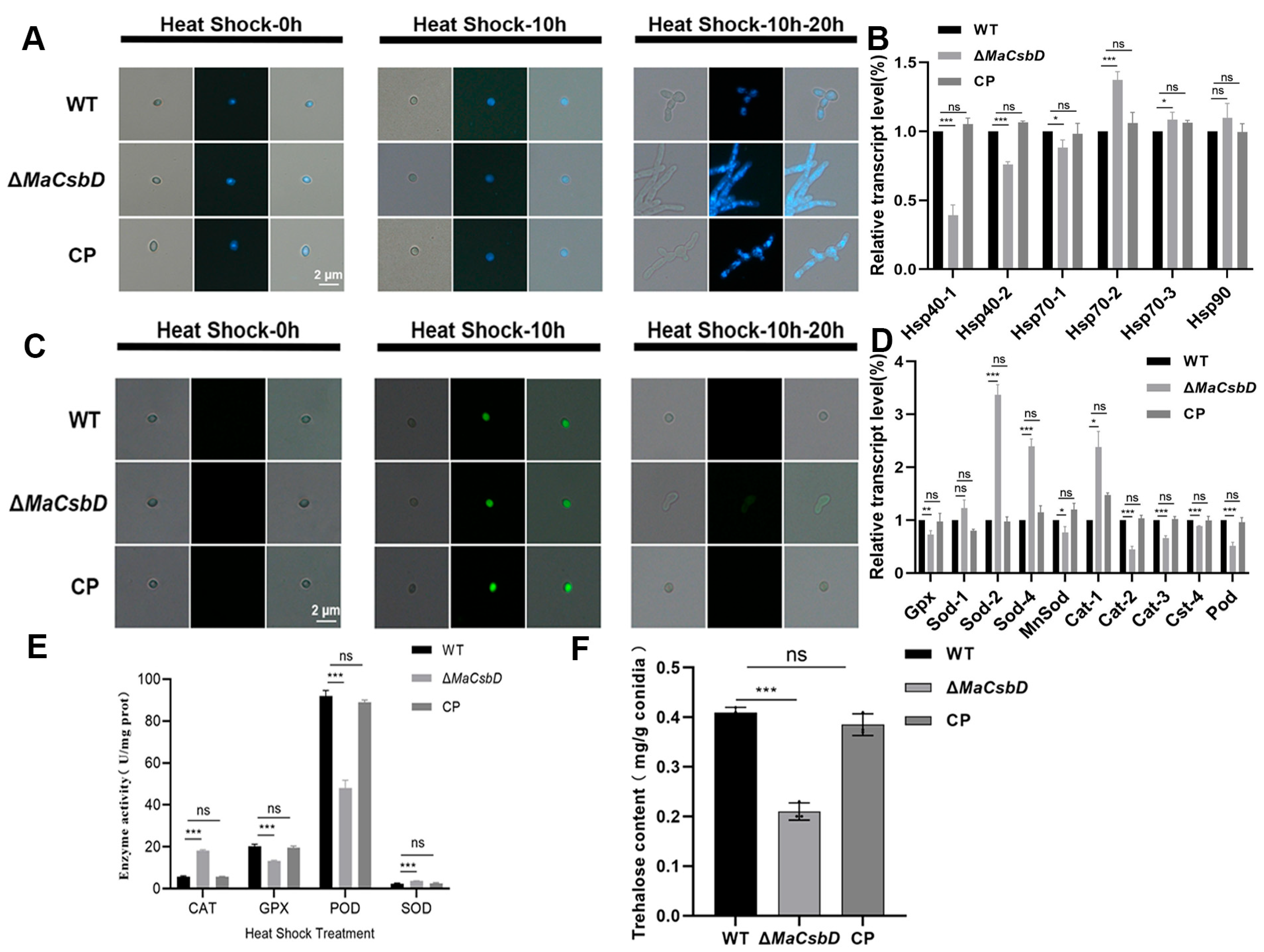
3.6. MaCsbD Mediates Thermotolerance Through Enhanced DNA Repair, HSP Induction, ROS Clearance, and Trehalose Accumulation in Conidia
3.7. MaCsbD Promotes UV-B Resistance by Facilitating DNA Repair and Antioxidant Defense
3.8. MaCsbD Deletion Does Not Impair Fungal Virulence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panwar, N.; Szczepaniec, A. Endophytic entomopathogenic fungi as biological control agents of insect pests. Pest Manag. Sci. 2024, 80, 6033–6040. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Yadav, P.K. Entomopathogenic fungi and their relevance in sustainable agriculture: A review. Cogent Food Agric. 2023, 9, 2180857. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef]
- Méndez-González, F.; Castillo-Minjarez, J.M.; Loera, O.; Favela-Torres, E. Current developments in the resistance, quality, and production of entomopathogenic fungi. World J. Microbiol. Biotechnol. 2022, 38, 115. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef]
- Gowtham, H.; Hema, P.; Murali, M.; Shilpa, N.; Nataraj, K.; Basavaraj, G.; Singh, S.B.; Aiyaz, M.; Udayashankar, A.; Amruthesh, K.N. Fungal endophytes as mitigators against biotic and abiotic stresses in crop plants. J. Fungi 2024, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Azimzadeh, P.; Leger, R.J.S. Strain improvement of fungal insecticides for controlling insect pests and vector-borne diseases. Curr. Opin. Microbiol. 2012, 15, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-H. Chapter Two—Subcellular biochemistry and biology of filamentous entomopathogenic fungi. In Advances in Applied Microbiology; Rangel, D.E.N., Ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 129, pp. 35–58. [Google Scholar]
- Ortiz-Urquiza, A.; Keyhani, N.O. Stress response signaling and virulence: Insights from entomopathogenic fungi. Curr. Genet. 2015, 61, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Newbury, J.; Peberdy, J.F. Characterization of the heat shock response in protoplasts of Aspergillus nidulans. Mycol. Res. 1996, 100, 1325–1332. [Google Scholar] [CrossRef]
- Kummasook, A.; Pongpom, P.; Vanittanakom, N. Cloning, characterization and differential expression of an hsp70 gene from the pathogenic dimorphic fungus, Penicillium marneffei. DNA Seq. J. DNA Seq. Mapp. 2007, 18, 385–394. [Google Scholar] [CrossRef]
- Feng, M.-G. Chapter Three—Recovery of insect-pathogenic fungi from solar UV damage: Molecular mechanisms and prospects. In Advances in Applied Microbiology; Rangel, D.E.N., Ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 129, pp. 59–82. [Google Scholar]
- Peng, H.; Zhang, Y.-L.; Ying, S.-H.; Feng, M.-G. Rad2, Rad14 and Rad26 recover Metarhizium robertsii from solar UV damage through photoreactivation in vivo. Microbiol. Res. 2024, 280, 127589. [Google Scholar] [CrossRef] [PubMed]
- Boylan, S.A.; Redfield, A.R.; Brody, M.S.; Price, C.W. Stress-induced activation of the sigma B transcription factor of Bacillus subtilis. J. Bacteriol. 1993, 175, 7931–7937. [Google Scholar] [CrossRef]
