Postharvest Disease Management of ‘Akizuki’ Pear in China: Identification of Fungal Pathogens and Control Efficacy of Chlorine Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Collection
2.2. Pathogen Isolation and Purification
2.3. Koch’s Postulates
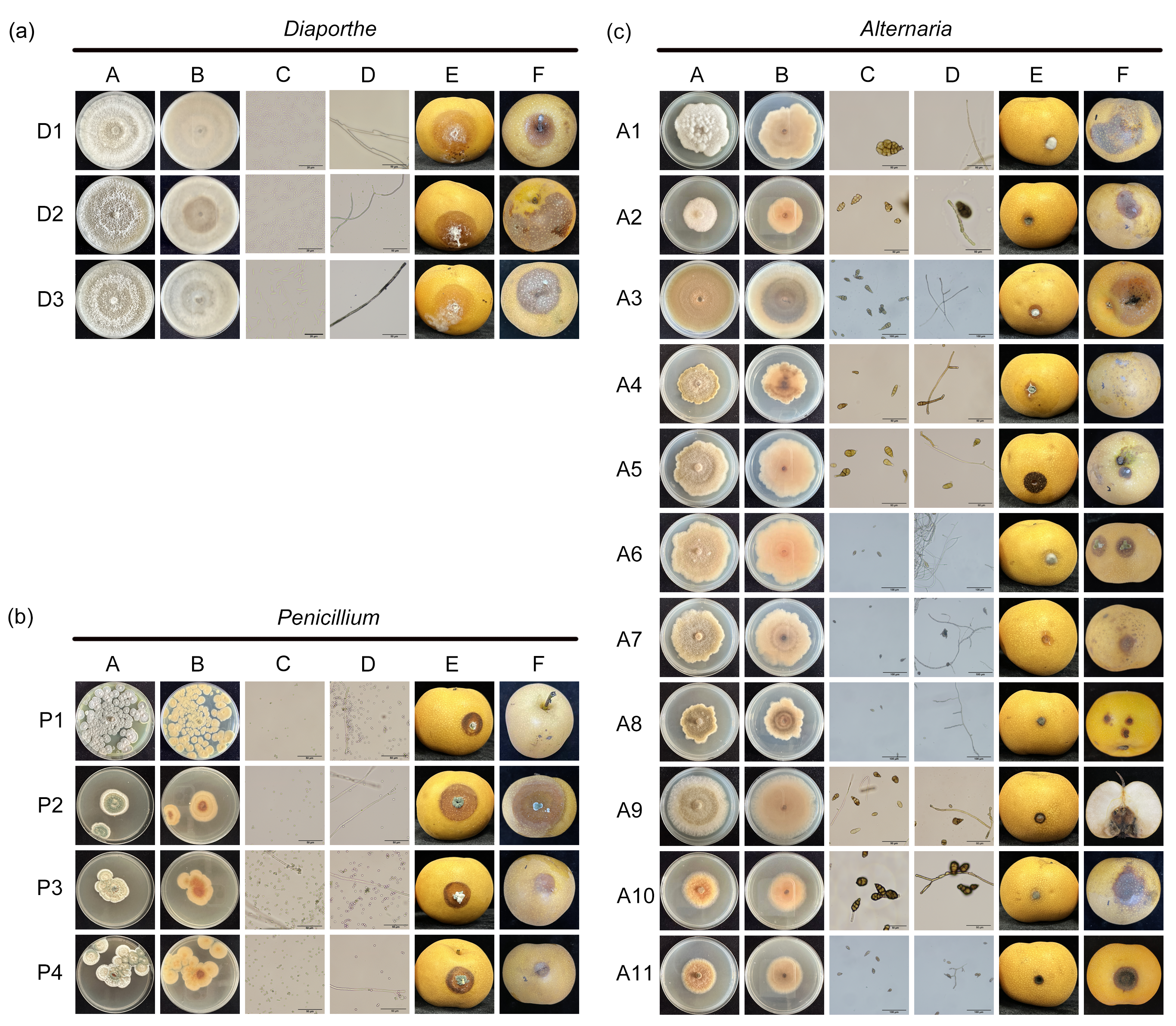
2.4. Morphological Identification
2.5. Molecular Biological Identification
2.6. Determination of the Antifungal Effect of ClO2
2.7. Determination of Fungicide Efficiency
2.8. Propidium Iodide Staining
2.9. Effect of ClO2 on the Control of Pathogenic Fungi in Fruits
2.10. Statistical Analysis
3. Results
3.1. Isolation and Morphological Identification of Pathogens from ‘Akizuki’ Pear
3.2. Pathogenicity Test of Pathogens
3.3. Molecular Biological Identification of Pathogens
3.4. Effect of ClO2 on the Development of Pathogens
3.5. Effect of ClO2 on the Cell Membrane of Pathogens
3.6. Effect of ClO2 on the Disease Incidence of ‘Akizuki’ Pear Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Zhang, H.H.; Wu, L.; Gu, S.F.; Xun, J.; Jia, B.; Ye, Z.F.; Heng, W.; Jin, X. An early asymptomatic diagnosis method for cork spot disorder in ‘Akizuki’ pear (Pyrus pyrifolia Nakai) using micro near infrared spectroscopy. Food Chem. X 2023, 19, 100851. [Google Scholar] [CrossRef]
- Xiong, J.H.; Gu, S.F.; Rao, Y.; Liu, L.; Zhang, X.D.; Wu, Y.T.; Jin, X. A multi-source feature stable learning method for rapid identification of cork spot disorder in ‘Akizuki’ pear. Postharvest Biol. Technol. 2025, 219, 113285. [Google Scholar] [CrossRef]
- Oyom, W.; Li, Y.C.; Prusky, D.; Zhang, Z.; Bi, Y.; Tahergorabi, R. Recent advances in postharvest technology of Asia pears fungi disease control: A review. Physiol. Mol. Plant Pathol. 2022, 117, 101771. [Google Scholar] [CrossRef]
- Sardella, D.; Muscat, A.; Brincat, J.P.; Gatt, R.; Decelis, S.; Valdramidis, V. A comprehensive review of the pear fungal diseases. Int. J. Fruit Sci. 2016, 16, 351–377. [Google Scholar] [CrossRef]
- Zhang, J.X.; Timmer, L.W. Preharvest application of fungicides for postharvest disease control on early season tangerine hybrids in Florida. Crop Prot. 2007, 26, 886–893. [Google Scholar] [CrossRef]
- Khokhar, I.; Jia, Y.; Mukhtar, I.; Wang, J.H.; Yan, Y.C. First report of Penicillium polonicum causing blue mold on stored pear (Pyrus bretschneideri) fruits in China. Plant Dis. 2019, 103, 3279. [Google Scholar] [CrossRef]
- DeShields, J.B.; Kc, A.N. Morphological and molecular characterization of Alternaria spp. isolated from European pears. Plant Dis. 2021, 105, 2531–2540. [Google Scholar] [CrossRef]
- Kwon, J.H.; Lee, C.J. Rhizopus soft rot on pear (Pyrus serotina) caused by Rhizopus stoloniferin Korea. Mycobiology 2006, 34, 151–153. [Google Scholar] [CrossRef]
