Evaluation of Fungal Sensitivity to Biosynthesized Copper-Oxide Nanoparticles (CuONPs) in Grapevine Tissues and Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Nanoparticles
2.2. Characterization of Nanoparticles
2.3. Application of Nanoparticles in Grapevine Plants
2.4. Isolation of Fungi
2.5. Virulence Test
2.6. In Vitro Antifungal Activity of Nanoparticles
2.7. Determination of Cu in Plant Tissues Treated with CuONPs
2.8. Processing of Plant Tissue for Transmission Electron Microscopy
2.9. Statistical Analysis
3. Results
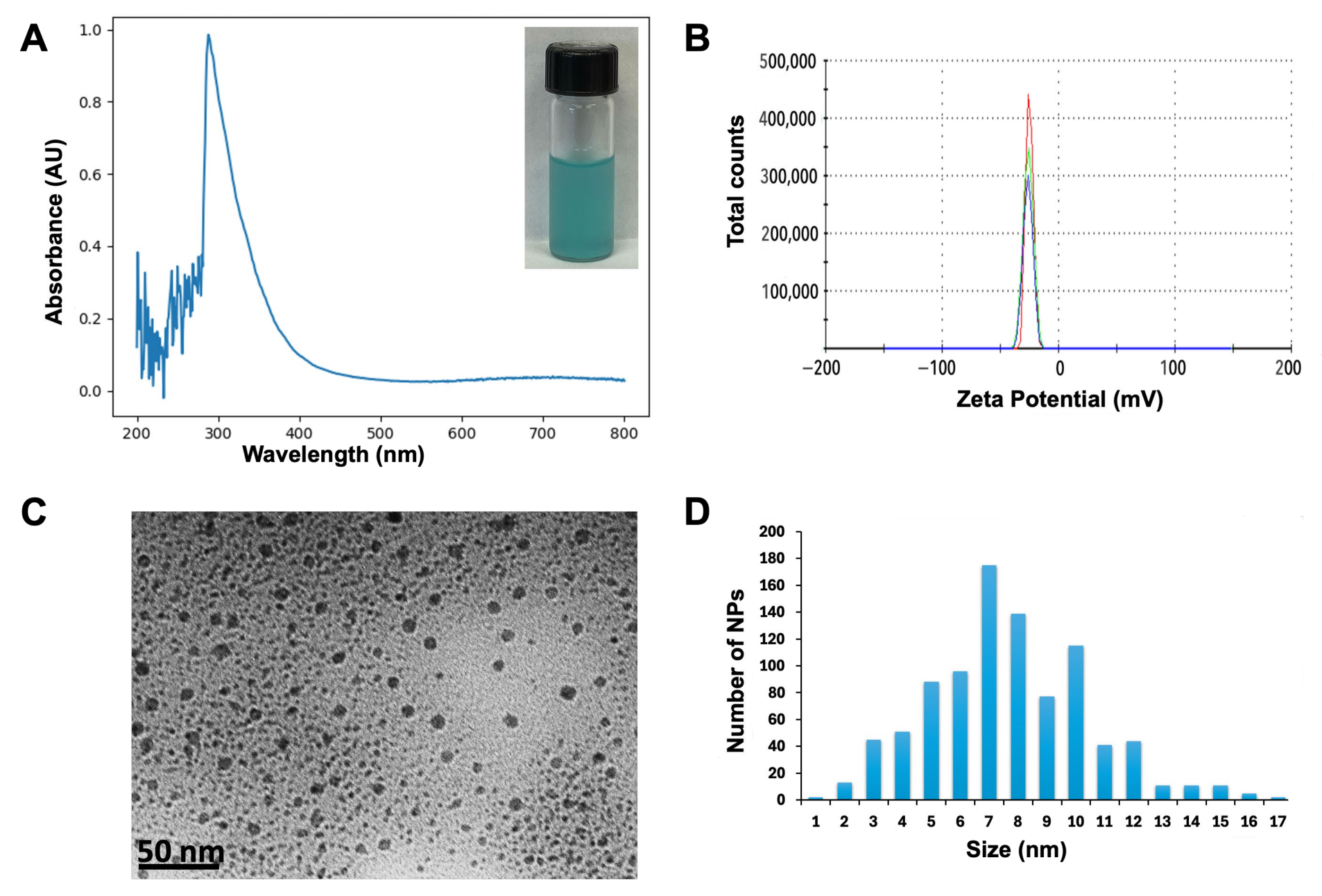
3.1. Nanoparticles Characterization
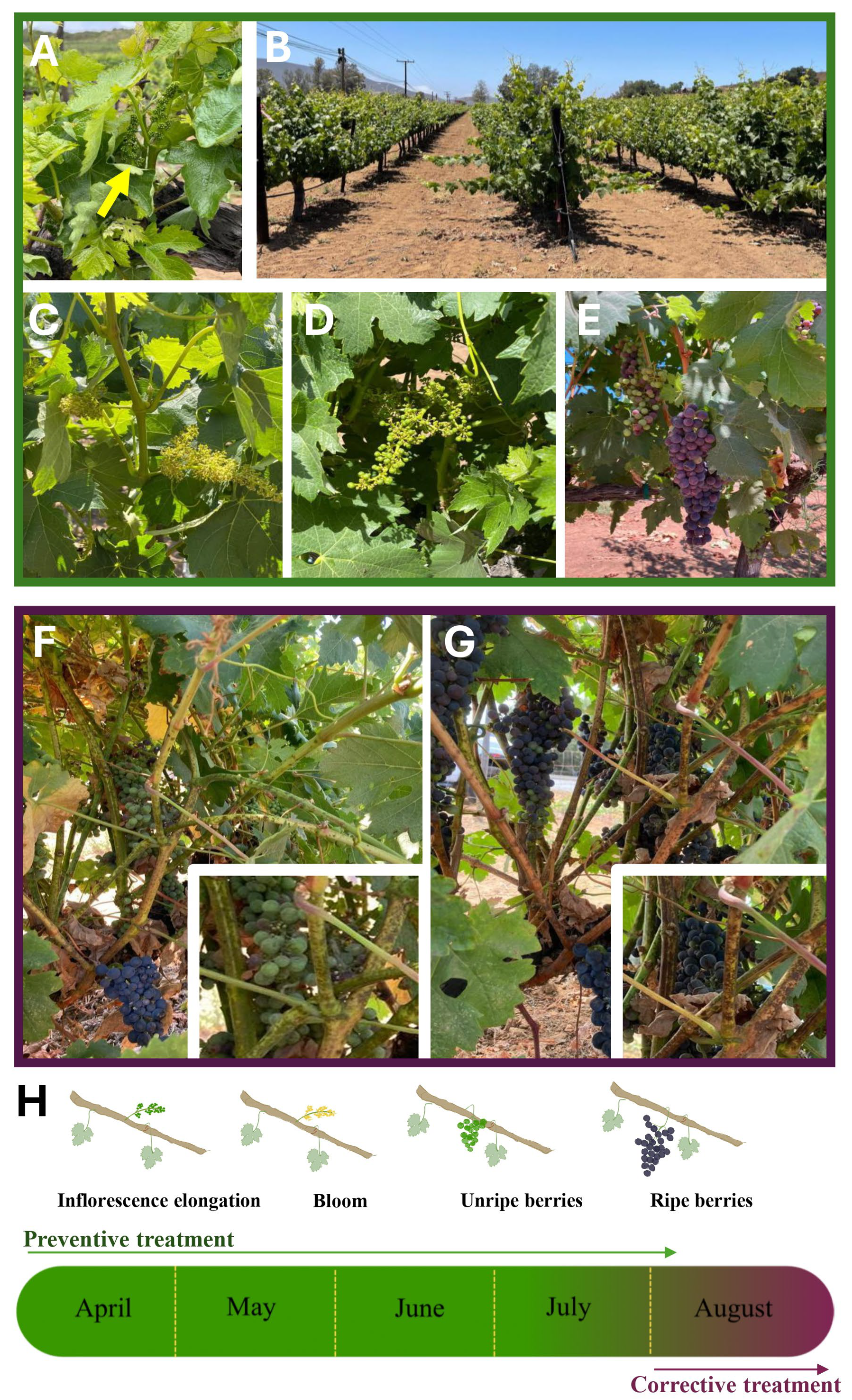
