Application and Mechanism of Action of Carvacrol Against Aspergillus niger Causing Postharvest Rot of Garlic Scapes (Allium sativum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Pathogen Isolation and Molecular Characterization
2.2.1. Pathogen Isolation
2.2.2. Pathogenicity Test
2.2.3. Morphological Characterization
2.2.4. Molecular Biological Characterization
2.3. In Vitro Antifungal Activity Test
2.4. Effect of Carvacrol on the Hyphae Morphology
2.5. Transcriptome and Proteomics Analysis
3. Results
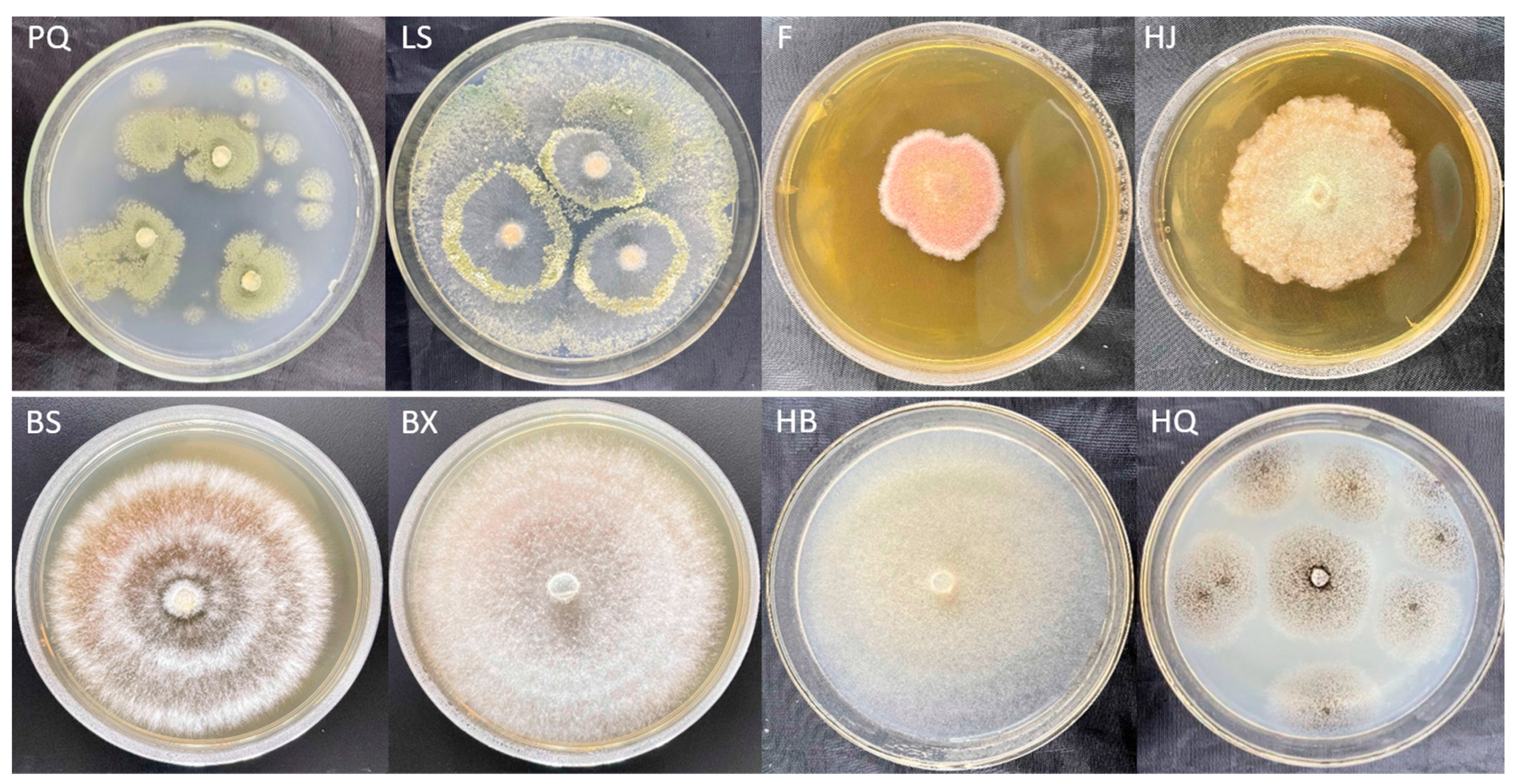
3.1. Pathogen Isolation and Pathogenicity Test
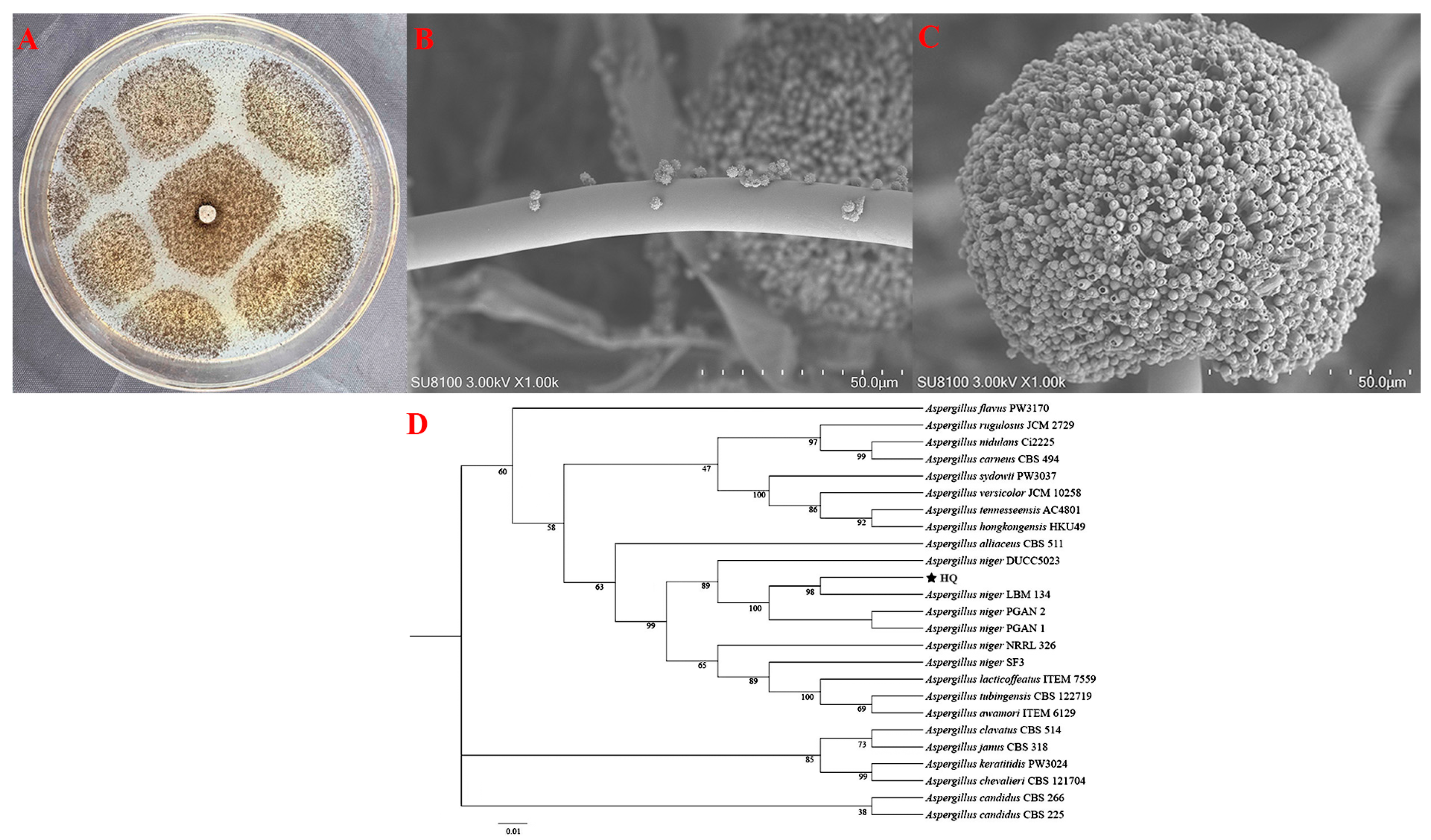
3.2. Fungal Identification
3.3. In Vitro Antifungal Activity of Carvacrol Against Aspergillus niger
3.4. Effect of Carvacrol on the Hyphae Morphology of Aspergillus niger
3.5. Quality Check of Transcriptome Sequencing Data
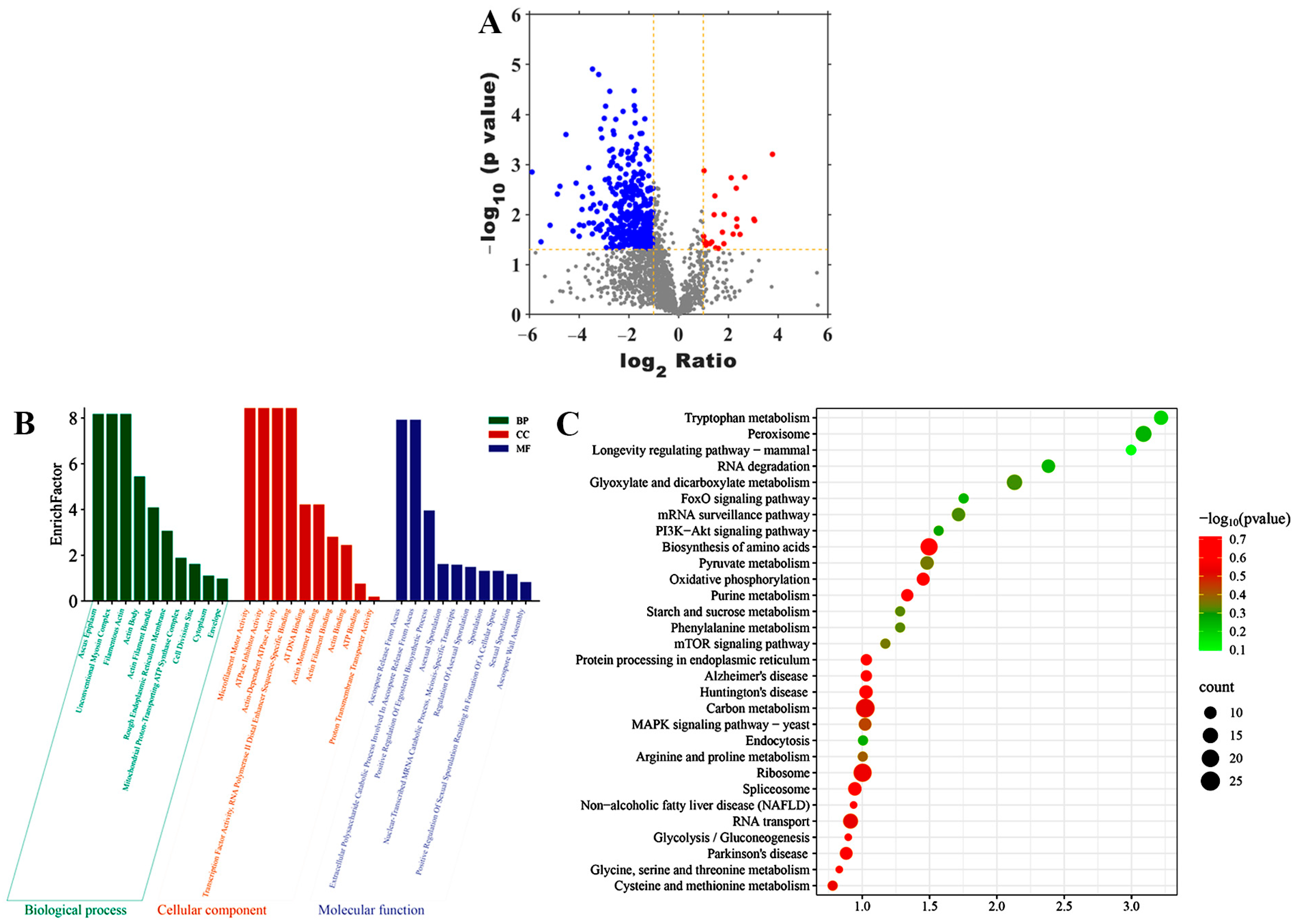
3.6. DEGs Identification and Bioinformatics Analysis
3.7. DEPs Identification and Bioinformatics Analysis
3.8. Combined Analysis of Transcriptomic and Proteomic Technology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.R.; Hu, Y.F.; Yan, R.X.; Hu, H.Y. Effect of high carbon dioxide injury on the physiological characteristics of fresh-cut garlic scapes. Sci. Hortic. 2019, 250, 359–365. [Google Scholar] [CrossRef]
- Li, G.Q.; Chen, P.F.; Zhao, Y.T.; Zeng, Q.H.; Ou, S.Y.; Zhang, Y.H.; Wang, P.C.; Chen, N.H.; Ou, J.Y. Isolation, structural characterization and anti-oxidant activity of a novel polysaccharide from garlic bolt. Carbohyd. Polym. 2021, 267, 118194. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.P.; Ho, C.T.; Lan, Y.Q.; Xiao, J.; Lu, M.W. Bioavailability, health benefits, and delivery systems of allicin: A review. J. Agric. Food Chem. 2023, 71, 19207–19220. [Google Scholar] [CrossRef]
