ABA and Ethylene Mediates Tomato Root Development Modulation During Endophytic Fungal Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Plant–Fungus Co-Cultivation and Sampling
2.2. Gene Selection
2.3. Gene Expression, Root Trait Measurement
2.4. Statistical Analysis
3. Results
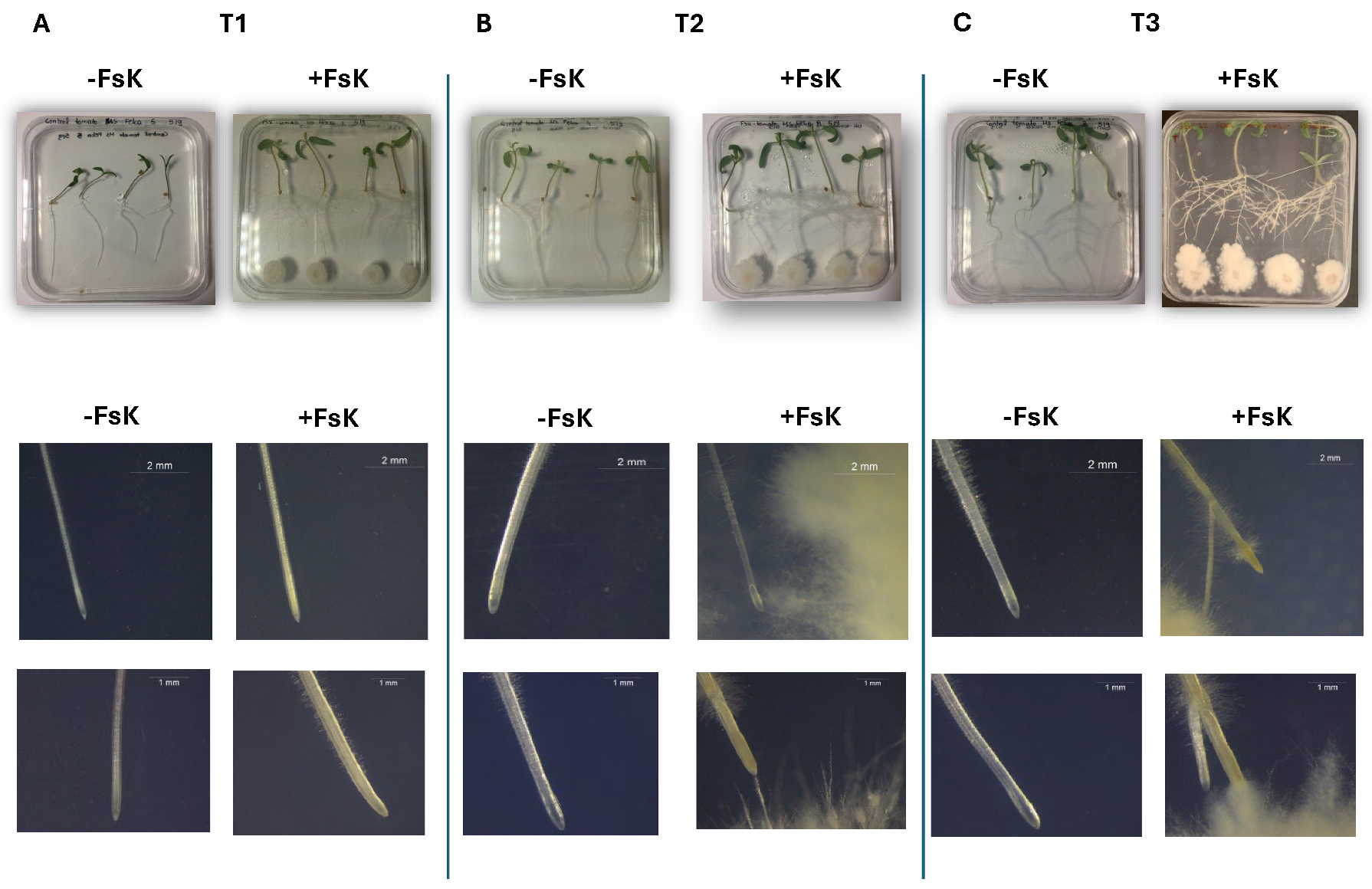
3.1. FsK Alters Root Architecture During Early Interaction
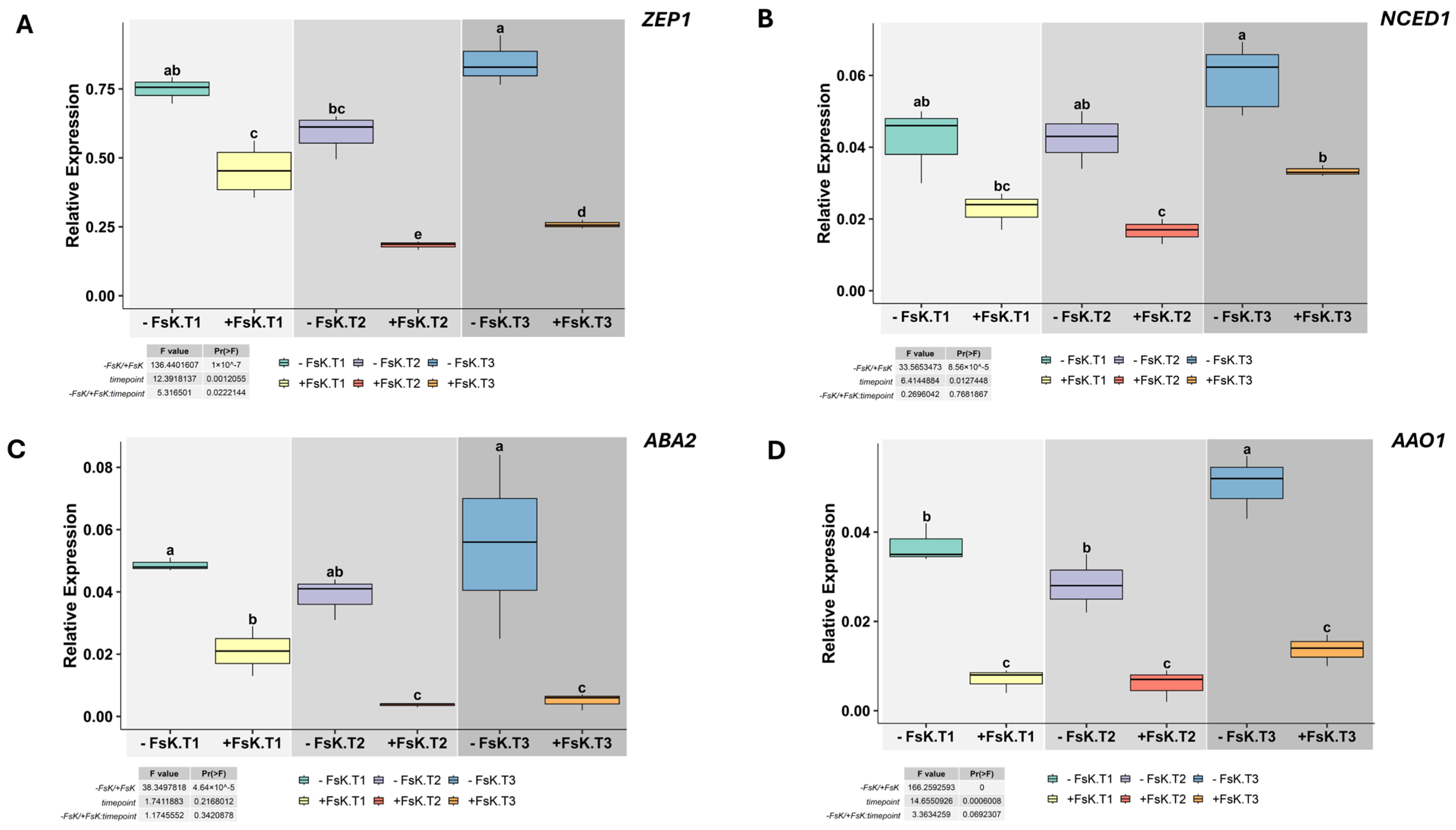
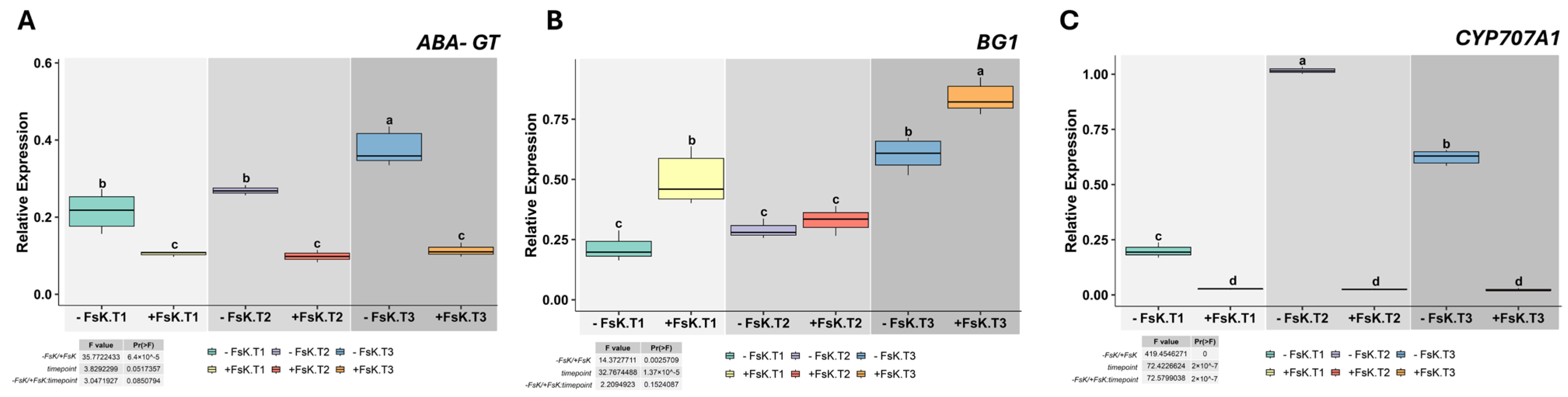
3.2. Tissue-Specific Modulation of ABA and Ethylene-Related Gene Expression in Tomato During Early Interaction with FsK
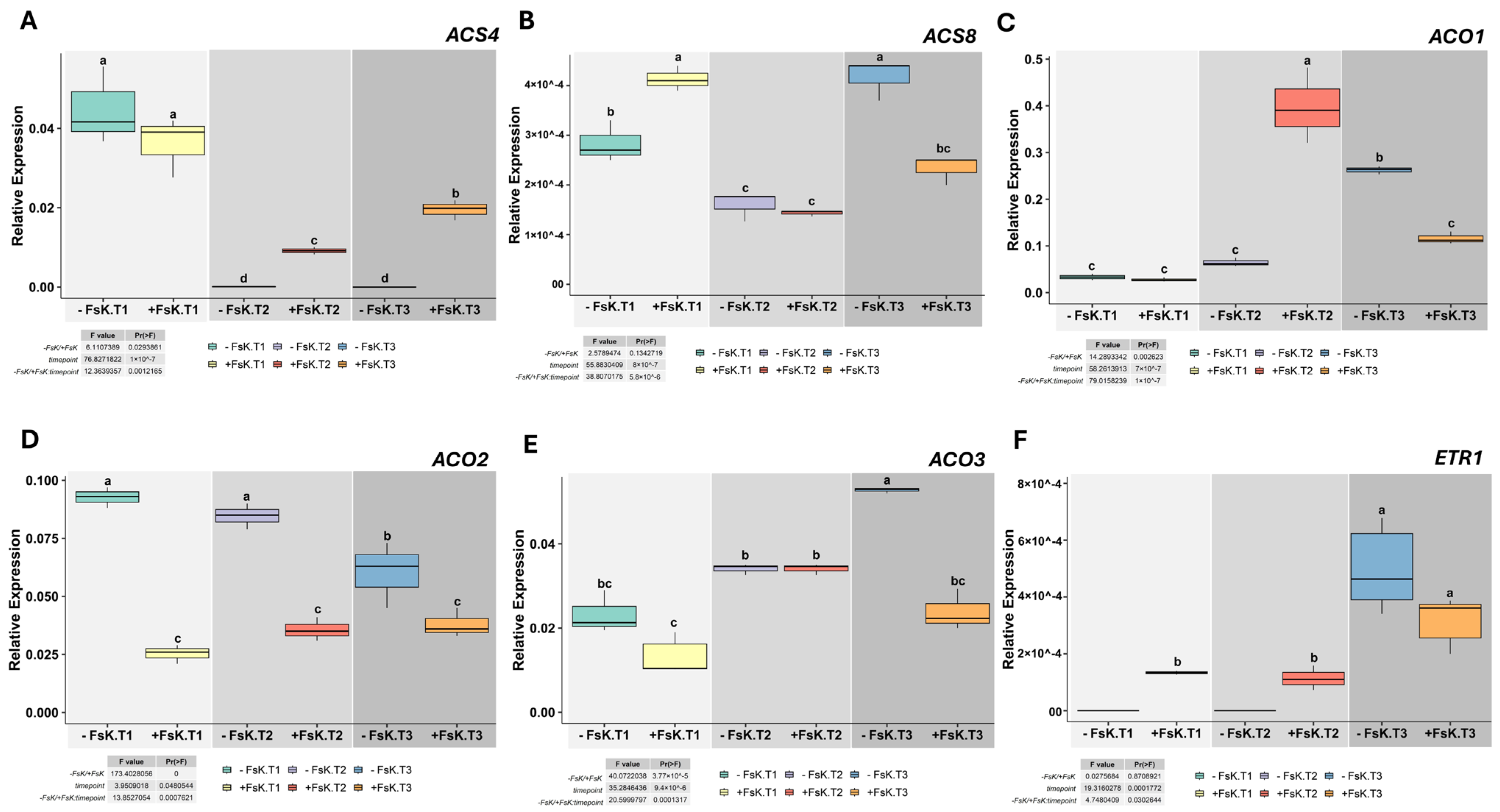
3.3. Temporal Regulation of Fungal Ethylene Biosynthesis Gene Expression During Host Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omomowo, O.I.; Babalola, O.O. Bacterial and Fungal Endophytes: Tiny Giants with Immense Beneficial Potential for Plant Growth and Sustainable Agricultural Productivity. Microorganisms 2019, 7, 481. [Google Scholar] [CrossRef]
- Jambon, I.; Thijs, S.; Weyens, N.; Vangronsveld, J. Harnessing Plant-Bacteria-Fungi Interactions to Improve Plant Growth and Degradation of Organic Pollutants. J. Plant Interact. 2018, 13, 119–130. [Google Scholar] [CrossRef]
- Chadha, N.; Mishra, M.; Prasad, R.; Varma, A. Root Endophytic Fungi: Research Update. J. Biol. Life Sci. 2014, 5, 135. [Google Scholar] [CrossRef]
- Mengistu, A.A. Endophytes: Colonization, Behaviour, and Their Role in Defense Mechanism. Int. J. Microbiol. 2020, 2020, 6927219. [Google Scholar] [CrossRef]
- Gautam, A.K.; Avasthi, S. Fungal endophytes: Potential biocontrol agents in agriculture. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Kumar, A., Singh, A.K., Choudhary, K.K., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 241–283. [Google Scholar]
- Zhao, J.; Zhou, L.; Wang, J.; Shan, T.; Zhong, L.; Liu, X.; Gao, X. Endophytic fungi for producing bioactive compounds originally from their host plants. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; Volume 1, pp. 567–576. [Google Scholar]