3.2. Plants Exposed to CuONPs as Preventive or Corrective Treatment Showed No Signs of Decay
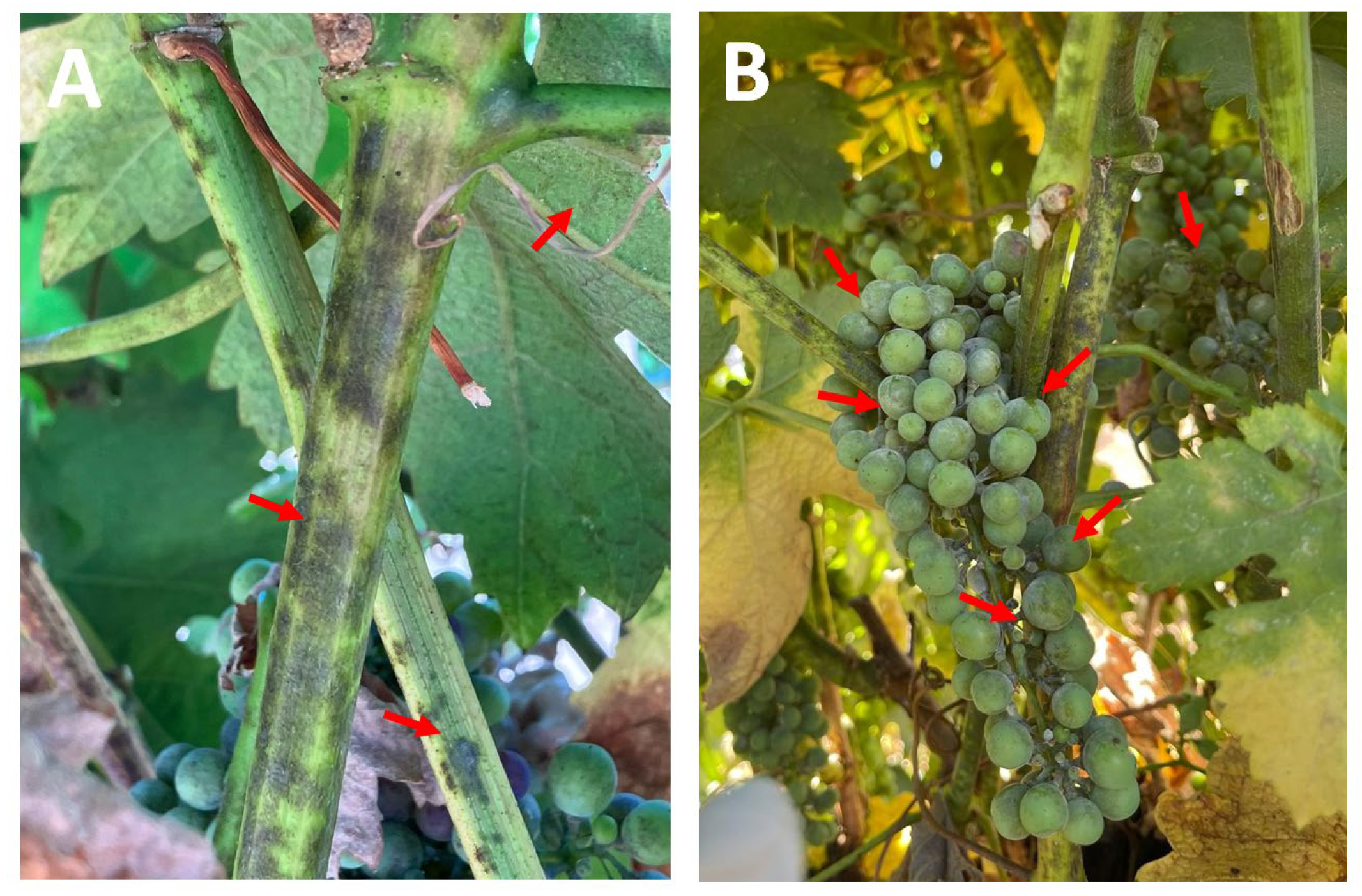
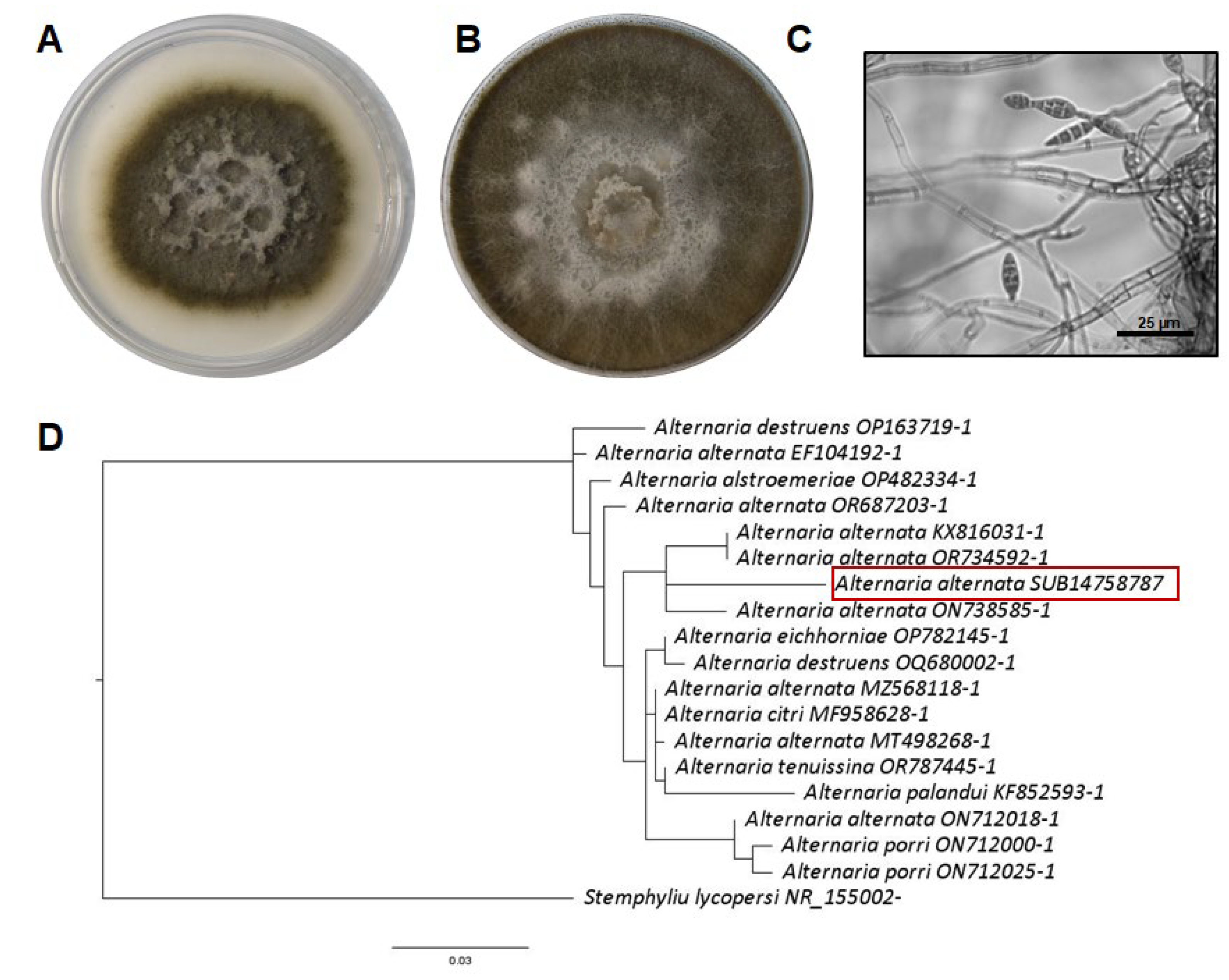
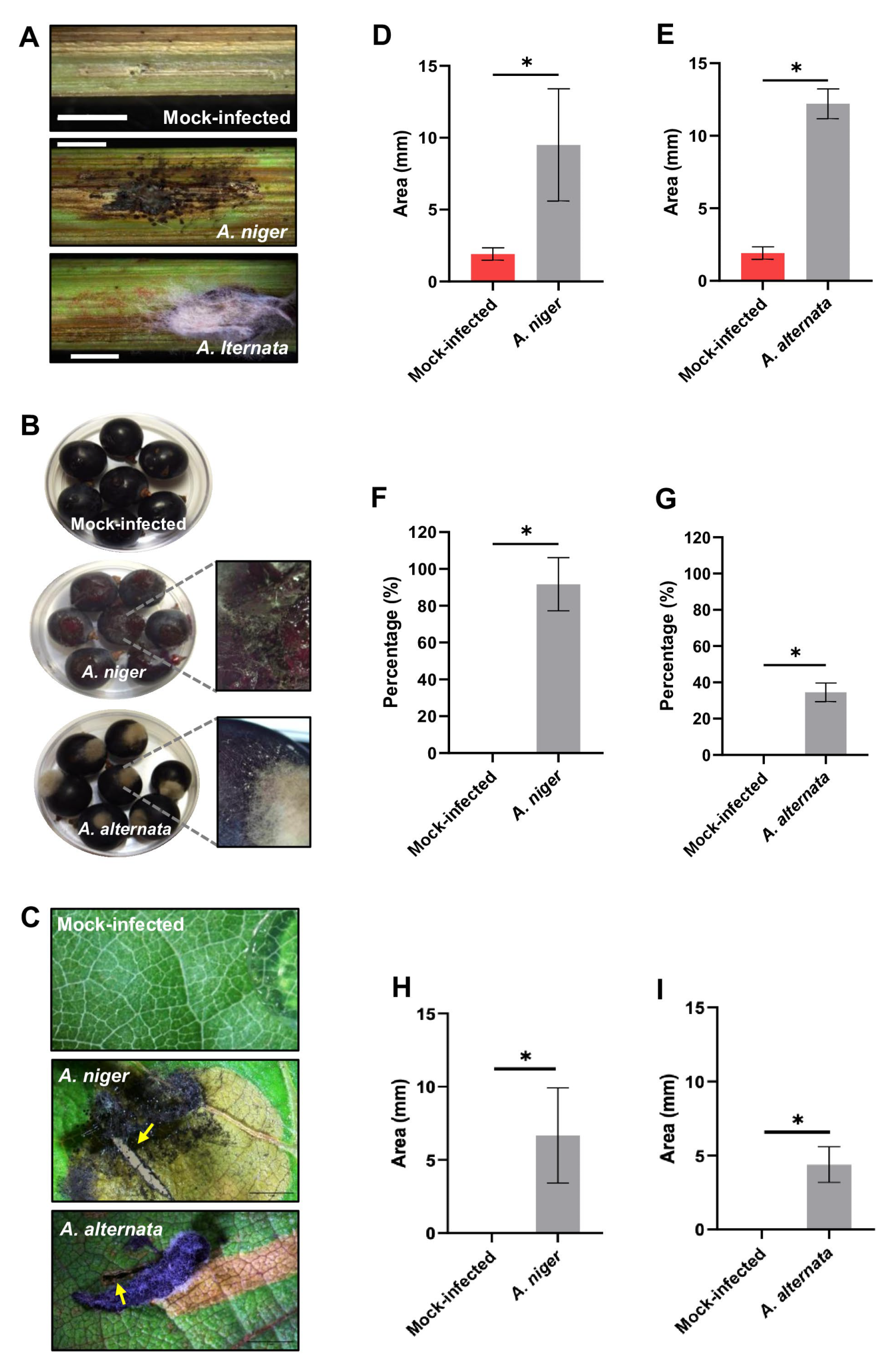
3.3. Plant Symptoms and Fungal Identification
3.4. Virulence Analysis of Fungal Isolates
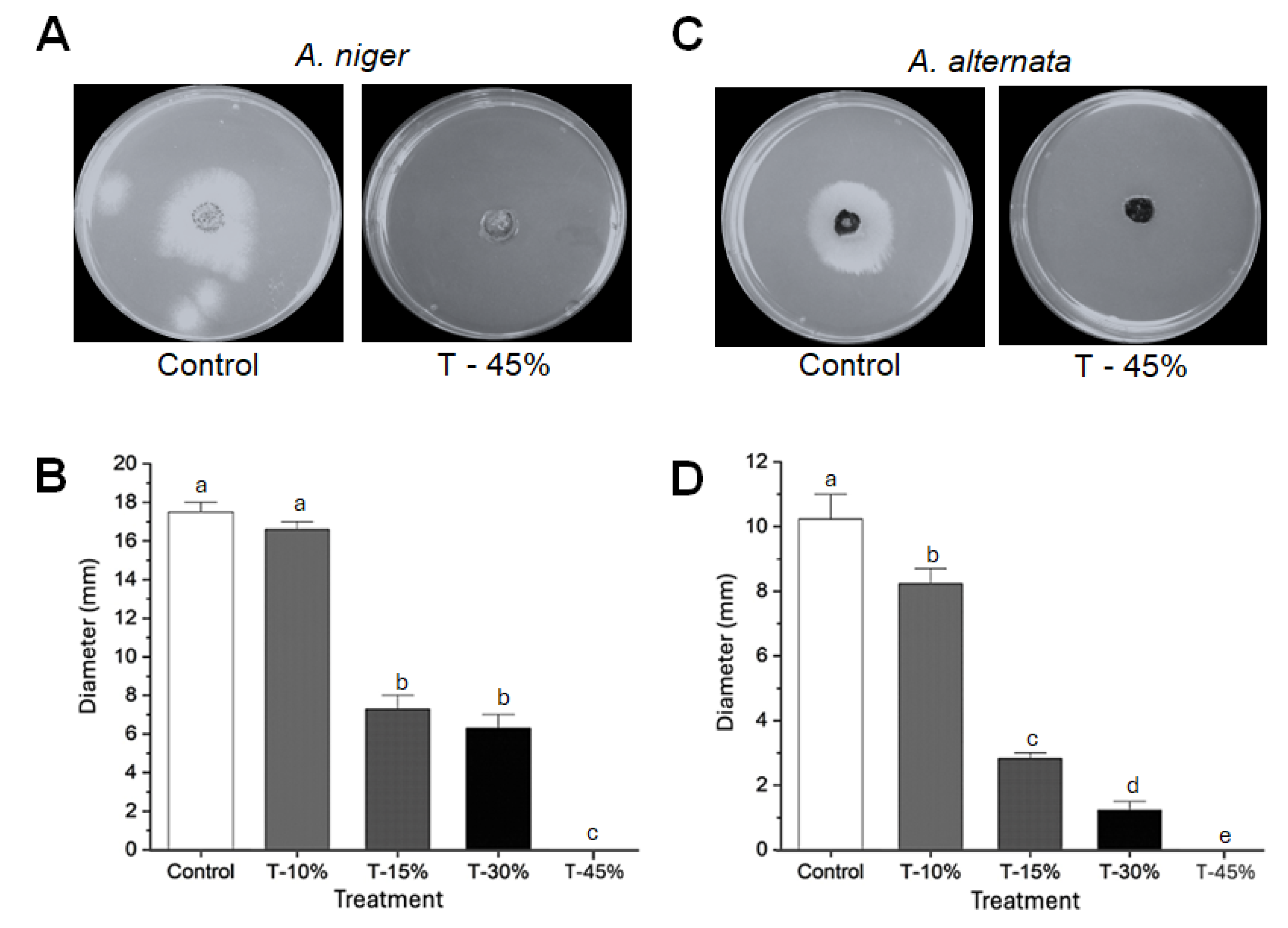
