Nine-Year Surveillance of Candida parapsilosis Candidemia in a Cardiothoracic ICU: Insights into Mortality and Resistance
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- da Silva Ruiz, L.; Montelli, A.C.; Sugizaki, M.F.; da Silva, E.G.; de Batista, G.C.; Moreira, D.; Paula, C.R. Outbreak of fungemia caused by Candida parapsilosis in a neonatal intensive care unit: Molecular investigation through microsatellite analysis. Rev. Iberoam. Micol. 2013, 30, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, F.G.; Gales, A.C.; Colombo, A.L. Systematic review of candidemia in Brazil: Unlocking historical trends and challenges in conducting surveys in middle-income countries. Mycopathologia 2024, 189, 60. [Google Scholar] [CrossRef]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Girão, E.; Levin, A.; Basso, M.; Gobara, S.; Gomes, L.; Medeiros, E.A.S.; Costa, S.F. Seven-year trend analysis of nosocomial candidemia and antifungal (fluconazole and caspofungin) use in Intensive Care Units at a Brazilian University Hospital. Med. Mycol. 2008, 46, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.M.; de Almeida Junior, J.N.; Magri, M.M.C.; Costa, S.F.; Guimarães, T. Epidemiological assessment and risk factors for mortality of bloodstream infections by Candida sp. and the impact of the COVID-19 pandemic era. J. Fungi 2024, 10, 268. [Google Scholar] [CrossRef]
- Escribano, P.; Guinea, J. Fluconazole-resistant Candida parapsilosis: A new emerging threat in the fungi arena. Front. Fungal Biol. 2022, 3, 1010782. [Google Scholar] [CrossRef]
- Yamin, D.; Akanmu, M.H.; Al Mutair, A.; Alhumaid, S.; Rabaan, A.A.; Hajissa, K. Global prevalence of antifungal-resistant Candida parapsilosis: A systematic review and meta-analysis. Trop. Med. Infect. Dis. 2022, 7, 188. [Google Scholar] [CrossRef]
- Thomaz, D.Y.; de Almeida, J.N., Jr.; Sejas, O.N.E.; Del Negro, G.M.B.; Carvalho, G.O.M.H.; Gimenes, V.M.F.; de Souza, M.E.B.; Arastehfar, A.; Camargo, C.H.; Motta, A.L.; et al. Environmental clonal spread of azole-resistant Candida parapsilosis with Erg11-Y132F mutation causing a large candidemia outbreak in a Brazilian cancer referral center. J. Fungi 2021, 7, 259. [Google Scholar] [CrossRef]
- Arastehfar, A.; Hilmioğlu-Polat, S.; Daneshnia, F.; Pan, W.; Hafez, A.; Fang, W.; Liao, W.; Şahbudak-Bal, Z.; Metin, D.Y.; De Almeida, J.N., Jr.; et al. Clonal candidemia outbreak by Candida parapsilosis carrying Y132F in Turkey: Evolution of a persisting challenge. Front. Cell Infect. Microbiol. 2021, 11, 676177. [Google Scholar]
- Bassetti, M.; Giacobbe, D.R.; Agvald-Ohman, C.; Akova, M.; Alastruey-Izquierdo, A.; Arikan-Akdagli, S.; Azoulay, E.; Blot, S.; Cornely, O.A.; Cuenca-Estrella, M.; et al. Invasive Fungal Diseases in Adult Patients in Intensive Care Unit (FUNDICU): 2024 consensus definitions from ESGCIP, EFISG, ESICM, ECMM, MSGERC, ISAC, and ISHAM. Intensive Care Med. 2024, 50, 502–515. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Agnelli, C.; Valerio, M.; Bouza, E.; Vena, A.; Guinea, J.; Martínez-Jiménez, M.d.C.; Marcos-Zambrano, L.J.; Escribano, P.; Muñoz, P. Persistent Candidemia in adults: Underlying causes and clinical significance in the antifungal stewardship era. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 607–614. [Google Scholar] [CrossRef]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeast, 3rd ed.; CLSI Guideline M44; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI Guidelines M27-Ed4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 2nd ed.; CLSI Guideline M60-Ed2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Pulcrano, G.; Roscetto, E.; Iula, V.D.; Panellis, D.; Rossano, F.; Catania, M.R. MALDI-TOF mass spectrometry and microsatellite markers to evaluate Candida parapsilosis transmission in neonatal intensive care units. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2919–2928. [Google Scholar] [CrossRef]