- Lahrmann, U.; Ding, Y.; Banhara, A.; Rath, M.; Hajirezaei, M.R.; Döhlemann, S.; Von Wirén, N.; Parniske, M.; Zuccaro, A. Host-Related Metabolic Cues Affect Colonization Strategies of a Root Endophyte. Proc. Natl. Acad. Sci. USA 2013, 110, 13965–13970. [Google Scholar] [CrossRef]
- Jia, M.; Chen, L.; Xin, H.L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. A Friendly Relationship Between Endophytic Fungi and Medicinal Plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef]
- Chithra, S.; Jasim, B.; Mathew, J.; Radhakrishnan, E.K. Endophytic Phomopsis Sp. Colonization in Oryza sativa Was Found to Result in Plant Growth Promotion and Piperine Production. Physiol. Plant. 2017, 160, 437–446. [Google Scholar] [CrossRef]
- Yao, Y.Q.; Lan, F.; Qiao, Y.M.; Wei, J.G.; Huang, R.S.; Li, L.B. Endophytic Fungi Harbored in the Root of Sophora Tonkinensis Gapnep: Diversity and Biocontrol Potential Against Phytopathogens. Microbiologyopen 2017, 6, e00437. [Google Scholar] [CrossRef] [PubMed]
- Leitão, A.L.; Enguita, F.J. Gibberellins in Penicillium Strains: Challenges for Endophyte-Plant Host Interactions Under Salinity Stress. Microbiol. Res. 2016, 183, 8–18. [Google Scholar] [CrossRef]
- Khan, R.; Shahzad, S.; Choudhary, M.I.; Khan, S.A.; Ahmad, A. Communities of Endophytic Fungi in Medicinal Plant Withania somnifera. Pak. J. Bot. 2010, 42, 1281–1287. [Google Scholar]
- Jahromi, F.; Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Influence of Salinity on the In Vitro Development of Glomus Intraradices and on the In Vivo Physiological and Molecular Responses of Mycorrhizal Lettuce Plants. Microb. Ecol. 2008, 55, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of Phosphorus and Nitrogen in the Rhizosphere and Plant Growth Promotion by Microorganisms. Plant Soil. 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kim, Y.H.; Kang, S.M.; Lee, I.J. Ameliorative Symbiosis of Endophyte (Penicillium funiculosum LHL06) under Salt Stress Elevated Plant Growth of Glycine max L. Plant Physiol. Biochem. 2011, 49, 852–861. [Google Scholar] [CrossRef]
