Silent Persistence: Molecular Evidence of Clonal Transmission in Fluconazole-Resistant Candida parapsilosis Hospital Outbreaks over Decades
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Isolation and Polymerase Chain Reaction (PCR)
2.2. Antifungal Susceptibility Test
2.3. Microsatellite Genotyping Analysis
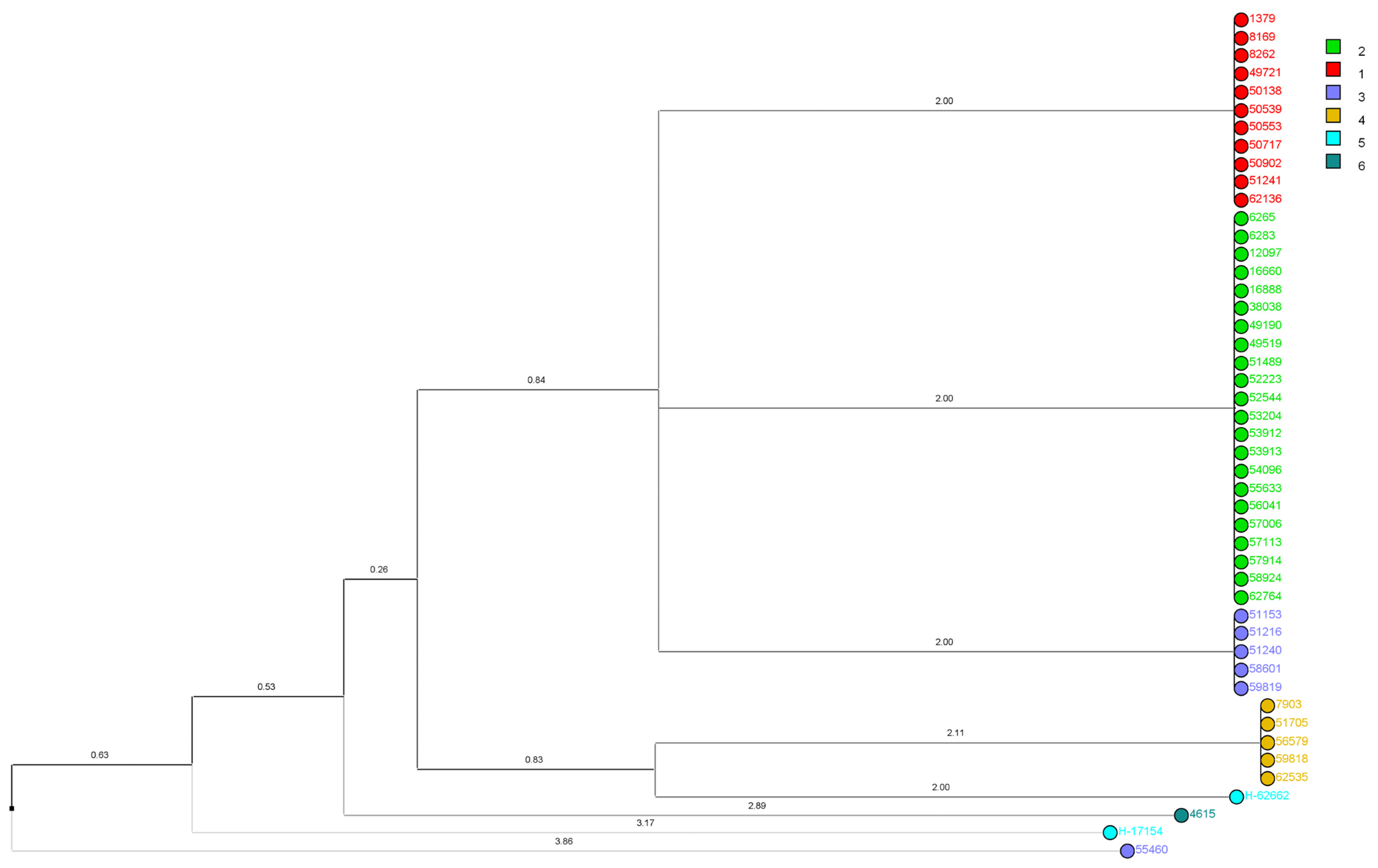
2.4. Phylogenetic Tree Construction
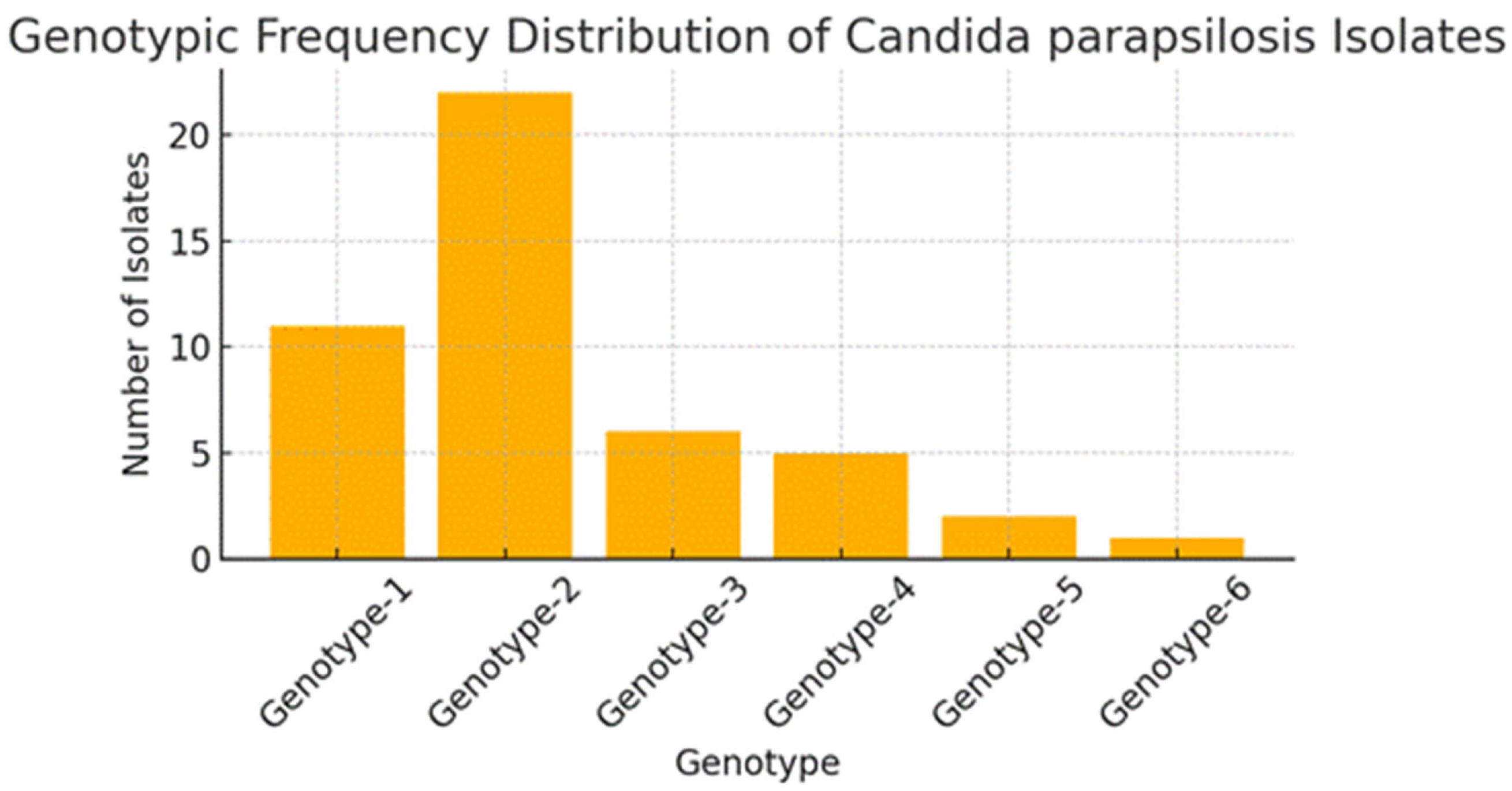
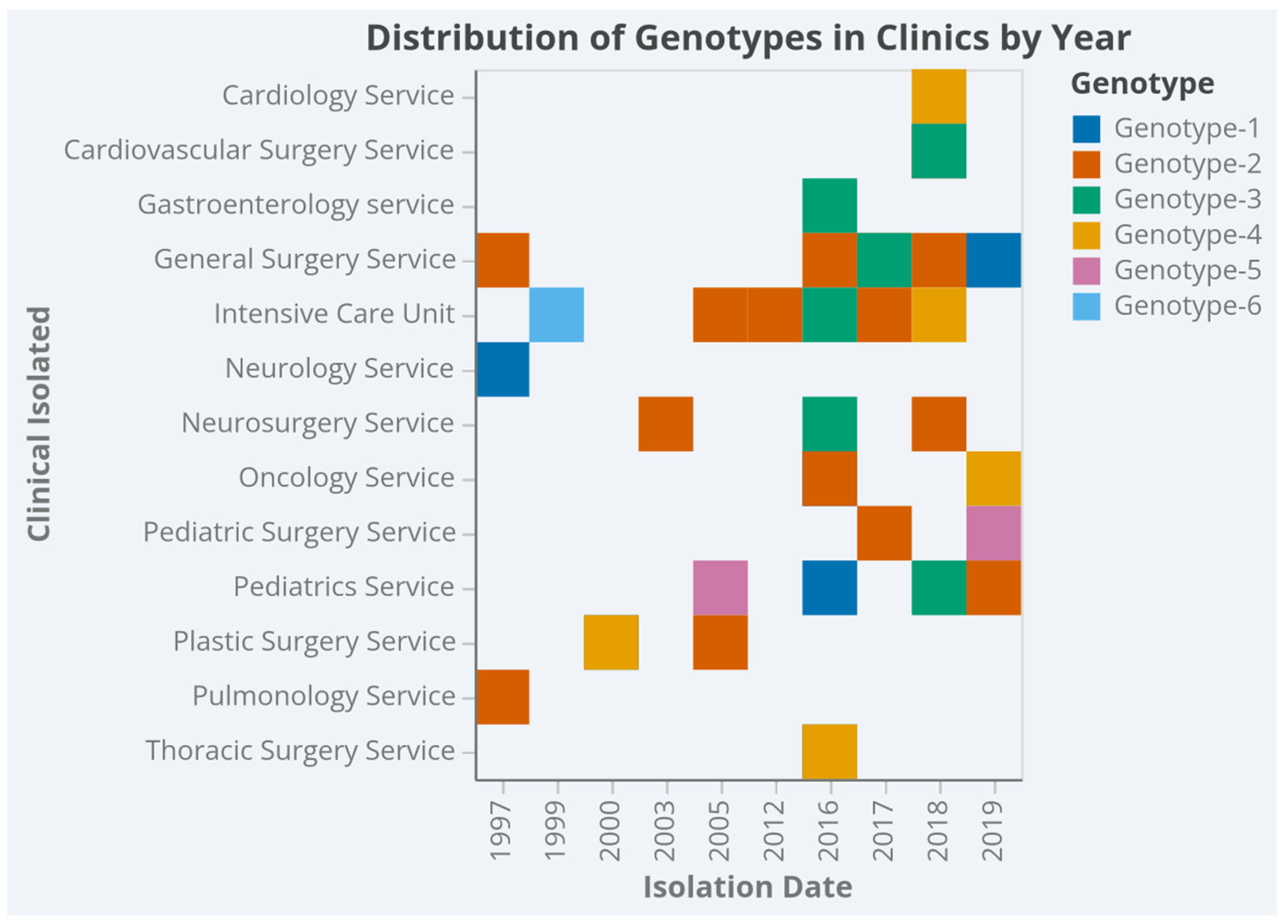
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sida, H.; Shah, P.; Pethani, J.; Patel, L.; Shah, H. Study of biofilm formation as a virulence marker in Candida species isolated from various clinical specimens. Int. J. Med. Sci. Public Health 2015, 5, 842. [Google Scholar] [CrossRef]
- Mukul, P. Characterization of Candida Species isolated from samples taken from patients with known Immunocompromised state presenting with Oral Thrush. J. Med. Sci. Clin. Res. 2018, 6, 765–768. [Google Scholar] [CrossRef]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef]
- Asogan, M.; Kim, H.; Kidd, S.; Alastruey-Izquierdo, A.; Govender, N.; Dao, A.; Shin, J.; Heim, J.; Ford, N.; Gigante, V.; et al. Candida parapsilosis: A systematic review to inform the World Health Organization fungal priority pathogens list. Med. Mycol. 2024, 62, myad131. [Google Scholar] [CrossRef]
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global guideline for the diagnosis and management of candidiasis: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293. [Google Scholar] [CrossRef]
- Díaz-García, J.; Machado, M.; Alcalá, L.; Reigadas, E.; Pérez-Ayala, A.; De La Pedrosa, E.G.-G.; Gónzalez-Romo, F.; Cuétara, M.S.; García-Esteban, C.; Quiles-Melero, I.; et al. Trends in antifungal resistance in Candida from a multicenter study conducted in Madrid (CANDIMAD study): Fluconazole-resistant C. parapsilosis spreading has gained traction in 2022. Antimicrob. Agents Chemother. 2023, 67, e0098623. [Google Scholar] [CrossRef]