- Javed, S.; Javaid, A.; Anwar, W.; Majeed, R.A.; Akhtar, R.; Naqvi, S.F. First report of botrytis bunch rot of grapes caused by Botrytis cinerea in Pakistan. Plant Dis. 2017, 101, 1036–1037. [Google Scholar] [CrossRef]
- Zhong, Q.P.; Xie, Y.Y.; Huo, G.H.; Cui, C.Y. Postharvest fruit rot of pear caused by Diaporthe eres in China. J. Plant Pathol. 2023, 105, 1153. [Google Scholar] [CrossRef]
- Van Campenhout, J.; Van Hemelrijck, W.; Grammen, C.; Bylemans, D. First report of Botryosphaeria dothidea in Belgium causing fruit rot on pear. Plant Dis. 2017, 101, 1672–1673. [Google Scholar] [CrossRef]
- Collum, T.D.; Evans, B.; Gottschalk, C. First report of Fusarium avenaceum causing postharvest decay of European pear in mid-atlantic United States. Plant Dis. 2023, 107, 2230. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Truter, M.; Groenewald, J.Z.; Crous, P.W. Large-spored Alternaria pathogens in section Porri disentangled. Stud. Mycol. 2014, 79, 1–47. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Xu, Y.Q.; Yolandani, Y.; Wang, Q.Q.; Wu, B.; Li, D. Biological control and other alternatives to chemical fungicides in controlling postharvest disease of fruits caused by Alternaria alternata and Botrytis cinerea. Food Innov. Adv. 2024, 3, 135–143. [Google Scholar] [CrossRef]
- Radulovic, J.; Lucic, M.; Nesic, A.; Onjia, A. Multivariate assessment and risk ranking of pesticide residues in citrus fruits. Foods 2023, 12, 2454. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Cash, J.N.; Zabik, M.J. Determination of degradation products and pathways of mancozeb and ethylenethiourea (ETU) in solutions due to ozone and chlorine dioxide treatments. J. Agric. Food Chem. 2003, 51, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Bridges, D.F.; Lacombe, A.; Wu, V.C.H. Integrity of the Escherichia coli O157:H7 cell wall and membranes after chlorine dioxide. Treatment. Front. Microbiol. 2020, 11, 888. [Google Scholar] [CrossRef]
- Guan, J.W.; Lacombe, A.; Rane, B.; Van Blair, J.; Zhang, Y.J.; Tang, J.M.; Sablani, S.; Wu, V.C.H. The stress response of Listeria monocytogenes inoculated on fresh apples exposed to gaseous chlorine dioxide. J. Food Saf. 2024, 44, e13126. [Google Scholar] [CrossRef]
- Guntiya, N.; Bussaban, B.; Faiyue, B.; Uthaibutra, J.; Saengnil, K. Application of gaseous chlorine dioxide for control of fungal fruit rot disease of harvested “Daw” longan. Sci. Hortic. 2016, 213, 164–172. [Google Scholar] [CrossRef]
- Fu, M.R.; Zhang, X.M.; Jin, T.; Li, B.Q.; Zhang, Z.Q.; Tian, S.P. Inhibitory of grey mold on green pepper and winter jujube by chlorine dioxide (ClO2) fumigation and its mechanisms. LWT-Food Sci. Technol. 2019, 100, 335–340. [Google Scholar] [CrossRef]