- Cosoveanu, A.; Chowdhary, K.; Cabrera, R.; Sharma, S. Role of phytohormones-producing fungal endophytes in plant–microbial interactions under stress. In Endophytes: Potential Source of Compounds of Commercial and Therapeutic Applications; Patil, R.H., Maheshwari, V.L., Eds.; Springer: Singapore, 2021; pp. 195–223. [Google Scholar]
- Mauch-Mani, B.; Mauch, F. The Role of Abscisic Acid in Plant-Pathogen Interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef]
- Constantin, M.E.; de Lamo, F.J.; Vlieger, B.V.; Rep, M.; Takken, F.L.W. Endophyte-Mediated Resistance in Tomato to Fusarium oxysporum Is Independent of ET, JA, and SA. Front. Plant Sci. 2019, 10, 979. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, L. Mechanisms of Induced. Annu. Rev. Microbiol. 1983, 37, 51–79. [Google Scholar] [CrossRef]
- Cardoso, A.A.; Gori, A.; Da-Silva, C.J.; Brunetti, C. Abscisic Acid Biosynthesis and Signaling in Plants: Key Targets to Improve Water Use Efficiency and Drought Tolerance. Appl. Sci. 2020, 10, 6322. [Google Scholar] [CrossRef]
- Simm, S.; Scharf, K.D.; Jegadeesan, S.; Chiusano, M.L.; Firon, N.; Schleiff, E. Survey of Genes Involved in Biosynthesis, Transport, and Signaling of Phytohormones with Focus on Solanum lycopersicum. Bioinform. Biol. Insights 2016, 10, 185–207. [Google Scholar] [CrossRef]
- Kende, H. Ethylene Biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 283–307. [Google Scholar] [CrossRef]
- Rehman, B.; Javed, J.; Rauf, M.; Khan, S.A.; Arif, M.; Hamayun, M.; Gul, H.; Khilji, S.A.; Sajid, Z.A.; Kim, W.C.; et al. ACC Deaminase-Producing Endophytic Fungal Consortia Promotes Drought Stress Tolerance in M. oleifera by Mitigating Ethylene and H2O2. Front. Plant Sci. 2022, 13, 967672. [Google Scholar] [CrossRef]
- Savada, R.P.; Ozga, J.A.; Jayasinghege, C.P.A.; Waduthanthri, K.D.; Reinecke, D.M. Heat Stress Differentially Modifies Ethylene Biosynthesis and Signaling in Pea Floral and Fruit Tissues. Plant Mol. Biol. 2017, 95, 313–331. [Google Scholar] [CrossRef]
- Dubois, M.; Claeys, H.; Van den Broeck, L.; Inzé, D. Time of Day Determines Arabidopsis Transcriptome and Growth Dynamics Under Mild Drought. Plant Cell Environ. 2017, 40, 180–189. [Google Scholar] [CrossRef]