- Sharma, V.; Paul, R.A.; Kaur, H.; Das, S.; Choudhary, H.; Rudramurthy, S.M.; Chakrabarti, A.; Ghosh, A.K. P019 A 5-year study on prevalence and molecular determinants of fluconazole resistance in C. parapsilosis spp. complex. Med. Mycol. 2022, 60, 44. [Google Scholar] [CrossRef]
- Ünal, N.; Spruijtenburg, B.; Arastehfar, A.; Gümral, R.; De Groot, T.; Meijer, E.F.J.; Türk-Dağı, H.; Birinci, A.; Hilmioğlu-Polat, S.; Meis, J.F.; et al. Multicentre Study of Candida parapsilosis Blood Isolates in Türkiye Highlights an Increasing Rate of Fluconazole Resistance and Emergence of Echinocandin and Multidrug Resistance. Mycoses 2024, 67, e70000. [Google Scholar] [CrossRef]
- Prigitano, A.; Blasi, E.; Calabrò, M.; Cavanna, C.; Cornetta, M.; Farina, C.; Grancini, A.; Innocenti, P.; Cascio, G.L.; Nicola, L.; et al. Yeast bloodstream infections in the COVID-19 patient: A multicenter Italian study (FICOV study). J. Fungi 2023, 9, 277. [Google Scholar] [CrossRef]
- Levin, A.S.; Costa, S.F.; Mussi, N.S.; Basso, M.; Sinto, S.I.; Machado, C.; Geiger, D.; Villares, M.; Schreiber, A.; Barone, A.; et al. Candida parapsilosis Fungemia Associated with Implantable and Semi-Implantable Central Venous Catheters and the Hands of Healthcare Workers. Diagn. Microbiol. Infect. Dis. 1998, 30, 243–249. [Google Scholar] [CrossRef]
- Qi, L.; Fan, W.; Xia, X.; Yao, L.; Liu, L.; Zhao, H.; Kong, X.; Liu, J. Nosocomial outbreak of Candida parapsilosis sensu stricto fungaemia in a neonatal intensive care unit in China. J. Hosp. Infect. 2018, 100, e246–e252. [Google Scholar] [CrossRef] [PubMed]
- Cacaci, M.; Squitieri, D.; Palmieri, V.; Torelli, R.; Perini, G.; Campolo, M.; Di Vito, M.; Papi, M.; Posteraro, B.; Sanguinetti, M.; et al. Curcumin-Functionalized Graphene Oxide Strongly Prevents Candida parapsilosis Adhesion and Biofilm Formation. Pharmaceuticals 2023, 16, 275. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, E.; Zarnowski, R.; Sanchez, H.; Covelli, A.S.; Westler, W.M.; Azadi, P.; Nett, J.; Mitchell, A.P.; Andes, D.R. Conservation and Divergence in the Candida Species Biofilm Matrix Mannan-Glucan Complex Structure, Function, and Genetic Control. mBio 2018, 9, e00451-18. [Google Scholar] [CrossRef] [PubMed]