3.5. CuONPs Showed Antifungal Activity In Vitro
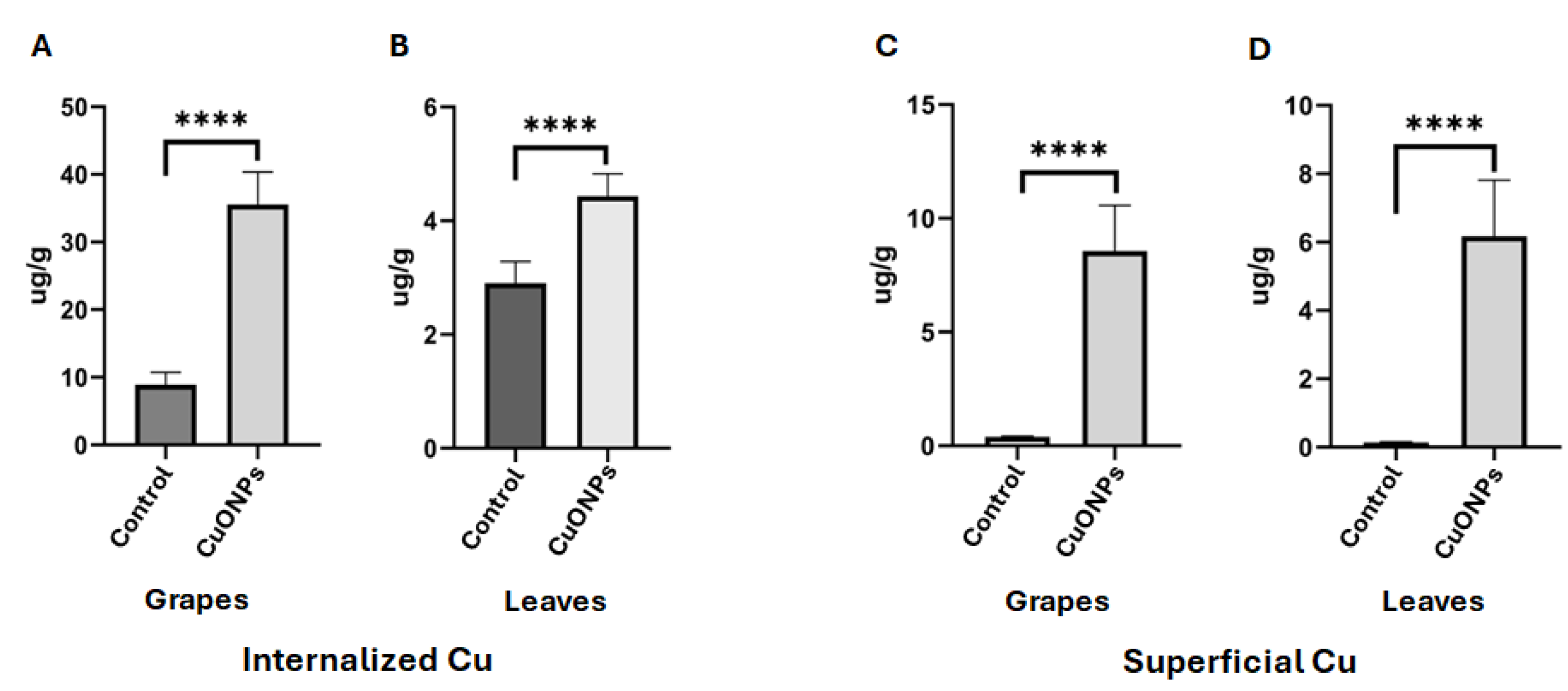
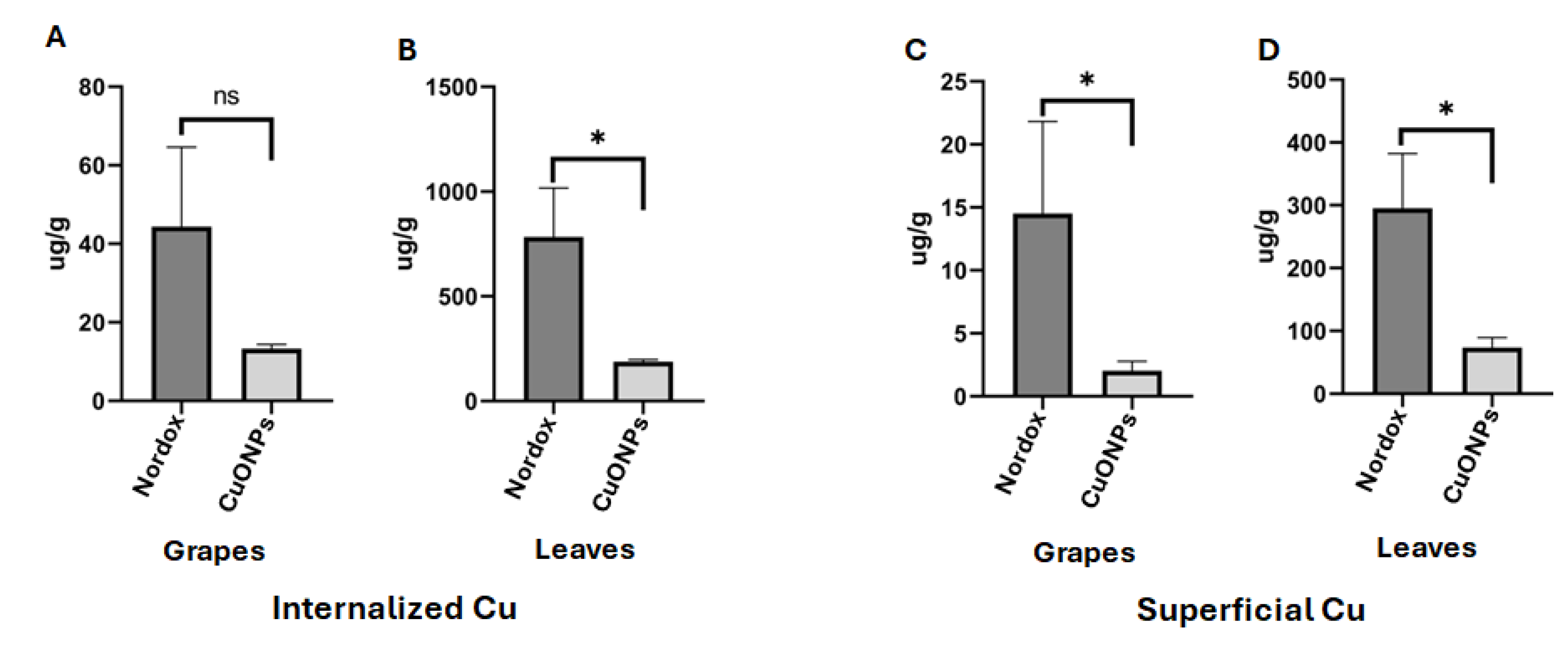
3.6. Plants Treated with CuONPs Showed Low Concentration of Cu in Tissues
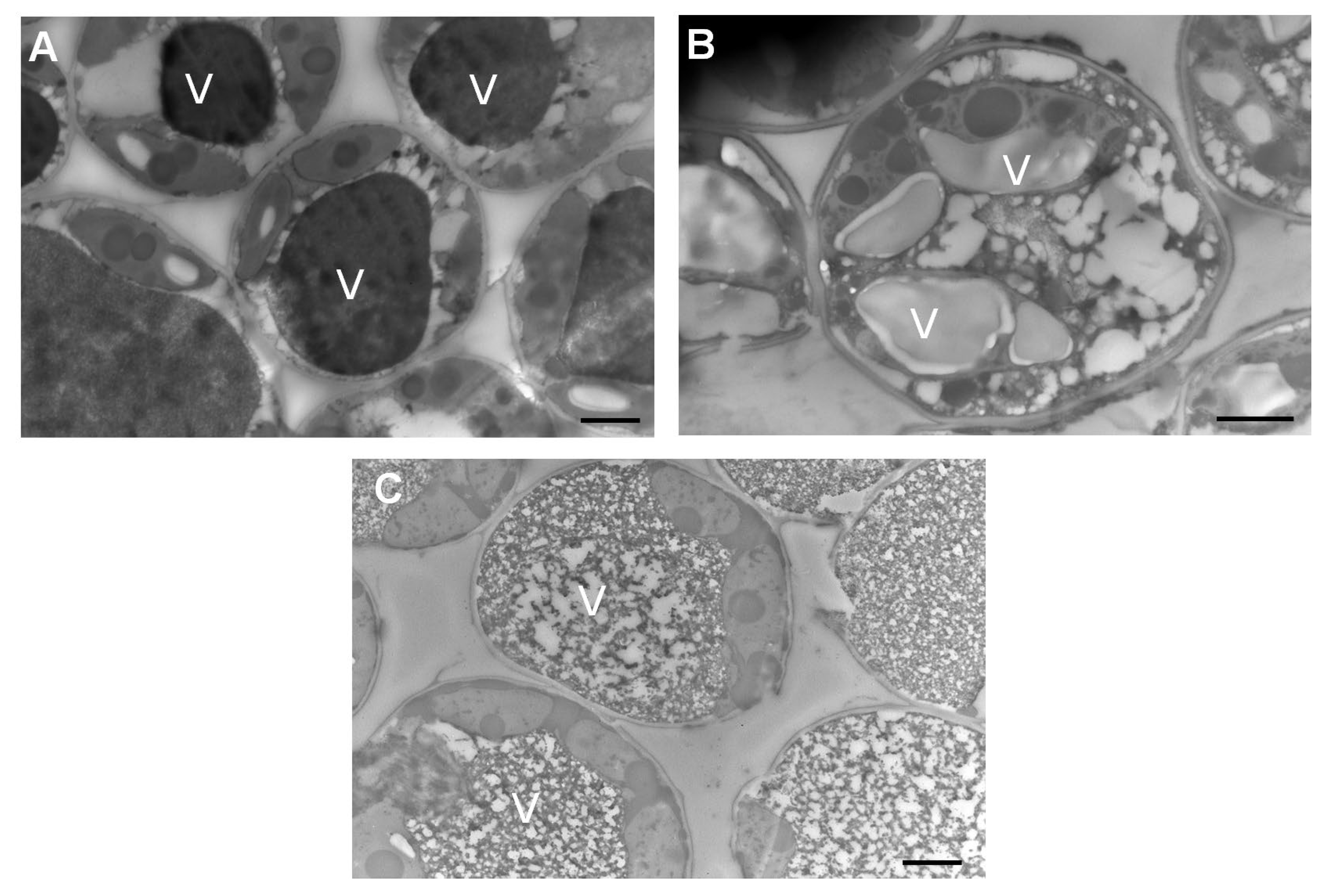
3.7. Leaves Exposed to CuONPs Maintain Cell Shape, but Excessive Exposure Provokes Apparent Metallic Accumulation in Vacuoles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gawande, A.; Sherekar, S. Grape dataset: A dataset for disease prediction and classification for machine learning applications through environmental parameters. Data Brief 2024, 54, 110546. [Google Scholar] [CrossRef]
- USDA. Foreign Agricultural Service. Fresh Apples, Grapes, and Pears: World Markets and Trade. 2024. Available online: https://www.fas.usda.gov/sites/default/files/2024-12/fruit.pdf (accessed on 13 March 2025).
- USDA. Foreign Agricultural Service. Production-Table Grapes. 2025. Available online: https://www.fas.usda.gov/data/production/commodity/0575100 (accessed on 4 August 2025).