- Rauf, M.; Arif, M.; Fisahn, J.; Xue, G.P.; Balazadeh, S.; Mueller-Roeber, B. NAC transcription factor speedy hyponastic growth regulates flooding-induced leaf movement in Arabidopsis. Plant Cell 2013, 25, 4941–4955. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Ntougias, S.; Zervakis, G.I.; Ehaliotis, C.; Haralampidis, K.; Papadopoulou, K.K. Role of Ethylene in the Protection of Tomato Plants against Soil-Borne Fungal Pathogens Conferred by an Endophytic Fusarium solani Strain. J. Exp. Bot. 2007, 58, 3853–3864. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Doupis, G.; Papadakis, I.E.; Ehaliotis, C.; Papadopoulou, K.K. Tolerance of Tomato Plants to Water Stress Is Improved by the Root Endophyte Fusarium solani FsK. Rhizosphere 2018, 6, 77–85. [Google Scholar] [CrossRef]
- Garantonakis, N.; Pappas, M.L.; Varikou, K.; Skiada, V.; Broufas, G.D.; Kavroulakis, N.; Papadopoulou, K.K. Tomato Inoculation with the Endophytic Strain Fusarium solani K Results in Reduced Feeding Damage by the Zoophytophagous Predator Nesidiocoris tenuis. Front. Ecol. Evol. 2018, 6, 126. [Google Scholar] [CrossRef]
- Pappas, M.L.; Liapoura, M.; Papantoniou, D.; Avramidou, M.; Kavroulakis, N.; Weinhold, A.; Broufas, G.D.; Papadopoulou, K.K. The Beneficial Endophytic Fungus Fusarium solani Strain K Alters Tomato Responses against Spider Mites to the Benefit of the Plant. Front. Plant Sci. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Merck. Potato Dextrose Agar. In Merck Microbiology Manual, 12th ed.; Merck: San Jose, CA, USA, 2006; Volume 12, pp. 1–2. [Google Scholar]
- Hasan, M.M.; Liu, X.-D.; Yao, G.-Q.; Liu, J.; Fang, X.-W. Ethylene-Mediated Stomatal Responses to Dehydration and Rehydration in Seed Plants. J. Exp. Bot. 2024, 75, 6719–6732. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, K.; Liang, B.; Du, Y.; Jiang, L.; Wang, J.; Kai, W.; Zhang, Y.; Zhai, X.; Chen, P.; et al. Suppressing ABA Uridine Diphosphate Glucosyltransferase (SlUGT75C1) Alters Fruit Ripening and the Stress Response in Tomato. Plant J. 2017, 91, 574–589. [Google Scholar] [CrossRef]
