Effect of Inoculation with Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis BGC AH01) on the Soil Bacterial Community Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Strains
2.2. Experimental Setup
2.3. Sample Collection
2.4. Sample Analysis
2.4.1. Mycorrhizal Colonization and Microelement Concentrations
2.4.2. Determination of Soil Physicochemical Properties
2.4.3. Soil Bacterial Community
2.5. Statistical Analysis
3. Results
3.1. Effects of AM Fungi Inoculation on Crop Growth and Soil Physicochemical Properties
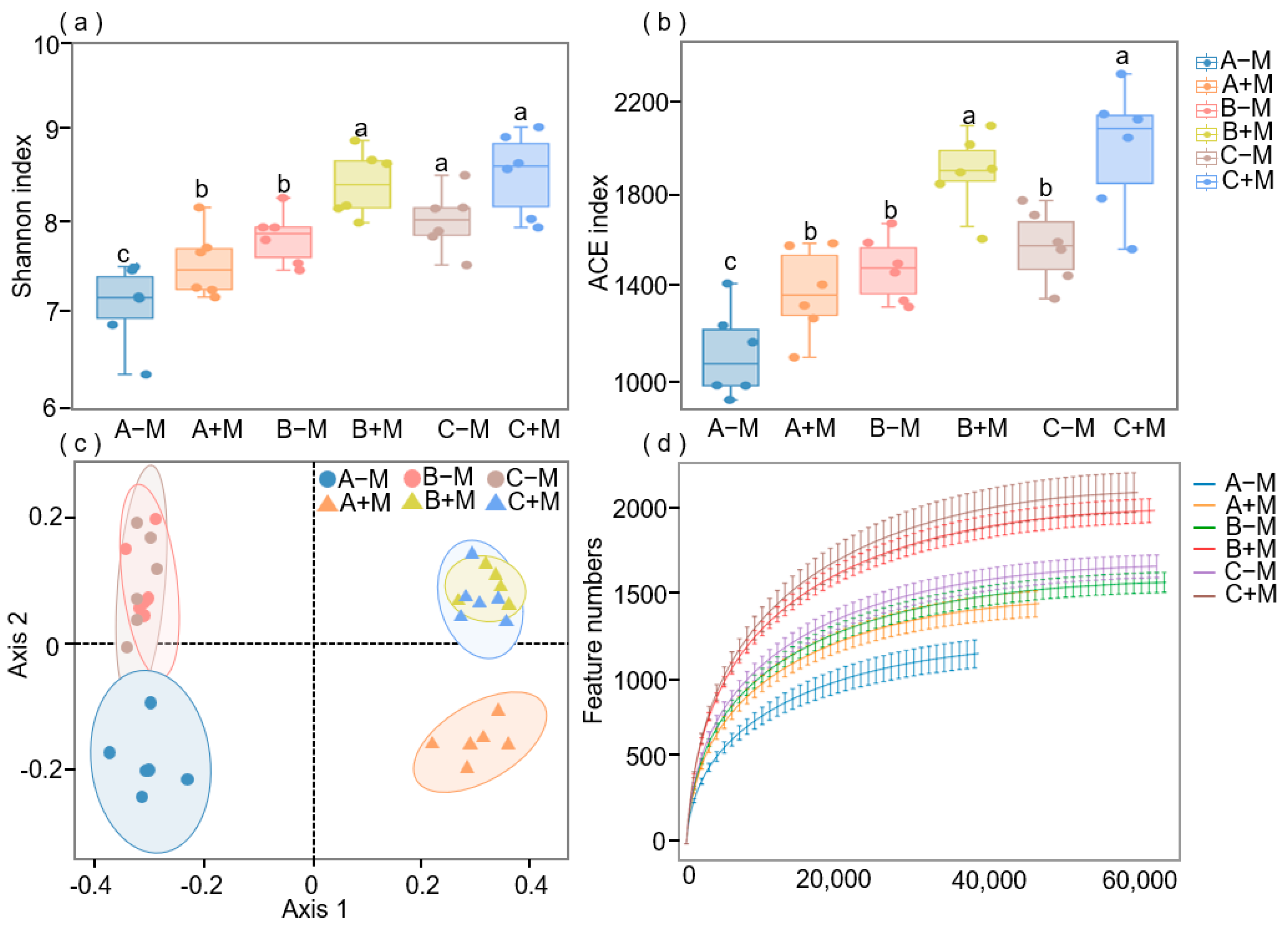
3.2. Effects of AM Fungi Inoculation on the Soil Bacterial Community Composition and Diversity
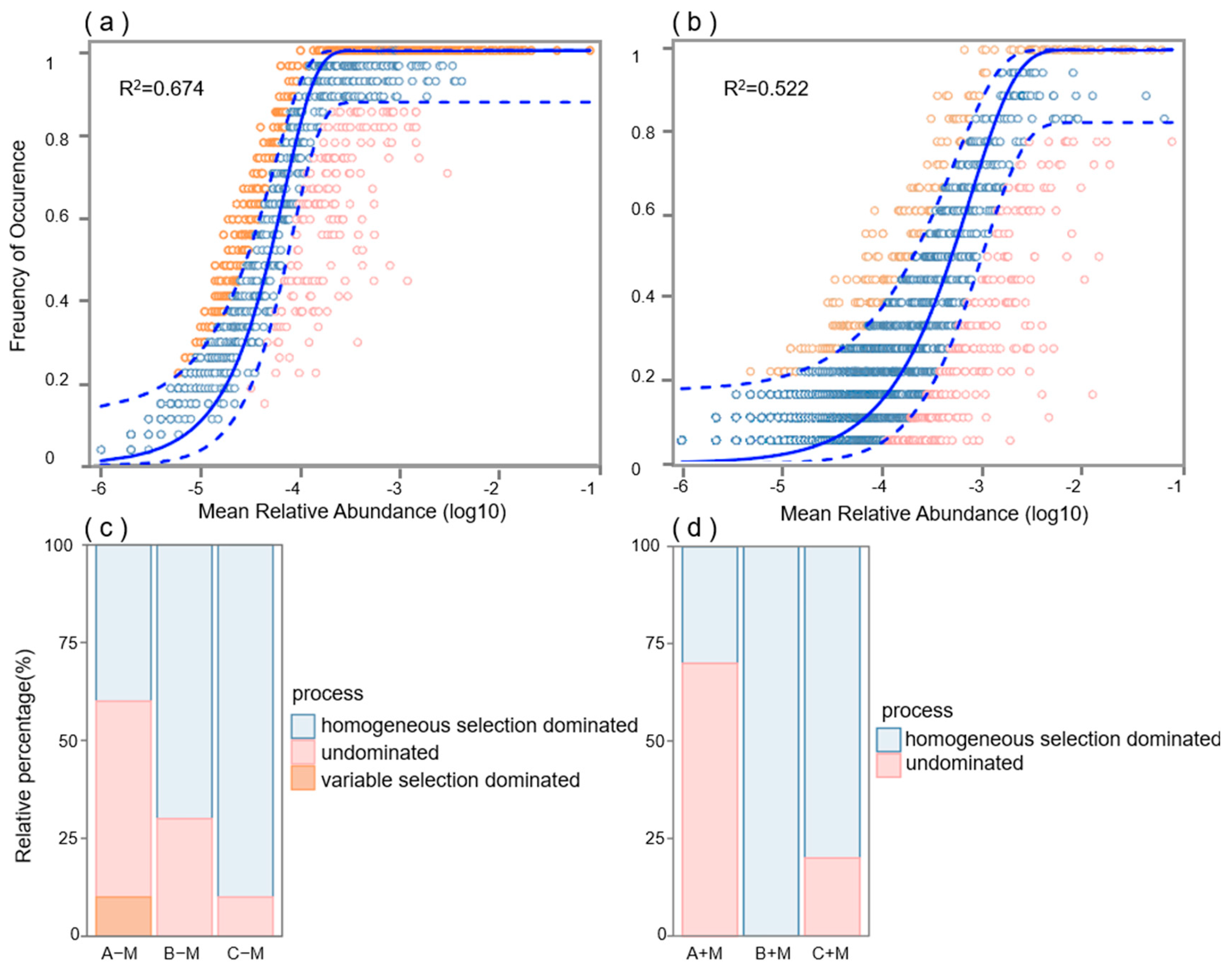
3.3. Effects of AM Fungi Inoculation on the Bacterial Community Network and Assembly
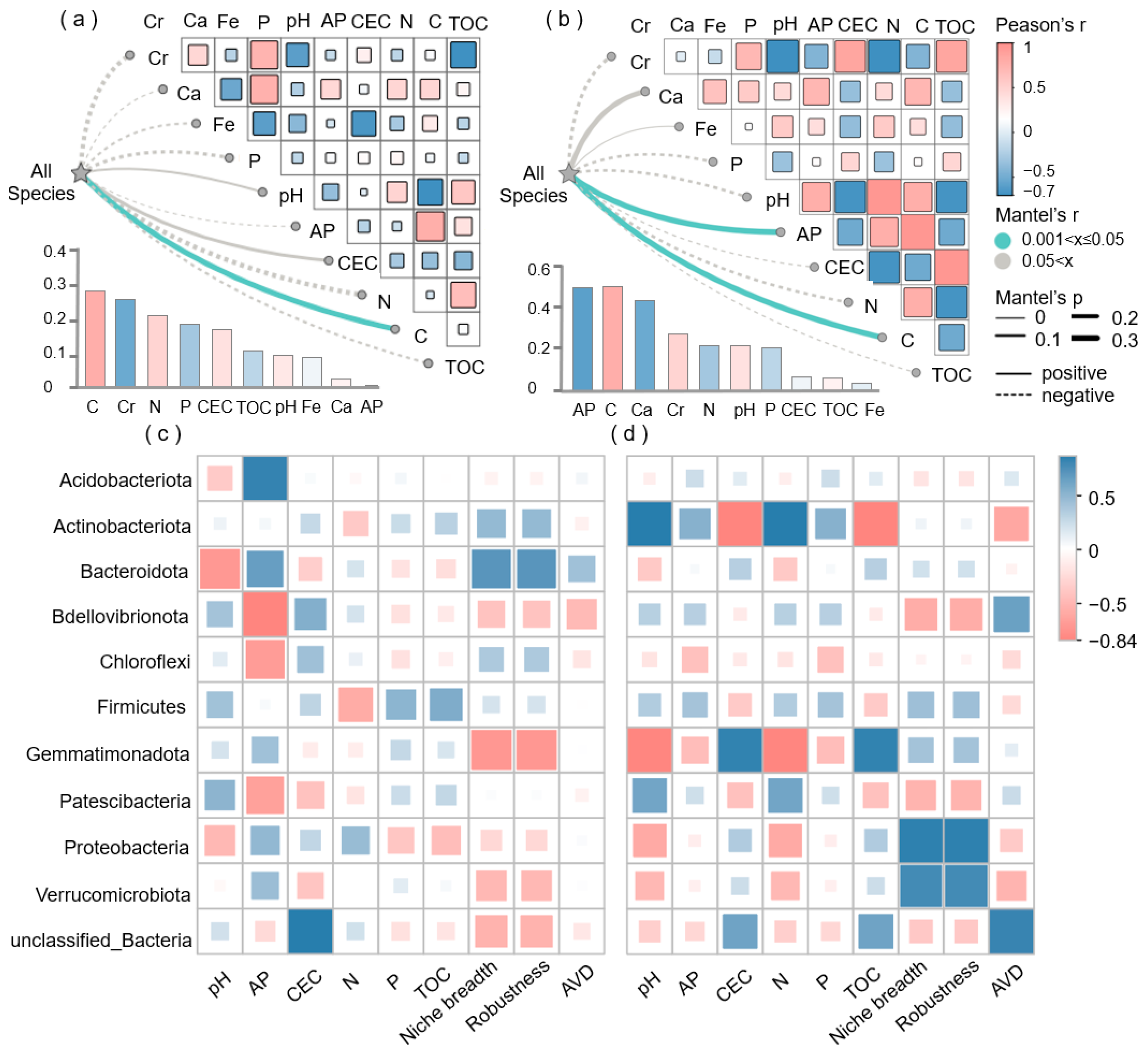
3.4. Relationships Between the Soil Bacterial Community and Soil Physicochemical Properties
4. Discussion
5. Limitations of the Experimental Design
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, S.; Feng, G.; Limpens, E.; Bonfante, P.; Xie, X.; Zhang, L. Cross-kingdom nutrient exchange in the plant–arbuscular mycorrhizal fungus–bacterium continuum. Nat. Rev. Microbiol. 2024, 22, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jin, Z.; George, T.S.; Feng, G.; Zhang, L. Arbuscular mycorrhizal fungi enhance plant phosphorus uptake through stimulating hyphosphere soil microbiome functional profiles for phosphorus turnover. New Phytol. 2023, 238, 2578–2593. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; George, T.S.; Feng, G. Concepts and consequences of the hyphosphere core microbiome for arbuscular mycorrhizal fungal fitness and function. New Phytol. 2023, 242, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Hassani, M.A.; Duran, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, Y.; Lou, Y.; Meng, J.; Shi, L.; Xia, F. Assembly strategies of the wheat root-associated microbiome in soils contaminated with phenanthrene and copper. J. Hazard. Mater. 2021, 412, 125340. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Wang, X.; Liu, S.; Luo, L.; Zeng, Q.; Wu, Y.; Zeng, Y.; Yang, Z.; Sheng, G. Emerging Nanochitosan for Sustainable Agriculture. Int. J. Mol. Sci. 2024, 25, 12261. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Zhang, X.; Ju, W.; Duan, C.; Wang, Y.; Fang, L. A novel extracellular enzyme stoichiometry method to evaluate soil heavy metal contamination: Evidence derived from microbial metabolic limitation. Sci. Total Environ. 2020, 738, 139709. [Google Scholar] [CrossRef]