- Gasperin-Bulbarela, J.; Licea-Navarro, A.F.; Pino-Villar, C.; Hernández-Martínez, R.; Carrillo-Tripp, J. First report of grapevine red blotch virus in Mexico. Plant Dis. 2019, 103, 381. [Google Scholar] [CrossRef]
- Garcia-Reséndiz, K.G.; Carrillo-Tripp, J. Grapevine viruses in Mexico: Studies and reports. Agro Product. 2022. [Google Scholar] [CrossRef]
- Rangel-Montoya, E.A.; Hernandez-Martinez, R. Identification and pathogenicity of Aspergillus species associated with vine canker in Mexico. J. Plant Pathol. 2024, 106, 1311–1324. [Google Scholar] [CrossRef]
- Rangel-Montoya, E.A.; Candolfi-Arballo, O.; Obrador-Sánchez, J.A.; Valenzuela-Solano, C.; Hernandez-Martinez, R. Enhancing epidemiological knowledge of Botryosphaeriaceae in Mexican vineyards. Phytopathol. Mediterr. 2024, 63, 191–206. [Google Scholar] [CrossRef]
- Berbegal, M.; Blasco, A.; Gaume, G.; Amorós, P.; Fernandes, A.; Ros-Lis, J.V.; Armengol, J. Evaluation of electrolyzed water to control fungal trunk pathogens in grapevine nurseries. Pest Manag. Sci. 2025, 81, 1740–1751. [Google Scholar] [CrossRef]
- Gawande, A.; Sherekar, S.; Gawande, R. Early prediction of grape disease attack using a hybrid classifier in association with IoT sensors. Heliyon 2024, 10, e38093. [Google Scholar] [CrossRef]
- Rajput, P.; Thakur, A.; Devi, P. Emerging agrochemicals contaminants: Current status, challenges, and technological solutions. Agrochemicals Detection, Treatment and Remediation. In Agrochemicals Detection, Treatment and Remediation; Elsvier: Amsterdam, The Netherlands, 2020; pp. 117–142. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Yin, Y.; Miao, J.; Shao, W.; Liu, X.; Zhao, Y.; Ma, Z. Fungicide resistance: Progress in understanding mechanisms, monitoring, and management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef]
- Castro-Longoria, E. Biosynthesis of Metal-Based Nanoparticles by Trichoderma and Its Potential Applications. In Advances in Trichoderma Biology for Agricultural Applications; Amaresan, N., Sankaranarayanan, A., Dwivedi, M.K., Druzhinina, I.S., Eds.; Fungal Biology; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Garcia-Marin, L.E.; Juarez-Moreno, K.; Vilchis-Nestor, A.R.; Castro-Longoria, E. Highly antifungal activity of biosynthesized copper oxide nanoparticles against Candida albicans. Nanomaterials 2022, 12, 3856. [Google Scholar] [CrossRef]
- Rivera-Mendoza, D.; Quiñones, B.; Huerta-Saquero, A.; Castro-Longoria, E. Antimicrobial activity of green synthesized silver and copper oxide nanoparticles against the foodborne pathogen Campylobacter jejuni. Antibiotics 2024, 13, 650. [Google Scholar] [CrossRef]
- Flores-Rábago, K.M.; Rivera-Mendoza, D.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Castro-Longoria, E. Antibacterial activity of biosynthesized copper oxide nanoparticles (CuONPs) using Ganoderma sessile. Antibiotics 2023, 12, 1251. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Sadek, M.E.; Shabana, Y.M.; Sayed-Ahmed, K.; Abou-Tabl, A.H. Antifungal activities of sulfur and copper nanoparticles against cucumber postharvest diseases caused by Botrytis cinerea and Sclerotinia sclerotiorum. J. Fungi 2022, 8, 412. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Gaba, S.; Rai, A.K.; Varma, A.; Prasad, R.; Goel, A. Biocontrol potential of mycogenic copper oxide nanoparticles against Alternaria brassicae. Front. Chem. 2022, 10, 966396. [Google Scholar] [CrossRef] [PubMed]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- BokowskI, L.V.V.; Sobrinho, R.B.; Armijo, C.J.; Dani, C.; Henriques, J.A.; Funchal, C. Method validation for determination of metals in Vitis labrusca L. grapevine leaf extracts by inductively coupled plasma mass spectrometry (ICP-MS). An. Acad. Bras. Ciências 2016, 88, 2247–2255. [Google Scholar] [CrossRef]
- Celotti, E.; Sadeghian, F.; Comuzzo, P.; Iacumin, L.; Zanzotti, R.; Giovannini, O.; Trioli, G.; Torraco, N.P.; Visintini, O.; Mian, G. Study for the development of a rapid and non-destructive method for copper analysis in vineyards towards a precision fungal defense strategy. BIO Web Conf. 2023, 68, 01025. [Google Scholar]
- Mian, G.; Comuzzo, P.; Iacumin Lcopperotti, R.; Celotti, E. Study to Optimize the Effectiveness of Copper Treatments for a Low Impact Viticulture. INFOWINE 2021. [Google Scholar] [CrossRef]
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Producción de Uva en México; Secretaría de Agricultura y Desarrollo Rural: Mexico City, Mexico, 2022; Available online: https://www.gob.mx/cms/uploads/attachment/file/771603/Producci_n_Uva_en_M_xico.pdf (accessed on 5 April 2025).