- Huang, X.Q.; Ren, J.; Li, P.H.; Feng, S.; Dong, P.; Ren, M.Z. Potential of microbial endophytes to enhance the resistance to postharvest diseases of fruit and vegetables. J. Sci. Food Agric. 2021, 101, 1744–1757. [Google Scholar] [CrossRef]
- Wang, Z.S.; Sui, Y.; Li, J.S.; Tian, X.L.; Wang, Q. Biological control of postharvest fungal decays in citrus: A review. Criti. Rev. Food Sci. Nutr. 2022, 62, 861–870. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Rayón-Díaz, E.; Hernández-Montiel, L.G.; Sánchez-Burgos, J.A.; Zamora-Gasga, V.M.; González-Estrada, R.R.; Gutiérrez-Martínez, P. Natural Compounds and Derivates: Alternative Treatments to Reduce Post-Harvest Losses in Fruits. AgriEngineering 2024, 6, 1022–1042. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Tjeerdsma-van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural requirements for the antimicrobial activity of carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef]
- Shu, C.; Kim-Lee, B.; Sun, X. Chitosan Coating Incorporated with Carvacrol Improves Postharvest Guava (Psidium guajava) Quality. Horticulturae 2024, 10, 80. [Google Scholar] [CrossRef]
- Yang, X.R.; Fang, S.Y.; Xie, Y.; Mei, J.; Jing Xie, J. Preservative Effects of Flaxseed Gum-Sodium Alginate Active Coatings Containing Carvacrol on Quality of Turbot (Scophthalmus maximus) during Cold Storage. Coatings 2024, 14, 338. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Stammati, A.; de Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3529–4088. [Google Scholar] [CrossRef]