- Prágai, Z.; Harwood, C.R. Regulatory interactions between the Pho and sigma(B)-dependent general stress regulons of Bacillus subtilis. Microbiology 2002, 148, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yuan, C.; Pan, F.; Zhou, X.; Fan, H.; Ma, Z. CsbD, a Novel Group B Streptococcal Stress Response Factor That Contributes to Bacterial Resistance against Environmental Bile Salts. J. Bacteriol. 2023, 205, e0044822. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.H.; Meng, Y.; Wang, D. Comparative gene expression analysis of Beauveria bassiana against Spodoptera frugiperda. PeerJ 2025, 13, e19591. [Google Scholar] [CrossRef]
- Li, Y.M.; Gao, J.Q.; Pei, X.Z.; Du, C.; Fan, C.; Yuan, W.J.; Bai, F.W. Production of L-alanyl-L-glutamine by immobilized Pichia pastoris GS115 expressing α-amino acid ester acyltransferase. Microb. Cell Factories 2019, 18, 27. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Song, L.; Xue, X.; Wang, S.; Li, J.; Jin, K.; Xia, Y. MaAts, an Alkylsulfatase, Contributes to Fungal Tolerances against UV-B Irradiation and Heat-Shock in Metarhizium acridum. J. Fungi 2022, 8, 270. [Google Scholar] [CrossRef]
- Lazo, G.R.; Stein, P.A.; Ludwig, R.A. A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Bio/Technology 1991, 9, 963–967. [Google Scholar] [CrossRef]
- Gao, P.; Li, M.; Jin, K.; Xia, Y. The homeobox gene MaH1 governs microcycle conidiation for increased conidial yield by mediating transcription of conidiation pattern shift-related genes in Metarhizium acridum. Appl. Microbiol. Biotechnol. 2019, 103, 2251–2262. [Google Scholar] [CrossRef]
- Oliveira, D.G.P.; Pauli, G.; Mascarin, G.M.; Delalibera, I. A protocol for determination of conidial viability of the fungal entomopathogens Beauveria bassiana and Metarhizium anisopliae from commercial products. J. Microbiol. Methods 2015, 119, 44–52. [Google Scholar] [CrossRef]
- Zou, Y.; Li, C.; Wang, S.; Xia, Y.; Jin, K. MaCts1, an Endochitinase, Is Involved in Conidial Germination, Conidial Yield, Stress Tolerances and Microcycle Conidiation in Metarhizium acridum. Biology 2022, 11, 1730. [Google Scholar] [CrossRef]
- Braga, G.U.; Flint, S.D.; Messias, C.L.; Anderson, A.J.; Roberts, D.W.J.M.R. Effect of UV-B on conidia and germlings of the entomopathogenic hyphomycete Metarhizium anisopliae. Mycol. Res. 2001, 105, 874–882. [Google Scholar] [CrossRef]
- Sun, X.; Shen, J.; Perrimon, N.; Kong, X.; Wang, D. The endoribonuclease Arlr is required to maintain lipid homeostasis by downregulating lipolytic genes during aging. Nat. Commun. 2023, 14, 6254. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xia, Y. A negative melanin regulator, Mamrn, regulates thermo and UV tolerance via distinct mechanisms in Metarhizium. Microbiol. Res. 2025, 297, 128190. [Google Scholar] [CrossRef]
- Song, T.; Li, C.; Jin, K.; Xia, Y. The Forkhead Box Gene, MaSep1, Negatively Regulates UV- and Thermo-Tolerances and Is Required for Microcycle Conidiation in Metarhizium acridum. J. Fungi 2024, 10, 544. [Google Scholar] [CrossRef]