- Wang, F.; Kertesz, M.A.; Feng, G. Phosphorus forms affect the hyphosphere bacterial community involved in soil organic phosphorus turnover. Mycorrhiza 2019, 29, 351–362. [Google Scholar] [CrossRef]
- Herman, D.J.; Firestone, M.K.; Nuccio, E.; Hodge, A. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol. Ecol. 2012, 80, 236–247. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef]
- He, J.; Zhang, L.; Van Dingenen, J.; Desmet, S.; Goormachtig, S.; Calonne-Salmon, M.; Declerck, S. Arbuscular mycorrhizal hyphae facilitate rhizobia dispersal and nodulation in legumes. ISME J. 2024, 18, wrae185. [Google Scholar] [CrossRef]
- Sun, T.; Li, G.; Mazarji, M.; Delaplace, P.; Yang, X.; Zhang, J.; Pan, J. Heavy metals drive microbial community assembly process in farmland with long-term biosolids application. J. Hazard. Mater. 2024, 468, 133845. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.L.; Billings, S.A.; Hirmas, D.; Li, L.; Zhang, X.; Ziegler, S.; Murenbeeld, K.; Ajami, H.; Guthrie, A.; Singha, K.; et al. Embracing the dynamic nature of soil structure: A paradigm illuminating the role of life in critical zones of the Anthropocene. Earth-Sci. Rev. 2021, 225, 103873. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2020, 402, 123829. [Google Scholar] [CrossRef]
- Emmett, B.D.; Lévesque-Tremblay, V.; Harrison, M.J. Conserved and reproducible bacterial communities associate with extraradical hyphae of arbuscular mycorrhizal fungi. ISME J. 2021, 15, 2276–2288. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, W.; Chen, B. Arbuscular mycorrhiza improved drought tolerance of maize seedlings by altering photosystem II efficiency and the levels of key metabolites. Chem. Biol. Technol. Agric. 2020, 7, 20. [Google Scholar] [CrossRef]