- Fekkar, A.; Blaize, M.; Bouglé, A.; Normand, A.-C.; Raoelina, A.; Kornblum, D.; Kamus, L.; Piarroux, R.; Imbert, S. Hospital Outbreak of Fluconazole-Resistant Candida parapsilosis: Arguments for Clonal Transmission and Long-Term Persistence. Antimicrob. Agents Chemother. 2023, 95, e02036-20. [Google Scholar] [CrossRef]
- Sanchez, V.; Vazquez, J.A.; Barth-Jones, D.; Dembry, L.; Sobel, J.D.; Zervos, M.J. Nosocomial acquisition of Candida parapsilosis: An epidemiologic study. Am. J. Med. 1993, 94, 577–582. [Google Scholar] [CrossRef]
- Lupetti, A.; Tavanti, A.; Davini, P.; Ghelardi, E.; Corsini, V.; Merusi, I.; Boldrini, A.; Campa, M.; Senesi, S. Horizontal Transmission of Candida parapsilosis Candidemia in a Neonatal Intensive Care Unit. J. Clin. Microbiol. 2002, 40, 2363–2369. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Ahmad, S.; Al-Sweih, N.; Hagen, F.; Meis, J.F.; Khan, Z. High-resolution fingerprinting of Candida parapsilosis isolates suggests persistence and transmission of infections among neonatal intensive care unit patients in Kuwait. Sci. Rep. 2019, 9, 1340. [Google Scholar] [CrossRef]
- Thomaz, D.Y.; Del Negro, G.M.B.; Ribeiro, L.B.; Da Silva, M.; Carvalho, G.O.M.H.; Camargo, C.H.; de Almeida, J.N.; Motta, A.L.; Siciliano, R.F.; Sejas, O.N.E.; et al. A Brazilian Inter-Hospital Candidemia Outbreak Caused by Fluconazole-Resistant Candida parapsilosis in the COVID-19 Era. J. Fungi 2022, 8, 100. [Google Scholar] [CrossRef]
- Sabino, R.; Sampaio, P.; Rosado, L.; Videira, Z.; Grenouillet, F.; Pais, C. Analysis of clinical and environmental Candida parapsilosis isolates by microsatellite genotyping—A tool for hospital infection surveillance. Clin. Microbiol. Infect. 2015, 21, 954.e1–954.e8. [Google Scholar] [CrossRef]