- Mou, W.; Li, D.; Bu, J.; Jiang, Y.; Khan, Z.U.; Luo, Z.; Mao, L.; Ying, T. Comprehensive Analysis of ABA Effects on Ethylene Biosynthesis and Signaling During Tomato Fruit Ripening. PLoS ONE 2016, 11, e0154072. [Google Scholar] [CrossRef]
- Jia, H.; Chen, S.; Liu, D.; Liesche, J.; Shi, C.; Wang, J.; Ren, M.; Wang, X.; Yang, J.; Shi, W.; et al. Ethylene-Induced Hydrogen Sulfide Negatively Regulates Ethylene Biosynthesis by Persulfidation of ACO in Tomato Under Osmotic Stress. Front. Plant Sci. 2018, 871, 1517. [Google Scholar] [CrossRef] [PubMed]
- Zhijin, Z.; Haiwen, Z.; Ruidan, Q.; Xue-Chen, W.; Rongfeng, H. Transcriptional Regulation of the Ethylene Response Factor Leerf2 in the Expression of Ethylene Biosynthesis Genes Controls Ethylene Production in Tomato and Tobacco. Plant Physiol. 2009, 150, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhu, J.K. Regulation of Abscisic Acid Biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef]
- Okamoto, M.; Tanaka, Y.; Abrams, S.R.; Kamiya, Y.; Seki, M.; Nambara, E. High Humidity Induces Abscisic Acid 8′-Hydroxylase in Stomata and Vasculature to Regulate Local and Systemic Abscisic Acid Responses in Arabidopsis. Plant Physiol. 2009, 149, 825–834. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Xie, Q.; Gong, Z. Abscisic acid. In Hormone Metabolism and Signaling in Plants; Li, J., Li, C., Smith, S.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 161–202. [Google Scholar]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in Stomatal Defense Against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Lee, K.H.; Dong, T.; Jeong, J.C.; Jin, J.B.; Kanno, Y.; Kim, D.H.; Kim, S.Y.; Seo, M.; Bressan, R.A.; et al. A Vacuolar β-Glucosidase Homolog That Possesses Glucose-Conjugated Abscisic Acid Hydrolyzing Activity Plays an Important Role in Osmotic Stress Responses in Arabidopsis. Plant Cell 2012, 24, 2184–2199. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arab. Book 2013, 11, e0166. [Google Scholar] [CrossRef]
- Gutjahr, C.; Paszkowski, U. Multiple Control Levels of Root System Remodeling in Arbuscular Mycorrhizal Symbiosis. Front. Plant Sci. 2013, 4, 204. [Google Scholar] [CrossRef]