- Yu, M.; Xie, W.; Zhang, X.; Zhang, S.; Wang, Y.; Hao, Z.; Chen, B. Arbuscular Mycorrhizal Fungi Can Compensate for the Loss of Indigenous Microbial Communities to Support the Growth of Liquorice (Glycyrrhiza uralensis Fisch.). Plants 2019, 9, 7. [Google Scholar] [CrossRef]
- Kakabouki, I.; Mavroeidis, A.; Tataridas, A.; Kousta, A.; Efthimiadou, A.; Karydogianni, S.; Katsenios, N.; Roussis, I.; Papastylianou, P. Effect of Rhizophagus irregularis on Growth and Quality of Cannabis sativa Seedlings. Plants 2021, 10, 1333. [Google Scholar] [CrossRef]
- Kokkoris, V.; Banchini, C.; Paré, L.; Abdellatif, L.; Séguin, S.; Hubbard, K.; Findlay, W.; Dalpé, Y.; Dettman, J.; Corradi, N.; et al. Rhizophagus irregularis, the model fungus in arbuscular mycorrhiza research, forms dimorphic spores. New Phytol. 2023, 242, 1771–1784. [Google Scholar] [CrossRef]
- Hestrin, R.; Kan, M.; Lafler, M.; Wollard, J.; Kimbrel, J.A.; Ray, P.; Blazewicz, S.J.; Stuart, R.; Craven, K.; Firestone, M.; et al. Plant-associated fungi support bacterial resilience following water limitation. ISME J. 2022, 16, 2752–2762. [Google Scholar] [CrossRef]
- Jin, Z.; Jiang, F.; Wang, L.; Declerck, S.; Feng, G.; Zhang, L. Arbuscular mycorrhizal fungi and Streptomyces: Brothers in arms to shape the structure and function of the hyphosphere microbiome in the early stage of interaction. Microbiome 2024, 12, 83. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Zeng, Q.; Wang, X.; Liu, S.; Wang, E.; Wu, Y.; Zeng, Y.; He, M.; Wang, Y. Chitosan hydrogel microspheres loaded with Bacillus subtilis promote plant growth and reduce chromium uptake. Int. J. Biol. Macromol. 2025, 286, 138401. [Google Scholar] [CrossRef]
- Gaudino, S.; Galas, C.; Belli, M.; Barbizzi, S.; de Zorzi, P.; Jaćimović, R.; Jeran, Z.; Pati, A.; Sansone, U. The role of different soil sample digestion methods on trace elements analysis: A comparison of ICP-MS and INAA measurement results. Accredit. Qual. Assur. 2007, 12, 84–93. [Google Scholar] [CrossRef]
- Fu, F.; Li, J.; Li, S.; Chen, W.; Ding, H.; Xiao, S.; Li, Y. Elevational distribution patterns and drivers of soil microbial diversity in the Sygera Mountains, southeastern Tibet, China. Catena 2023, 221, 106738. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S.; Hu, J.; Hou, H.; Li, K.; Fan, Q.; Wang, F.; Li, L.; Cui, X.; Liu, D.; et al. Soil sample sizes for DNA extraction substantially affect the examination of microbial diversity and co-occurrence patterns but not abundance. Soil Biol. Biochem. 2023, 177, 108902. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Xiang, X.; Sun, R.; Yang, T.; He, D.; Zhang, K.; Ni, Y.; Zhu, Y.G.; Adams, J.M.; et al. Spatial scale affects the relative role of stochasticity versus determinism in soil bacterial communities in wheat fields across the North China Plain. Microbiome 2018, 6, 27. [Google Scholar] [CrossRef]
- Liu, Y.X.; Qin, Y.; Chen, T.; Lu, M.; Qian, X.; Guo, X.; Bai, Y. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell 2021, 12, 315–330. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Liu, Y.R.; Delgado-Baquerizo, M.; Wang, J.T.; Hu, H.W.; Yang, Z.M.; He, J.Z. New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol. Biochem. 2018, 118, 35–41. [Google Scholar] [CrossRef]
- Xun, W.B.; Zhao, J.; Xue, C.; Zhang, G.S.; Ran, W.; Wang, B.R.; Shen, Q.R.; Zhang, R.F. Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ. Microbiol 2016, 18, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, H.; Wang, Z.; Liu, J.; Li, Y.; Xia, L.; Song, S. Critical steps in the restoration of coal mine soils: Microbial-accelerated soil reconstruction. J. Environ. Manag. 2024, 368, 122200. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, H.; Liu, X.; Zhang, Y.; Chao, L.; Liu, H.; Bao, Y. Changes of root AMF community structure and colonization levels under distribution pattern of geographical substitute for four Stipa species in arid steppe. Microbiol. Res. 2023, 271, 127371. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, H.; Wang, Y.; Wang, M.; Xie, X.; Chang, H.; Wang, L.; Qu, J.; Sun, K.; He, W.; et al. Mycorrhizal symbiosis modulates the rhizosphere microbiota to promote rhizobia–legume symbiosis. Mol. Plant 2021, 14, 503–516. [Google Scholar] [CrossRef]
- Gou, X.; Kong, W.; Sadowsky, M.J.; Chang, X.; Qiu, L.; Liu, W.; Shao, M.; Wei, X. Global responses of soil bacteria and fungi to inoculation with arbuscular mycorrhizal fungi. Catena 2024, 237, 107817. [Google Scholar] [CrossRef]