- Cilo, B.D.; Agca, H.; Ener, B. Identification of Candida parapsilosis Complex Strains Isolated from Blood Samples at Species Level and Determination of Their Antifungal Susceptibilities. Türk Mikrobiyoloji Cemiy. Derg. 2019, 49, 61–66. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility of Yeasts, 4th ed.; CLSI Standard M27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Performance Standards for Antifungal Susceptibility of Yeasts, 3rd ed.; CLSI supplement M27M44S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Neji, S.; Hadrich, I.; Ilahi, A.; Trabelsi, H.; Chelly, H.; Mahfoudh, N.; Cheikhrouhou, F.; Sellami, H.; Makni, F.; Ayadi, A. Molecular Genotyping of Candida parapsilosis Species Complex. Mycopathologia 2018, 183, 765–775. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Hilmioğlu-Polat, S.; Fang, W.; Yaşar, M.; Polat, F.; Metin, D.Y.; Rigole, P.; Coenye, T.; Ilkit, M.; et al. First Report of Candidemia Clonal Outbreak Caused by Emerging Fluconazole-Resistant Candida parapsilosis Isolates Harboring Y132F and/or Y132F+K143R in Turkey. Antimicrob. Agents Chemother. 2020, 64, e01001-20. [Google Scholar] [CrossRef]
- Sabino, R.; Sampaio, P.; Rosado, L.; Stevens, D.A.; Clemons, K.V.; Pais, C. New Polymorphic Microsatellite Markers Able to Distinguish among Candida parapsilosis Sensu Stricto Isolates. J. Clin. Microbiol. 2010, 48, 1677–1682. [Google Scholar] [CrossRef]
- Hunter, P.R. Reproducibility and indices of discriminatory power of microbial typing methods. J. Clin. Microbiol. 1990, 28, 1903–1905. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Desnos-Ollivier, M.; Bórmida, V.; Poirier, P.; Nourrisson, C.; Pan, D.; Bretagne, S.; Puime, A.; Dromer, F.; Uruguayan Invasive Fungal Infection Network. Population Structure of Candida parapsilosis: No Genetic Difference Between French and Uruguayan Isolates Using Microsatellite Length Polymorphism. Mycopathologia 2017, 183, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Escribano, P.; Guinea, J. Fluconazole-resistant Candida parapsilosis: A new emerging threat in the fungi arena. Front. Fungal Biol. 2022, 3, 1010782. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, L.R.; Eshaghi, A.; Hota, S.; Poutanen, S.M.; Johnstone, J.; De Luca, D.G.; Bharat, A.; Patel, S.N.; Kus, J.V. First Canadian report of transmission of fluconazole-resistant Candida parapsilosis within two hospital networks confirmed by genomic analysis. J. Clin. Microbiol. 2024, 62, e0116123. [Google Scholar] [CrossRef]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18. [Google Scholar] [CrossRef]
- Reiss, E.; Lasker, B.A.; Lott, T.J.; Bendel, C.M.; Kaufman, D.A.; Hazen, K.C.; McGowan, K.L.; Lockhart, S.R. Genotyping of Candida parapsilosis from three neonatal intensive care units (NICUs) using a panel of five multilocus microsatellite markers: Broad genetic diversity and a cluster of related strains in one NICU. Infect. Genet. Evol. 2012, 12, 1654–1660. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, S.-Y.; Chen, S.C.; Xiao, M.; Kong, F.; Wang, H.; Ning, Y.-T.; Lu, M.-Y.; Sun, T.-S.; Hou, X.; et al. Molecular Characterization of Candida parapsilosis by Microsatellite Typing and Emergence of Clonal Antifungal Drug Resistant Strains in a Multicenter Surveillance in China. Front. Microbiol. 2020, 11, 1320. [Google Scholar] [CrossRef]
- Romeo, O.; Delfino, D.; Cascio, A.; Lo Passo, C.; Amorini, M.; Romeo, D.; Pernice, I. Microsatellite-based genotyping of Candida parapsilosis sensu stricto isolates reveals dominance and persistence of a particular epidemiological clone among neonatal intensive care unit patients. Infect. Genet. Evol. 2012, 13, 105–108. [Google Scholar] [CrossRef]
- Hernández-Castro, R.; Arroyo-Escalante, S.; Carrillo-Casas, E.M.; Moncada-Barrón, D.; Álvarez-Verona, E.; Hernández-Delgado, L.; Torres-Narváez, P.; Lavalle-Villalobos, A. Outbreak of Candida parapsilosis in a neonatal intensive care unit: A health care workers source. Eur. J. Pediatr. 2009, 169, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.D.N.; Rodrigues, E.C.A.; Costa, S.F.; Van Der Heijden, I.M.; Dantas, K.C.; Lobo, R.D.; Basso, M.; Varkulja, G.F.; Krebs, V.L.J.; Gibelli, M.A.B.C.; et al. Candida parapsilosis candidaemia in a neonatal unit over 7 years: A case series study. BMJ Open 2012, 2, e000992. [Google Scholar] [CrossRef] [PubMed]