- Falkow, S. Molecular Koch’s Postulates Applied to Microbial Pathogenicity. Rev. Infect. Dis. 1988, 10, S274–S276. [Google Scholar] [CrossRef]
- Bello, M.A.; Ruiz-León, Y.; Sandoval-Sierra, J.V.; Rezinciuc, S.; Diéguez-Uribeondo, J. Scanning Electron Microscopy (SEM) Protocols for Problematic Plant, Oomycete, and Fungal Samples. J. Vis. Exp. 2017, 120, 55031. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Li, X.L.; Wang, T.Y.; Zeng, M.D.; Gu, H.; Li, L.L.; Li, C.W. First report of powdery mildew caused by Erysiphe astragali on Gueldenstaedtia stenophylla in China. Plant Dis. 2020, 104, 3067. [Google Scholar] [CrossRef]
- Husna, A.; Zakaria, L.; Mohamed Nor, N.M.I. Fusarium commune associated with wilt and root rot disease in rice. Plant Pathol. 2020, 70, 123–132. [Google Scholar] [CrossRef]
- Kumar, S.; Dudley, J.; Nei, M.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.S.; Li, P.; Liu, Q.; Zhang, F.M.; Wang, D.; Mei, W.T.; Wang, X. Design, Synthesis, Antibacterial, and Antifungal Activity Evaluation of 1,3,4-Oxadiazole Thioether Derivatives Incorporating a Methylsulfonyl Moiety. Russ. J. Gen. Chem. 2025, 95, 742–747. [Google Scholar] [CrossRef]
- Chattapadhyay, T.K.; Dureja, P. Antifungal activity of 4-methyl-6-alkyl-2H-pyran-2-ones. J. Agric. Food Chem. 2006, 54, 2129–2133. [Google Scholar] [CrossRef]
- Kechin, A.; Boyarskikh, U.; Kel, A.; Filipenko, M. cutPrimers: A new tool for accurate cutting of primers from reads of targeted next generation sequencing. J. Comput. Biol. 2017, 24, 1138–1143. [Google Scholar] [CrossRef]