- Wu, S.; Fu, W.; Rillig, M.C.; Chen, B.; Zhu, Y.G.; Huang, L. Soil organic matter dynamics mediated by arbuscular mycorrhizal fungi—An updated conceptual framework. New Phytol. 2023, 242, 1417–1425. [Google Scholar] [CrossRef]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.; Zhang, N.; Miao, Y.; Shen, Q.; Zhang, R. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 2021, 9, 35. [Google Scholar] [CrossRef]
- Zampieri, E.; Sillo, F.; Metelli, G.; Cucu, M.A.; Montesano, V.; Quagliata, G.; Philipp, L.; Brescia, F.; Conte, A.; Giovannini, L.; et al. Insights into the influence of intercropping and arbuscular mycorrhizal inoculation on two modern durum wheat cultivars and their associated microbiota. Biol. Fertil. Soils 2024, 61, 85–107. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, K.; Wang, Z.; Liu, D.; Li, T.; Hou, H.; Zhang, Z.; Chen, D.; Zhang, S.; Yu, A.; et al. Soil microbial subcommunity assembly mechanisms are highly variable and intimately linked to their ecological and functional traits. Mol. Ecol. 2024, 33, e17302. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; George, T.S.; Feng, G. A core microbiome in the hyphosphere of arbuscular mycorrhizal fungi has functional significance in organic phosphorus mineralization. New Phytol. 2022, 238, 859–873. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Yang, Y.; Xu, Y.; Zhang, J.; Lu, Y. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-Q.; Meng, L.-L.; Kuča, K.; Wu, Q.-S. The mechanism of arbuscular mycorrhizal fungi-alleviated manganese toxicity in plants: A review. Plant Physiol. Biochem. 2024, 213, 108808. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Bi, Y.; Xiao, L.; Sun, J. An arbuscular mycorrhizal fungus ameliorates plant growth and hormones after moderate root damage due to simulated coal mining subsidence: A microcosm study. Environ. Sci. Pollut. Res. 2019, 26, 11053–11061. [Google Scholar] [CrossRef]
- Nikitin, D.A.; Semenov, M.V.; Chernov, T.I.; Ksenofontova, N.A.; Zhelezova, A.D.; Ivanova, E.A.; Khitrov, N.B.; Stepanov, A.L. Microbiological Indicators of Soil Ecological Functions: A Review. Eurasian Soil Sci. 2022, 55, 221–234. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Z.; Chen, J.; Xu, H.; Ma, S.; Dippold, M.A.; Kuzyakov, Y. Long-term nitrogen and phosphorus fertilization reveals that phosphorus limitation shapes the microbial community composition and functions in tropical montane forest soil. Sci. Total Environ. 2023, 854, 158709. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Zhang, X.; Ju, W.; Duan, C.; Guo, X.; Wang, Y.; Fang, L. Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]