- Dizbay, M.; Kalkanci, A.; Sezer, B.E.; Aktas, F.; Aydogan, S.; Fidan, I.; Kustimur, S.; Sugita, T. Molecular investigation of a fungemia outbreak due to Candida parapsilosis in an intensive care unit. Braz. J. Infect. Dis. 2008, 12, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lin, Y.-H.; Chen, K.-W.; Lii, J.; Teng, H.-J.; Li, S.-Y. Molecular epidemiology and antifungal susceptibility of Candida parapsilosis sensu stricto, Candida orthopsilosis, and Candida metapsilosis in Taiwan. Diagn. Microbiol. Infect. Dis. 2010, 68, 284–292. [Google Scholar] [CrossRef]
- Daneshnia, F.; De Almeida Júnior, J.N.; Ilkit, M.; Lombardi, L.; Perry, A.M.; Gao, M.; Nobile, C.J.; Egger, M.; Perlin, D.S.; Zhai, B.; et al. Worldwide emergence of fluconazole-resistant Candida parapsilosis: Current framework and future research roadmap. Lancet Microbe 2023, 4, e470–e480. [Google Scholar] [CrossRef]
- Caggiano, G.; Fioriti, S.; Morroni, G.; Apollonio, F.; Triggiano, F.; D’Achille, G.; Stefanizzi, P.; Dalfino, L.; Ronga, L.; Mosca, A.; et al. Genotypic and phenotypic characteristics of Candida parapsilosis bloodstream isolates: Health Care Associated Infections in a teaching Hospital in Italy. J. Infect. Public Health 2024, 17, 967–974. [Google Scholar] [CrossRef]
- Almirante, B.; RodríGuez, D.; Cuenca-Estrella, M.; Almela, M.; Sanchez, F.; Ayats, J.; Alonso-Tarres, C.; Rodriguez-Tudela, J.L.; Pahissa, A. Epidemiology, Risk Factors, and Prognosis of Candida parapsilosis Bloodstream Infections: Case-Control Population-Based Surveillance Study of Patients in Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2006, 44, 1681–1685. [Google Scholar] [CrossRef]
- Miyake, A.; Gotoh, K.; Iwahashi, J.; Togo, A.; Horita, R.; Miura, M.; Kinoshita, M.; Ohta, K.; Yamashita, Y.; Watanabe, H. Characteristics of Biofilms Formed by C. parapsilosis Causing an Outbreak in a Neonatal Intensive Care Unit. J. Fungi 2022, 8, 700. [Google Scholar] [CrossRef]