- Arango, J.; Rubino, M.; Auras, R.; Gillett, J.; Schilder, A.; Grzesiak, A.L. Evaluation of chlorine dioxide as an antimicrobial against Botrytis cinerea in California strawberries. Food Packag. Shelf Life 2016, 9, 45–54. [Google Scholar] [CrossRef]
- Suh, M.J.; Simpson, A.M.A.; Mitch, W.A. Purified chlorine dioxide as an alternative to chlorine disinfection to minimize chlorate formation during postharvest produce washing. Environ. Sci. Technol. 2023, 57, 12063–12071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Fu, M. Inhibitory effect of chlorine dioxide (ClO2) fumigation on growth and patulin production and its mechanism in Penicillum expansum. LWT-Food Sci. Technol. 2018, 96, 335–343. [Google Scholar] [CrossRef]
- Saengnil, K.; Chumyam, A.; Faiyue, B.; Uthaibutr, J. Use of chlorine dioxide fumigation to alleviate enzymatic browning of harvested “Daw” longan pericarp during storage under ambient conditions. Postharvest Biol. Technol. 2014, 91, 49–56. [Google Scholar] [CrossRef]
- Bastide, F.; Sérandat, I.; Gombert, J.; Laurent, E.; Morel, E.; Kolopp, J.; Guillermin, P.L.; Hamon, B.; Simoneau, P.; Berruyer, R. Characterization of fungal pathogens (Diaporthe angelicae and D. eres) responsible for umbel browning and stem necrosis on carrot in France. Plant Pathol. 2017, 66, 239–253. [Google Scholar] [CrossRef]
- Masschel, W. Spectrophotometric determination of chlorine dioxide with Acid Chrome Violet K. Anal. Chem. 1966, 38, 1839. [Google Scholar] [CrossRef]
- Rouissi, W.; Ugolini, L.; Martini, C.; Lazzeri, L.; Mari, M. Control of postharvest fungal pathogens by antifungal compounds from Penicillium expansum. J. Food Prot. 2013, 76, 1879–1886. [Google Scholar] [CrossRef]
- Chen, M.X.; Zhong, L.K.; Zhang, Z.X.; Peng, C.B.; Ke, D.X.; Gan, P.; Wang, Z.R.; Wei, R.F.; Liu, W.; Yang, J.F. Isolation and identification of Colletotrichum as fungal pathogen from tea and preliminary fungicide screening. Qual. Assur. Saf. Crops Foods 2022, 14, 92–101. [Google Scholar] [CrossRef]
- Duan, Y.X.; Xu, Y.; Wang, R.; Ma, C.H. Investigation and prevention of cork spot disorder in ‘Akizuki’ pear (Pyrus pyrifoliaNakai). Hortscience 2019, 54, 480–486. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhu, Y.L.; Ji, P.Y.; Li, A.Q.; Qiu, Z.Y.; Chen, Y.Y.; Wang, R.; Ma, C.H.; Song, J.K.; Cui, Z.H. Mineral and metabolome analyses provide insights into the cork spot disorder on ‘Akizuki’ pear fruit. Horticulturae 2023, 9, 818. [Google Scholar] [CrossRef]
- Guo, Y.S.; Crous, P.W.; Bai, Q.; Fu, M.; Yang, M.M.; Wang, X.H.; Du, Y.M.; Hong, N.; Xu, W.X.; Wang, G.P. High diversity of Diaporthe species associated with pear shoot canker in China. Persoonia 2020, 45, 132–162. [Google Scholar] [CrossRef]