- Sui, Y.; Li, S.P.; Fu, X.Q.; Joe Zhao, Z.Z.; Xing, S. Bioinformatics analyses of combined databases identify shared differentially expressed genes in cancer and autoimmune disease. J. Transl. Med. 2023, 21, 109. [Google Scholar] [CrossRef]
- Teng, Z.Y.; Yu, Y.J.; Zhu, Z.J.; Hong, S.B.; Yang, B.X.; Zang, Y.X. Melatonin elevated Sclerotinia sclerotiorum resistance via modulation of ATP and glucosinolate biosynthesis in Brassica rapa ssp. pekinensis. J. Proteomics 2021, 243, 104264. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Prot. Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Yu, L.; Wang, W.L.; Zeng, S.; Chen, Z.; Yang, A.M.; Shi, J.; Zhao, X.Z.; Song, B.A. Label-free quantitative proteomics analysis of cytosinpeptidemycin responses in southern rice black-streaked dwarf virus-infected rice. Pestic. Biochem. Phys. 2018, 147, 20–26. [Google Scholar] [CrossRef]
- Yang, M.W.; Yu, L.; Li, P.; Mu, B.; Wen, J. Effect of carvacrol on the inhibitory of gas-producing spoilage microbes and volatile flavor in fermented pepper (Capsicum annuum L.). LWT 2024, 206, 116621. [Google Scholar] [CrossRef]
- Zhang, J.H.; Ma, S.; Du, S.L.; Chen, S.Y.; Sun, H.L. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Šimović, M.; Delaš, F.; Gradvol, V.; Kocevski, D.; Pavlović, H. Antifungal effect of eugenol and carvacrol against foodborne pathogens Aspergillus carbonarius and Penicillium roqueforti in improving safety of fresh-cut watermelon. J. Intercult. Ethnopharmacol. 2014, 3, 91–96. [Google Scholar] [CrossRef]