- Seemann, C.; Heck, C.; Voß, S.; Schmoll, J.; Enderle, E.; Schwarz, D.; Requena, N. Root Cortex Development Is Fine-Tuned by the Interplay of MIGs, SCL3 and DELLAs During Arbuscular Mycorrhizal Symbiosis. New Phytol. 2022, 233, 948–965. [Google Scholar] [CrossRef] [PubMed]
- Shahollari, B.; Vadassery, J.; Varma, A.; Oelmüller, R. A Leucine-Rich Repeat Protein Is Required for Growth Promotion and Enhanced Seed Production Mediated by the Endophytic Fungus Piriformospora indica in Arabidopsis thaliana. Plant J. 2007, 50, 1–13. [Google Scholar] [CrossRef]
- Etemadi, M.; Gutjahr, C.; Couzigou, J.M.; Zouine, M.; Lauressergues, D.; Timmers, A.; Audran, C.; Bouzayen, M.; Bécard, G.; Combier, J.P. Auxin Perception Is Required for Arbuscule Development in Arbuscular Mycorrhizal Symbiosis. Plant Physiol. 2014, 166, 281–292. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma Virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth Through an Auxin-Dependent Mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Ramírez-Flores, M.R.; Bello-Bello, E.; Rellán-Álvarez, R.; Sawers, R.J.H.; Olalde-Portugal, V. Inoculation with the Mycorrhizal Fungus Rhizophagus irregularis Modulates the Relationship Between Root Growth and Nutrient Content in Maize (Zea mays Ssp. mays L.). Plant Direct 2019, 3, e00192. [Google Scholar] [CrossRef]
- Xu, L.; Wang, A.; Wang, J.; Wei, Q.; Zhang, W. Piriformospora indica Confers Drought Tolerance on Zea mays L. Through Enhancement of Antioxidant Activity and Expression of Drought-Related Genes. Crop J. 2017, 5, 251–258. [Google Scholar] [CrossRef]
- Li, L.; Feng, Y.; Qi, F.; Hao, R. Research Progress of Piriformospora indica in Improving Plant Growth and Stress Resistance to Plant. J. Fungi 2023, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- Peskan-Berghöfer, T.; Vilches-Barro, A.; Müller, T.M.; Glawischnig, E.; Reichelt, M.; Gershenzon, J.; Rausch, T. Sustained Exposure to Abscisic Acid Enhances the Colonization Potential of the Mutualist Fungus Piriformospora indica on Arabidopsis thaliana Roots. New Phytol. 2015, 208, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.; Arif, M. Drought-Tolerant Fungal Microbes, Aspergillus oryzae and Aspergillus fumigatus, Elevate Physiohormonal and Antioxidant Responses of Maize Under Drought Stress. Front. Microbiol. 2024, 15, 1488639. [Google Scholar] [CrossRef]