- Geng, M.Y.; Wang, J. First report of black rot on persimmon fruits caused by Diaporthe eres in China. Plant Dis. 2023, 107, 3292. [Google Scholar] [CrossRef]
- Mei, P.Y.; Song, X.H.; Zhu, Z.Y.; Li, L.Y. First report of Diaporthe eres causing root rot of coptis chinensis. Plant Dis. 2021, 105, 1854. [Google Scholar] [CrossRef]
- Stosic, S.; Ristic, D.; Savkovic, Z.; Grbic, M.L.; Vukojevic, J.; Zivkovic, S. Penicillium and Talaromyces species as postharvest pathogens of pear Fruit (Pyrus communis) in Serbia. Plant Dis. 2021, 105, 3510–3521. [Google Scholar] [CrossRef] [PubMed]
- Cancino, S.; Lolas, M.; Galdós, L.; Hernández, Y.; Ferrada, E.; Riveros, P.; Blanco-Ulate, B.; Díaz, G.A. Occurrence of Alternaria alternata and A. tenuissima causing black rot in cherry fruits (Prunus avium) in Central Chile. Plant Dis. 2023, 107, 12. [Google Scholar] [CrossRef]
- Chen, G.; Yang, L.; Luo, H.L.; Huang, Y.C.; Ju, Y.; Wei, Y.W.; Sun, J.M. First report of leaf spot on passion fruit in China caused by Alternaria alternata. Plant Dis. 2023, 107, 4. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Choi, E.D.; Kim, Y.; Park, S.Y. First report of fruit scab caused by Alternaria alternata on Actinidia chinensis in Korea. Plant Dis. 2024, 108, 9. [Google Scholar] [CrossRef]
- Prodromou, I.; Thomidis, T.; Zamhounis, A. First report of Penicillium expansum (Link) Thom. causing post-harvest fruit rot of kiwifruit in northern greece. Plant Dis. 2018, 102, 1851. [Google Scholar] [CrossRef]
- Azam, M.; Shahid, A.A.; Majeed, R.A.; Ali, M.; Ahmad, N.; Haider, M.S. First report of Penicillium biourgeianum causing post-harvest fruit rot of apple in pakistan. Plant Dis. 2016, 100, 1778–1779. [Google Scholar] [CrossRef]
- Xu, M.Q.; Yang, Q.Y.; Boateng, N.A.S.; Ahima, J.; Dou, Y.; Zhang, H.Y. Ultrastructure observation and transcriptome analysis of Penicillium expansum invasion in postharvest pears. Postharvest Biol. Technol. 2020, 165, 111198. [Google Scholar] [CrossRef]
- Khokhar, I.; Chen, J.M.; Wang, J.H.; Jia, Y.; Yan, Y.C.; Mukhtar, I. First report of postharvest blue mold decay caused by Penicillium expansum on Lemon (Citrus limon) fruit in China. Plant Dis. 2021, 105, 3747. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jeong, J.J.; Jin, H.; Kim, W.; Yu, G.D.; Kim, K.D. Inhibitory effects of gaseous chlorine dioxide against Diaporthe batatas isolated from stored sweetpotato. Plant Pathol. J. 2019, 35, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Zhou, B.; Luo, Y.G.; Ference, C.; Baldwin, E.; Harrison, K.; Bai, J.H.; Fla State Hort, S. The application of controlled-release chlorine dioxide pouches for preservation of cherry/grape tomatoes. Proc. Fla. State Hortic. Soc. 2017, 130, 204–205. [Google Scholar]