- Jiang, X.D.; Feng, K.J.; Yang, X.P. In vitro Antifungal Activity and Mechanism of Action of Tea Polyphenols and Tea Saponin against Rhizopus stolonifer. J. Mol. Microbiol. Biotech. 2015, 25, 269–276. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Inter. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- Yue, J.C.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Inter. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Roncero, C.; Celador, R.; Sánchez, N.; García, P.; Sánchez, Y. The Role of the Cell Integrity Pathway in Septum Assembly in Yeast. J. Fungi 2021, 7, 729. [Google Scholar] [CrossRef]
- Thomas, C.F., Jr.; Vohra, P.K.; Park, J.G.; Puri, V.; Limper, A.H.; Kottom, T.J. Pneumocystis carinii BCK1 functions in a mitogen-activated protein kinase cascade regulating fungal cell-wall assembly. FEBS Lett. 2003, 548, 59–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.; Zhu, J.; Ying, S.H.; Feng, M.G. Three Mitogen-Activated Protein Kinases Required for Cell Wall Integrity Contribute Greatly to Biocontrol Potential of a Fungal Entomopathogen. PLoS ONE 2014, 9, e87948. [Google Scholar] [CrossRef]
- Sanz, A.B.; García, R.; Rodríguez-Peña, J.M.; Arroyo, J. The CWI Pathway: Regulation of the Transcriptional Adaptive Response to Cell Wall Stress in Yeast. J. Fungi 2018, 4, 1. [Google Scholar] [CrossRef]
- Hu, X.L.; Yang, P.; Chai, C.D.; Liu, J.; Sun, H.H.; Wu, Y.N.; Zhang, M.J.; Zhang, M.; Liu, X.T.; Yu, H.J. Structural and mechanistic insights into fungal β-1,3-glucan synthase FKS1. Nature 2023, 616, 190–198. [Google Scholar] [CrossRef] [PubMed]




| Concentration (μg/L) | Inhibition Rate (%) | EC50 (μg/L) 1 |
|---|---|---|