- Arastehfar, A.; Hilmioğlu-Polat, S.; Daneshnia, F.; Pan, W.; Hafez, A.; Fang, W.; Liao, W.; Şahbudak-Bal, Z.; Metin, D.Y.; De Almeida, J.N., Jr.; et al. Clonal Candidemia Outbreak by Candida parapsilosis Carrying Y132F in Turkey: Evolution of a Persisting Challenge. Front. Cell. Infect. Microbiol. 2021, 11, 676177. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Desnos-Ollivier, M.; Bretagne, S.; Dromer, F. The risk and clinical outcome of candidemia depending on underlying malignancy. Intensive Care Med. 2017, 43, 652–662. [Google Scholar] [CrossRef]
- Pulcrano, G.; Roscetto, E.; Iula, V.D.; Panellis, D.; Rossano, F.; Catania, M.R. MALDI-TOF mass spectrometry and microsatellite markers to evaluate Candida parapsilosis transmission in neonatal intensive care units. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2919–2928. [Google Scholar] [CrossRef]
- Magobo, R.E.; Naicker, S.D.; Wadula, J.; Nchabeleng, M.; Coovadia, Y.; Hoosen, A.; Lockhart, S.R.; Govender, N.P.; TRAC-South Africa Group. Detection of neonatal unit clusters of Candida parapsilosis fungaemia by microsatellite genotyping: Results from laboratory-based sentinel surveillance, South Africa, 2009–2010. Mycoses 2017, 60, 320–327. [Google Scholar] [CrossRef]
- Brunetti, G.; Navazio, A.; Giuliani, A.; Giordano, A.; Proli, E.; Antonelli, G.; Raponi, G. Candida blood stream infections observed between 2011 and 2016 in a large Italian University Hospital: A time-based retrospective analysis on epidemiology, biofilm production, antifungal agents consumption and drug-susceptibility. PLoS ONE 2019, 14, e0224678. [Google Scholar] [CrossRef]
- Bailly, S.; Maubon, D.; Fournier, P.; Pelloux, H.; Schwebel, C.; Chapuis, C.; Foroni, L.; Cornet, M.; Timsit, J. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp.—Trends over 10 years. J. Infect. 2016, 72, 103–111. [Google Scholar] [CrossRef]