- Liu, X.; Jiao, W.X.; Du, Y.M.; Chen, Q.M.; Sun, Z.B.; Fu, M.R. Chlorine dioxide controls green mold caused by Penicillium digitatum in citrus fruits and the mechanism involved. J. Agric. Food Chem. 2020, 68, 13897–13905. [Google Scholar] [CrossRef]
- Hatamzadeh, S.; Oghaz, N.A.; Rahnama, K.; Noori, F. Comparison of the antifungal activity of chlorine dioxide, peracetic acid and some chemical fungicides in post-harvest management of Penicillium digitatum and Botrytis cinerea infecting sweet orange and strawberry fruits. Agric. Res. 2024, 13, 72–84. [Google Scholar] [CrossRef]
- Zhang, T.C.; Huang, C.G.; Chong, X.H.; Jing, X.H.; Huang, H.J.; Deng, R.G.; Fang, J.J. Chlorine dioxide delays enzymatic browning in postharvest cherimoya and enables establishment of kinetics substrate model. Horticulturae 2024, 10, 901. [Google Scholar] [CrossRef]
- Singh, S.; Maji, P.K.; Lee, Y.S.; Gaikwad, K.K. Applications of gaseous chlorine dioxide for antimicrobial food packaging: A review. Environ. Chem. Lett. 2021, 19, 253–270. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chen, L.; Hang, Z.Y.; Tian, C.; Jiang, H.Y.; Fu, M.; Lyu, C. Effect of chlorine dioxide (ClO2) combined with different oxygen permeability package treatments on postharvest antioxidant activity and storage life of ‘Liaofeng’ grapes. Food Control 2025, 168, 110902. [Google Scholar] [CrossRef]
- Sun, H.X.; Chen, Z.P.; Zhao, Y.; An, J.J.; Hang, H.H.; Liu, R. Polyvinyl alcohol film with chlorine dioxide microcapsules can be used for blueberry preservation by slow-release of chlorine dioxide gas. Front. Nutr. 2023, 10, 1177950. [Google Scholar] [CrossRef]






| Isolates | The Closest Matching GenBank Taxa | GenBank Accession Nos. | ||
|---|---|---|---|---|
| ITS | tef1 | β-tub | ||
| A1 | Alternaria alternata | PX148910 | PX410011 | |
| A2 | Alternaria alternata | PX148902 | PX410009 | |
| A3 | Alternaria alternata | PX148911 | PX410010 | |
| A4 | Alternaria alternata | PX148904 | PX410019 | |
| A5 | Alternaria alternata | PX148909 | PX410018 | |
| A6 | Alternaria alternata | PX148906 | PX410013 | |
| A7 | Alternaria alternata | PX148907 | PX410014 | |
| A8 | Alternaria alternata | PX148903 | PX410012 | |
| A9 | Alternaria alternata | PX148912 | PX410017 | |
| A10 | Alternaria alternata | PX148905 | PX410015 | |
| A11 | Alternaria alternata | PX148908 | PX410016 | |
| AP002 | Alternaria alternata | OK103568.1 | OK120224.1 | |
| W-1 | Alternaria alternata | MG189596.1 | MZ750391.1 | |
| SF-003 | Alternaria alternata | ON053451.1 | ON055374.1 | |
| Egy-T4 | Alternaria solani | MT996273.1 | MT478030.1 | |