| 25 | 5.86 ± 0.65 | 75.99 ± 2.31 |
| 50 | 17.71 ± 1.32 | |
| 75 | 51.45 ± 2.02 | |
| 100 | 60.66 ± 2.06 | |
| 125 | 85.23 ± 1.98 | |
| 150 | 94.93 ± 2.34 |
| Samples | Raw Reads (Mb) | Clean Reads (Mb) | GC Content (%) | Clean Reads ≥ Q30 (%) |
|---|---|---|---|---|
| HC-1 | 44.18 | 42.24 | 55.04 | 93.17 |
| HC-2 | 46.68 | 44.64 | 55.27 | 93.33 |
| HC-3 | 41.03 | 39.32 | 55.34 | 94.22 |
| HX-1 | 63.06 | 56.33 | 54.70 | 93.52 |
| HX-2 | 74.14 | 70.13 | 54.00 | 94.03 |
| HX-3 | 78.79 | 58.69 | 54.39 | 93.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wu, W.; He, C.; Tan, B.; Tang, S.; Yu, L. Application and Mechanism of Action of Carvacrol Against Aspergillus niger Causing Postharvest Rot of Garlic Scapes (Allium sativum L.). J. Fungi 2025, 11, 709. https://doi.org/10.3390/jof11100709
Li P, Wu W, He C, Tan B, Tang S, Yu L. Application and Mechanism of Action of Carvacrol Against Aspergillus niger Causing Postharvest Rot of Garlic Scapes (Allium sativum L.). Journal of Fungi. 2025; 11(10):709. https://doi.org/10.3390/jof11100709
Chicago/Turabian StyleLi, Pei, Wenqing Wu, Can He, Boxi Tan, Shijing Tang, and Lu Yu. 2025. "Application and Mechanism of Action of Carvacrol Against Aspergillus niger Causing Postharvest Rot of Garlic Scapes (Allium sativum L.)" Journal of Fungi 11, no. 10: 709. https://doi.org/10.3390/jof11100709
APA StyleLi, P., Wu, W., He, C., Tan, B., Tang, S., & Yu, L. (2025). Application and Mechanism of Action of Carvacrol Against Aspergillus niger Causing Postharvest Rot of Garlic Scapes (Allium sativum L.). Journal of Fungi, 11(10), 709. https://doi.org/10.3390/jof11100709