- Morales-vargas, A.T.; López-Ramírez, V.; Álvarez-Mejía, C.; Vázquez-Martínez, J. Endophytic Fungi for Crops Adaptation to Abiotic Stresses. Microorganisms 2024, 12, 1357. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Plett, J.M.; Lopez-Raez, J.A.; Reid, D. Editorial: The Role of Plant Hormones in Plant-Microbe Symbioses. Front. Plant Sci. 2019, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Ansari, M.W.; Tuteja, N.; Wattal, R.K. Ethylene Mediates Plant-Beneficial Fungi Interaction That Leads to Increased Nutrient Uptake, Improved Physiological Attributes, and Enhanced Plant Tolerance Under Salinity Stress. In Global Climate Change and Plant Stress Management; Ansari, M.W., Singh, A.K., Tuteja, N., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 361–370. [Google Scholar] [CrossRef]
- Khalloufi, M.; Martínez-Andújar, C.; Karray-Bouraouib, N.; Pérez-Alfocea, F.; Albacete, A. The Crosstalk Interaction of Ethylene, Gibberellins, and Arbuscular Mycorrhiza Improves Growth in Salinized Tomato Plants by Modulating the Hormonal Balance. J. Plant Physiol. 2024, 303, 154336. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.; Heil, M. Costs and Trade-Offs Associated with Induced Resistance. Physiol. Mol. Plant Pathol. 2007, 71, 3–17. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Rovenich, H.; Jeena, G.; Nizam, S.; Tissier, A.; Balcke, G.U.; Mahdi, L.K.; Bonkowski, M.; Langen, G.; Zuccaro, A. The Inconspicuous Gatekeeper: Endophytic Serendipita Vermifera Acts as Extended Plant Protection Barrier in the Rhizosphere. New Phytol. 2019, 224, 886–901. [Google Scholar] [CrossRef]
- Daneshkhah, R.; Grundler, F.M.W.; Wieczorek, K. The Role of MPK6 as Mediator of Ethylene/Jasmonic Acid Signaling in Serendipita indica-Colonized Arabidopsis Roots. Plant Mol. Biol. Rep. 2018, 36, 284–294. [Google Scholar] [CrossRef]
- Chagué, V.; Elad, Y.; Barakat, R.; Tudzynski, P.; Sharon, A. Biosynthesis in Botrytis Cinerea. FEMS Microbiol. Ecol. 2002, 40, 143–149. [Google Scholar] [CrossRef]