| SAs16 | Alternaria solani | MG525490.1 | MG525516.1 | |
| Egy-T6 | Alternaria linariae | MT996275.1 | MT996281.1 | |
| Egy-T5 | Alternaria linariae | MT996274.1 | MT996280.1 | |
| CBS 105.41 | Alternaria linariae | NR_136092.1 | KJ718528.1 | |
| Egy-P1 | Alternaria solani | MT476733.1 | MT478033.1 | |
| SICAUCC 19-0001 | Rhizopus stolonifera | MN267051.1 | MN159909.1 | |
| CBS 150.83 | Rhizopus stolonifera | AB113022.1 | AB512254.1 | |
| CBS 609.82 | Rhizopus stolonifera | AB113023.1 | AB512268.1 | |
| D1 | Diaporthe eres | PX148848 | PX289532 | |
| D2 | Diaporthe eres | PX148846 | PX393511 | |
| D3 | Diaporthe eres | PX148848 | PX393512 | |
| MN05 | Diaporthe longicolla | OL843914.1 | OL999083.1 | |
| Goodhue 1 | Diaporthe longicolla | OL843916.1 | OL999081.1 | |
| MN10 | Diaporthe longicolla | OL843919.1 | OL999085.1 | |
| FAU499 | Diaporthe sojae | KJ590717.1 | KJ610873.1 | |
| FAU635 | Diaporthe sojae | KJ590719.1 | KJ610875.1 | |
| ACJY44 | Diaporthe sojae | MW578676.1 | MW598122.1 | |
| YLX73 | Diaporthe ellipsoidea | OM538397.1 | OM654882.1 | |
| YLX11 | Diaporthe ellipsoidea | OM538389.1 | OM654877.1 | |
| YLX77 | Diaporthe ellipsoidea | OM538398.1 | OM654879.1 | |
| YYH13 | Diaporthe hongkongensis | PQ049734.1 | PP975435.1 | |
| HT-1 | Diaporthe hongkongensis | MT740484.1 | MT749776.1 | |
| D58 | Diaporthe hongkongensis | PP383967.1 | PP412806.1 | |
| SXCX2-1 | Diaporthe eres | MT877020.1 | MT874938.1 | |
| HGUP192112 | Diaporthe eres | MZ724720.1 | MZ724004.1 | |
| CFCC 53146 | Diaporthe eres | MN266201.1 | MN315471.1 | |
| JFRL 04-12 | Diaporthe fusicola | ON994259.1 | OP076826.1 | |
| JFRL 04-11 | Diaporthe fusicola | ON994258.1 | OP076825.1 | |
| MUCC3589 | Diaporthe amygdali | OR897081.1 | OR913141.1 | |
| MUCC3592 | Diaporthe amygdali | OR913144.1 | OR897084.1 | |
| MUCC3590 | Diaporthe amygdali | OR897082.1 | OR913142.1 | |
| RWP-47 | Rhizopus stolonifera | MH348275.1 | MH370152.1 | |
| P1 | Penicillium citrinum | PX376074 | PX373324 | |
| P2 | Penicillium expansum | PX254693 | PX373325 | |
| P3 | Penicillium expansum | PX254694 | PX393513 | |
| P4 | Penicillium expansum | PX254695 | PX393514 | |
| Aby4 | Penicillium expansum | OR426630.1 | OL802926.1 | |
| YC-IK11 | Penicillium expansum | MK850332.1 | MK862430.1 | |
| HF2P11 | Penicillium expansum | OP178985.1 | OP562802.1 | |
| CV1267 | Penicillium crustosum | JX091401.1 | JX091537.1 | |
| PM23 | Penicillium crustosum | ON116669.1 | ON155603.1 | |
| CV1529 | Penicillium crustosum | JX091538.1 | JX091402.1 | |
| QPA3 | Penicillium solitum | MK660355.1 | MK675786.1 | |
| Q2M5 | Penicillium solitum | MK660346.1 | MK675777.1 | |
| M124 | Penicillium solitum | MK660333.1 | MK675764.1 | |