- Xu, J.; Xu, J.; Shi, T.; Zhang, Y.; Chen, F.; Yang, C.; Guo, X.; Liu, G.; Shao, D.; Leong, K.W.; et al. Probiotic-Inspired Nanomedicine Restores Intestinal Homeostasis in Colitis by Regulating Redox Balance, Immune Responses, and the Gut Microbiome. Adv. Mater. 2023, 35, e2207890. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Xia, Y.; Jin, K. MaAreB, a GATA Transcription Factor, Is Involved in Nitrogen Source Utilization, Stress Tolerances and Virulence in Metarhizium acridum. J. Fungi 2021, 7, 512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Li, Q.; Zhang, Z.; Huang, Y.; Wu, Y.; Li, X.; Huang, M.; Huang, Y.; Jian, J. Selection and evaluation of stable reference genes for quantitative real-time PCR in the head kidney leukocyte of Oreochromis niloticus. Aquac. Rep. 2023, 31, 101660. [Google Scholar] [CrossRef]
- Sanchez, Y.; Taulien, J.; Borkovich, K.A.; Lindquist, S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992, 11, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.R.; Lindquist, S. Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 1998, 94, 73–82. [Google Scholar] [CrossRef]
- Fraikin, G.Y.; Belenikina, N.S.; Rubin, A.B. [Photochemical Processes of Cell DNA Damage by UV Radiation of Various Wavelengths: Biological Consequences]. Mol. Biol. 2024, 58, 3–21. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Fleer, R.; Naumovski, L.; Nicolet, C.M.; Robinson, G.W.; Weiss, W.A.; Yang, E. Nucleotide excision repair genes from the yeast Saccharomyces cerevisiae. Basic Life Sci. 1986, 39, 231–242. [Google Scholar] [CrossRef]
- Sancar, G.B. Enzymatic photoreactivation: 50 years and counting. Mutat. Res. 2000, 451, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Käfer, E.; Mayor, O. Genetic analysis of DNA repair in Aspergillus: Evidence for different types of MMS-sensitive hyperrec mutants. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1986, 161, 119–134. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.A.; Hirasawa, T.; Shimizu, H. Differential importance of trehalose accumulation in Saccharomyces cerevisiae in response to various environmental stresses. J. Biosci. Bioeng. 2010, 109, 262–266. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, K.; Xia, Y. MaCsbD Mediates Thermotolerance and UV-B Resistance in Metarhizium acridum by Regulating DNA Repair, Antioxidant Defense, and Protective Metabolites. J. Fungi 2025, 11, 838. https://doi.org/10.3390/jof11120838
Li X, Li K, Xia Y. MaCsbD Mediates Thermotolerance and UV-B Resistance in Metarhizium acridum by Regulating DNA Repair, Antioxidant Defense, and Protective Metabolites. Journal of Fungi. 2025; 11(12):838. https://doi.org/10.3390/jof11120838
Chicago/Turabian StyleLi, Xinyu, Ke Li, and Yuxian Xia. 2025. "MaCsbD Mediates Thermotolerance and UV-B Resistance in Metarhizium acridum by Regulating DNA Repair, Antioxidant Defense, and Protective Metabolites" Journal of Fungi 11, no. 12: 838. https://doi.org/10.3390/jof11120838
APA StyleLi, X., Li, K., & Xia, Y. (2025). MaCsbD Mediates Thermotolerance and UV-B Resistance in Metarhizium acridum by Regulating DNA Repair, Antioxidant Defense, and Protective Metabolites. Journal of Fungi, 11(12), 838. https://doi.org/10.3390/jof11120838