- Benidire, L.; Madline, A.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere 2021, 262, 127803. [Google Scholar] [CrossRef]
- Hacquard, S.; Kracher, B.; Hiruma, K.; Münch, P.C.; Garrido-Oter, R.; Thon, M.R.; Weimann, A.; Damm, U.; Dallery, J.-F.; Hainaut, M.; et al. Survival trade-offs in plant roots during colonization by closely related beneficial and pathogenic fungi. Nat. Commun. 2016, 7, 11362. [Google Scholar] [CrossRef]


| Treatment | Shoot Dry Weight (g·pot −1) | Root Dry Weight (g·pot −1) |
|---|---|---|
| –M | 13.3 ± 1.8 b | 3.2 ± 0.3 a |
| +M | 20.5 ± 0.5 a | 4.3 ± 0.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Jing, X.; Wang, Y.; Ma, Y.; Shu, X.; Fu, W.; Xing, S.; Liu, W.; Ye, Q.; Zhu, Y.; et al. Effect of Inoculation with Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis BGC AH01) on the Soil Bacterial Community Assembly. J. Fungi 2025, 11, 739. https://doi.org/10.3390/jof11100739
Wang X, Jing X, Wang Y, Ma Y, Shu X, Fu W, Xing S, Liu W, Ye Q, Zhu Y, et al. Effect of Inoculation with Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis BGC AH01) on the Soil Bacterial Community Assembly. Journal of Fungi. 2025; 11(10):739. https://doi.org/10.3390/jof11100739
Chicago/Turabian StyleWang, Xueli, Xuemin Jing, Yan Wang, Youran Ma, Xiangyang Shu, Wei Fu, Shuping Xing, Weijia Liu, Qinxin Ye, Yalan Zhu, and et al. 2025. "Effect of Inoculation with Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis BGC AH01) on the Soil Bacterial Community Assembly" Journal of Fungi 11, no. 10: 739. https://doi.org/10.3390/jof11100739
APA StyleWang, X., Jing, X., Wang, Y., Ma, Y., Shu, X., Fu, W., Xing, S., Liu, W., Ye, Q., Zhu, Y., Ren, P., Zhang, X., Chen, B., & Wang, X. (2025). Effect of Inoculation with Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis BGC AH01) on the Soil Bacterial Community Assembly. Journal of Fungi, 11(10), 739. https://doi.org/10.3390/jof11100739