| Antifungal Agents | MIC Ranges (µg/mL) | MIC 50 (µg/mL) | MIC 90 (µg/mL) | Percent of Non-Susceptible /Non-Wild Type Isolates |
|---|---|---|---|---|
| Fluconazole | 4–>64 | 8 | 32 | 100 (≥4 µg/mL) |
| Voriconazole | 0.06–1 | 0.125 | 0.25 | 14.9 (≥0.25 µg/mL) |
| İtraconazole | 0.06–0.5 | 0.06 | 0.25 | none |
| Posaconazole | ≤0.03 –0.125 | 0.06 | 0.125 | none |
| Amphotericin B | 0.5–1 | 0.5 | 1 | none |
| Anidulafungin | 0.5–2 | 0.5 | 1 | none |
| Marker | Number of Alleles | Allele Sizes | Number of Repeats | Allele Frequencies | Number of Genotypes | Genotype Frequencies | Heterozygosity Rate (%) | DP * |
|---|---|---|---|---|---|---|---|---|
| CP1 | 3 | 224–302 | 1–40 | 0.0213–0.9574 | 3 | 0.0110–0.8123 | 0 | 0.0842 |
| CP4 | 4 | 249–286 | 1–19 | 0.0213–0.8511 | 3 | 0.0222–0.6691 | 0.0425 | 0.2683 |
| B5 | 3 | 140–148 | 1–5 | 0.1277–0.6170 | 3 | 0.0676–0.3901 | 0 | 0.5495 |
| Total | 0.7114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semet, C.; Kazak, E.; Ak-Aksoy, S.; Ağca, H.; Ener, B. Silent Persistence: Molecular Evidence of Clonal Transmission in Fluconazole-Resistant Candida parapsilosis Hospital Outbreaks over Decades. J. Fungi 2025, 11, 802. https://doi.org/10.3390/jof11110802
Semet C, Kazak E, Ak-Aksoy S, Ağca H, Ener B. Silent Persistence: Molecular Evidence of Clonal Transmission in Fluconazole-Resistant Candida parapsilosis Hospital Outbreaks over Decades. Journal of Fungi. 2025; 11(11):802. https://doi.org/10.3390/jof11110802
Chicago/Turabian StyleSemet, Cihan, Esra Kazak, Seçil Ak-Aksoy, Harun Ağca, and Beyza Ener. 2025. "Silent Persistence: Molecular Evidence of Clonal Transmission in Fluconazole-Resistant Candida parapsilosis Hospital Outbreaks over Decades" Journal of Fungi 11, no. 11: 802. https://doi.org/10.3390/jof11110802
APA StyleSemet, C., Kazak, E., Ak-Aksoy, S., Ağca, H., & Ener, B. (2025). Silent Persistence: Molecular Evidence of Clonal Transmission in Fluconazole-Resistant Candida parapsilosis Hospital Outbreaks over Decades. Journal of Fungi, 11(11), 802. https://doi.org/10.3390/jof11110802