- García-Reséndiz, K.G.; Moyano-Briones, G.; López-Simancas, P.; Úrbez-Torres, J.R.; Hernández-Martínez, R.; Carrillo-Tripp, J. Epidemiological survey of grapevine leafroll and red blotch diseases in Baja California, Mexico. Am. J. Enol. Vitic. 2025, 76, 0760007. [Google Scholar] [CrossRef]
- Szegedi, E.; Civerolo, E.L. Bacterial disease of grapevine. Int. J. Hortic. Sci. 2011, 17, 45–49. [Google Scholar] [CrossRef]
- Fröbel, S.; Zyprian, E. Colonization of different grapevine tissues by Plasmopara viticola—A histological study. Front. Plant Sci. 2019, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Viret, O.; Keller, M.; Jaudzems, V.G.; Cole, F.M. Botrytis cinerea infection of grape flowers: Light and electron microscopical studies of infection sites. Phytopathology 2004, 94, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; Schena, L.; De Cicco, V.; Ippolito, A. Early detection of Botrytis cinerea latent infections as a tool to improve postharvest quality of table grapes. Postharvest Biol. Technol. 2012, 68, 64–71. [Google Scholar] [CrossRef]
- Swift, J.G.; Buttrose, M.S.; Possingham, J.V. Stomata and starch in grape berries. VITIS—J. Grapevine Res. 1973, 12, 38. [Google Scholar] [CrossRef]
- Nakagawa, S.; Komatsu, H.; Yuda, E.J. A Study of micro-morphology of grape berry surface during their development with special reference to stoma. J. Jpn. Soc. Hort. Sci. 1980, 49, 1–7. [Google Scholar] [CrossRef]
- Gonçalves da Silva, A.; Antonio Pavan, M.; Saraiva Muniz, M.; Antonio Tonin, T.A.; Pelizer, T. Nutrient availability in the soil and its absorption, transport, and redistribution in vines. Commun. Soil Sci. Plant Anal. 2008, 39, 1507–1516. [Google Scholar] [CrossRef]
- Teszlák, P.; Kocsis, M.; Scarpellini, A.; Jakab, G.; Kőrösi, L. Foliar exposure of grapevine (Vitis vinifera L.) to TiO2 nanoparticles under field conditions: Photosynthetic response and flavonol profile. Photosynthetica 2018, 56, 1378–1386. [Google Scholar] [CrossRef]
- Herman, E.M.; Hankins, C.N.; Shannon, L.M. Bark and leaf lectins of Sophora japonica are sequestered in protein-storage vacuoles. Plant Physiol. 1988, 86, 1027–1031. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A. Pesticide residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 967–973. [Google Scholar] [CrossRef]
- Provenzano, M.R.; El Bilali, H.; Simeone, V.; Baser, N.; Mondelli, D.; Cesari, G. Copper contents in grapes and wines from a Mediterranean organic vineyard. Food Chem. 2010, 122, 1338–1343. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Souza-da Costa, B.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Nanotechnology: Recent advances in viticulture and enology. J. Sci. Food Agric. 2021, 101, 6156–6166. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A. The role of nanoparticles in wine science: Innovations and applications. Nanomaterials 2025, 15, 175. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Ramírez-Rodríguez, G.B.; Carmona, F.J.; Martínez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainable viticulture: Foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2021, 101, 1307–1313. [Google Scholar] [CrossRef]
- Gaiotti, F.; Lucchetta, M.; Rodegher, G.; Lorenzoni, D.; Longo, E.; Boselli, E.; Cesco, S.; Belfiore, N.; Lovat, L.; Delgado-López, J.M.; et al. Urea-doped calcium phosphate nanoparticles as sustainable nitrogen nanofertilizers for viticulture: Implications on yield and quality of pinot gris grapevines. Agronomy 2021, 11, 1026. [Google Scholar] [CrossRef]
- Vizitiu, D.E.; Sardarescu, D.I.; Fierascu, I.; Fierascu, R.C.; Soare, L.C.; Ungureanu, C.; Buciumeanu, E.C.; Guta, I.C.; Pandelea, L.M. Grapevine plants management using natural extracts and phytosynthesized silver nanoparticles. Materials 2022, 15, 8188. [Google Scholar] [CrossRef]
- Rashad, Y.M.; El-Sharkawy, H.H.; Belal, B.E.; Abdel Razik, E.S.; Galilah, D.A. Silica nanoparticles as a probable anti-oomycete compound against downy mildew, and yield and quality enhancer in grapevines: Field evaluation, molecular, physiological, ultrastructural, and toxicity investigations. Front. Plant Sci. 2021, 12, 763365. [Google Scholar] [CrossRef]
- Kassemeyer, H.H. Fungi of Grapes. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, F., Fröhlich, J., Eds.; Springer International Publishing AG: Basel, Switzerland, 2017; pp. 103–132. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Soto, D.; Campos-Jiménez, E.; Cabello-Pasini, A.; Garcia-Marin, L.E.; Meza-Villezcas, A.; Castro-Longoria, E. Evaluation of Fungal Sensitivity to Biosynthesized Copper-Oxide Nanoparticles (CuONPs) in Grapevine Tissues and Fruits. J. Fungi 2025, 11, 719. https://doi.org/10.3390/jof11100719
Martínez-Soto D, Campos-Jiménez E, Cabello-Pasini A, Garcia-Marin LE, Meza-Villezcas A, Castro-Longoria E. Evaluation of Fungal Sensitivity to Biosynthesized Copper-Oxide Nanoparticles (CuONPs) in Grapevine Tissues and Fruits. Journal of Fungi. 2025; 11(10):719. https://doi.org/10.3390/jof11100719
Chicago/Turabian StyleMartínez-Soto, Domingo, Erisneida Campos-Jiménez, Alejandro Cabello-Pasini, Luis Enrique Garcia-Marin, Anaid Meza-Villezcas, and Ernestina Castro-Longoria. 2025. "Evaluation of Fungal Sensitivity to Biosynthesized Copper-Oxide Nanoparticles (CuONPs) in Grapevine Tissues and Fruits" Journal of Fungi 11, no. 10: 719. https://doi.org/10.3390/jof11100719
APA StyleMartínez-Soto, D., Campos-Jiménez, E., Cabello-Pasini, A., Garcia-Marin, L. E., Meza-Villezcas, A., & Castro-Longoria, E. (2025). Evaluation of Fungal Sensitivity to Biosynthesized Copper-Oxide Nanoparticles (CuONPs) in Grapevine Tissues and Fruits. Journal of Fungi, 11(10), 719. https://doi.org/10.3390/jof11100719