- Gu, S.; Xie, L.; Guan, Q.; Sheng, X.; Fang, Y.; Wang, X. Effect of Ethylene Production by Four Pathogenic Fungi on the Postharvest Diseases of Green Pepper (Capsicum annuum L.). Int. J. Food Microbiol. 2024, 418, 110729. [Google Scholar] [CrossRef]
- Du, M.; Zhai, Q.; Deng, L.; Li, S.; Li, H.; Yan, L.; Huang, Z.; Wang, B.; Jiang, H.; Huang, T.; et al. Closely Related NAC Transcription Factors of Tomato Differentially Regulate Stomatal Closure and Reopening during Pathogen Attack. Plant Cell 2014, 26, 3167–3184. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espinoza, V.A.; López-Climent, M.F.; Casaretto, J.A.; Gómez-Cadenas, A. Water Stress Responses of Tomato Mutants Impaired in Hormone Biosynthesis Reveal Abscisic Acid, Jasmonic Acid and Salicylic Acid Interactions. Front. Plant Sci. 2015, 6, 997. [Google Scholar] [CrossRef] [PubMed]
- Jegadeesan, S.; Beery, A.; Altahan, L.; Meir, S.; Pressman, E.; Firon, N. Ethylene Production and Signaling in Tomato (Solanum lycopersicum) Pollen Grains Is Responsive to Heat Stress Conditions. Plant Reprod. 2018, 31, 367–383. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feka, M.; Chousein, B.; Tsiouri, O.; Papadopoulou, K.K. ABA and Ethylene Mediates Tomato Root Development Modulation During Endophytic Fungal Interaction. J. Fungi 2025, 11, 707. https://doi.org/10.3390/jof11100707
Feka M, Chousein B, Tsiouri O, Papadopoulou KK. ABA and Ethylene Mediates Tomato Root Development Modulation During Endophytic Fungal Interaction. Journal of Fungi. 2025; 11(10):707. https://doi.org/10.3390/jof11100707
Chicago/Turabian StyleFeka, Maria, Bilge Chousein, Olga Tsiouri, and Kalliope K. Papadopoulou. 2025. "ABA and Ethylene Mediates Tomato Root Development Modulation During Endophytic Fungal Interaction" Journal of Fungi 11, no. 10: 707. https://doi.org/10.3390/jof11100707
APA StyleFeka, M., Chousein, B., Tsiouri, O., & Papadopoulou, K. K. (2025). ABA and Ethylene Mediates Tomato Root Development Modulation During Endophytic Fungal Interaction. Journal of Fungi, 11(10), 707. https://doi.org/10.3390/jof11100707