| CBS 306.48 | Penicillium chrysogenum | MH856357.1 | ||
| CBS 355.48 | Penicillium chrysogenum | MH856388.1 | JF909948.1 | |
| CBS 498.73 | Penicillium roqueforti | MH860759.1 | HQ442359.1 | |
| PM9 | Penicillium roqueforti | ON116661.1 | ON155595.1 | |
| EFA 548.2 | Penicillium roqueforti | OK323188.1 | OK148549.1 | |
| PM20 | Penicillium brevicompactum | ON116667.1 | ON155601.1 | |
| CV1821 | Penicillium brevicompactum | JX091534.1 | JX091399.1 | |
| CV1492 | Penicillium brevicompactum | JX091533.1 | JX091398.1 | |
| NG54 | Penicillium citrinum | OP464912.1 | OP502874.1 | |
| CBS 122451 | Penicillium citrinum | GU944572.1 | GU944544.1 | |
| 202F8R-AC | Penicillium citrinum | MZ410306.1 | MZ369129.1 | |
| CBS 173.81 | Penicillium asturianum | MH861321.1 | KF296470.1 | |
| CBS 219.30 | Penicillium oxalicum | MH855125.1 | KF296462.1 | |
| 5648 | Penicillium oxalicum | KJ527449.1 | KJ527414.1 | |
| W357 | Penicillium oxalicum | MH567091.1 | MH593522.1 | |
| DTO 301-I9 | Penicillium glabrum | KM189803.1 | KM089053.1 | |
| DTO 301-I3 | Penicillium glabrum | KM189798.1 | KM089048.1 | |
| DTO 301-I1 | Penicillium glabrum | KM189797.1 | KM089047.1 | |
| RWP-47 | Rhizopus stolonifera | MH348275.1 | MH370152.1 | |
| Fungal Species | Regression Equation | EC50 (mg/L) | R2 |
|---|---|---|---|
| Alternaria alternata | y = 4.8672x − 6.7795 | 24.71 | 0.918 |
| Diaporthe eres | y = 6.0360x − 9.3617 | 35.56 | 0.852 |
| Penicillium expansum | y = 16.0517x − 26.0532 | 41.98 | 0.835 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Zhang, L.; Zhang, Y.; Cheng, Y.; Chen, C.; Wang, Y.; Guan, J. Postharvest Disease Management of ‘Akizuki’ Pear in China: Identification of Fungal Pathogens and Control Efficacy of Chlorine Dioxide. J. Fungi 2025, 11, 694. https://doi.org/10.3390/jof11100694
Jiang H, Zhang L, Zhang Y, Cheng Y, Chen C, Wang Y, Guan J. Postharvest Disease Management of ‘Akizuki’ Pear in China: Identification of Fungal Pathogens and Control Efficacy of Chlorine Dioxide. Journal of Fungi. 2025; 11(10):694. https://doi.org/10.3390/jof11100694
Chicago/Turabian StyleJiang, Haichao, Lixin Zhang, Yang Zhang, Yudou Cheng, Cunkun Chen, Yongxia Wang, and Junfeng Guan. 2025. "Postharvest Disease Management of ‘Akizuki’ Pear in China: Identification of Fungal Pathogens and Control Efficacy of Chlorine Dioxide" Journal of Fungi 11, no. 10: 694. https://doi.org/10.3390/jof11100694
APA StyleJiang, H., Zhang, L., Zhang, Y., Cheng, Y., Chen, C., Wang, Y., & Guan, J. (2025). Postharvest Disease Management of ‘Akizuki’ Pear in China: Identification of Fungal Pathogens and Control Efficacy of Chlorine Dioxide. Journal of Fungi, 11(10), 694. https://doi.org/10.3390/jof11100694