- Braga, P.R.; Cruz, I.L.; Ortiz, I.; Barreiros, G.; Nouér, S.A.; Nucci, M. Secular trends of candidemia at a Brazilian tertiary care teaching hospital. Braz. J. Infect. Dis. 2018, 22, 273–277. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Trucchi, C.; Ansaldi, F.; Antonelli, M.; Adamkova, V.; Alicino, C.; Almyroudi, M.-P.; Atchade, E.; et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: Results of the EUCANDICU project. Crit. Care 2019, 23, 219. [Google Scholar] [CrossRef]
- Colombo, A.L.; Nucci, M.; Park, B.J.; Nouér, S.A.; Arthington-Skaggs, B.; da Matta, D.A.; Warnock, D.; Morgan, J. Brazilian Network Candidemia Study. Epidemiology of candidemia in Brazil: A nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 2006, 44, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, T.; Nucci, M.; Mendonça, J.S.; Martinez, R.; Brito, L.R.; Silva, N.; Moretti, M.L.; Salomão, R.; Colombo, A.L. Epidemiology and predictors of a poor outcome in elderly patients with candidemia. Int. J. Infect. Dis. 2012, 16, e442–e447. [Google Scholar] [CrossRef]
- Ramos-Martínez, A.; Pintos-Pascual, I.; Guinea, J.; Gutiérrez-Villanueva, A.; Gutiérrez-Abreu, E.; Díaz-García, J.; Asensio, Á.; Iranzo, R.; Sánchez-Romero, I.; Muñoz-Algarra, M.; et al. Impact of the COVID-19 pandemic on the clinical profile of candidemia and the incidence of fungemia due to fluconazole-resistant Candida parapsilosis. J. Fungi 2022, 8, 451. [Google Scholar] [CrossRef]
- Thomaz, D.Y.; Del Negro, G.M.B.; Ribeiro, L.B.; da Silva, M.; Carvalho, G.O.M.H.; Camargo, C.H.; de Almeida, J.N.; Motta, A.L.; Siciliano, R.F.; Sejas, O.N.E.; et al. A Brazilian inter-hospital candidemia outbreak caused by fluconazole-resistant Candida parapsilosis in the COVID-19 era. J. Fungi 2022, 8, 100. [Google Scholar]
- Mattos, K.; Rodrigues, L.C.; de Oliveira, K.M.P.; Diniz, P.F.; Marques, L.I.; Araujo, A.A.; Chang, M.R. Variability in the clinical distributions of Candida species and the emergence of azole-resistant non-Candida albicans species in public hospitals in the Midwest region of Brazil. Rev. Soc. Bras. Med. Trop. 2017, 50, 843–847. [Google Scholar] [CrossRef]
- Poissy, J.; Damonti, L.; Bignon, A.; Khanna, N.; Von Kietzell, M.; Schrenzel, J.; Funginos, T.; Boggian, K.; Neofytos, D.; Vuotto, F.; et al. Risk factors for candidemia: A prospective matched case-control study. Crit. Care 2020, 24, 109. [Google Scholar] [CrossRef]
- Hirai, Y.; Asahata, S.; Ainoda, Y.; Goto, A.; Fujita, T.; Totsuka, K. Nosocomial Candida parapsilosis candidemia: Risk factors, antifungal susceptibility and outcome. J. Hosp. Infect. 2014, 87, 54–58. [Google Scholar] [CrossRef]
- Kutlu, M.; Sayın-Kutlu, S.; Alp-Çavuş, S.; Şirin, M.C.; Tülek, N.; Alkan, E.B.; Kaya, O.; Kutsoylu, O.E.; Şenol-Akar, Ş.; Turhan, Ö.; et al. Mortality-associated factors of candidemia: A multi-center prospective cohort in Turkey. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 597–607. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Díaz-Martín, A.; García-Cabrera, E.; Ruiz Pérez de Pipaón, M.; Hernández-Caballero, C.; Lepe-Jiménez, J.A. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 206–213. [Google Scholar] [CrossRef]
- Puig-Asensio, M.; Pemán, J.; Zaragoza, R.; Garnacho-Montero, J.; Martín-Mazuelos, E.; Cuenca-Estrella, M.; Almirante, B. Impact of therapeutic strategies on the prognosis of candidemia in the ICU. Crit. Care Med. 2014, 42, 1423–1432. [Google Scholar] [CrossRef]
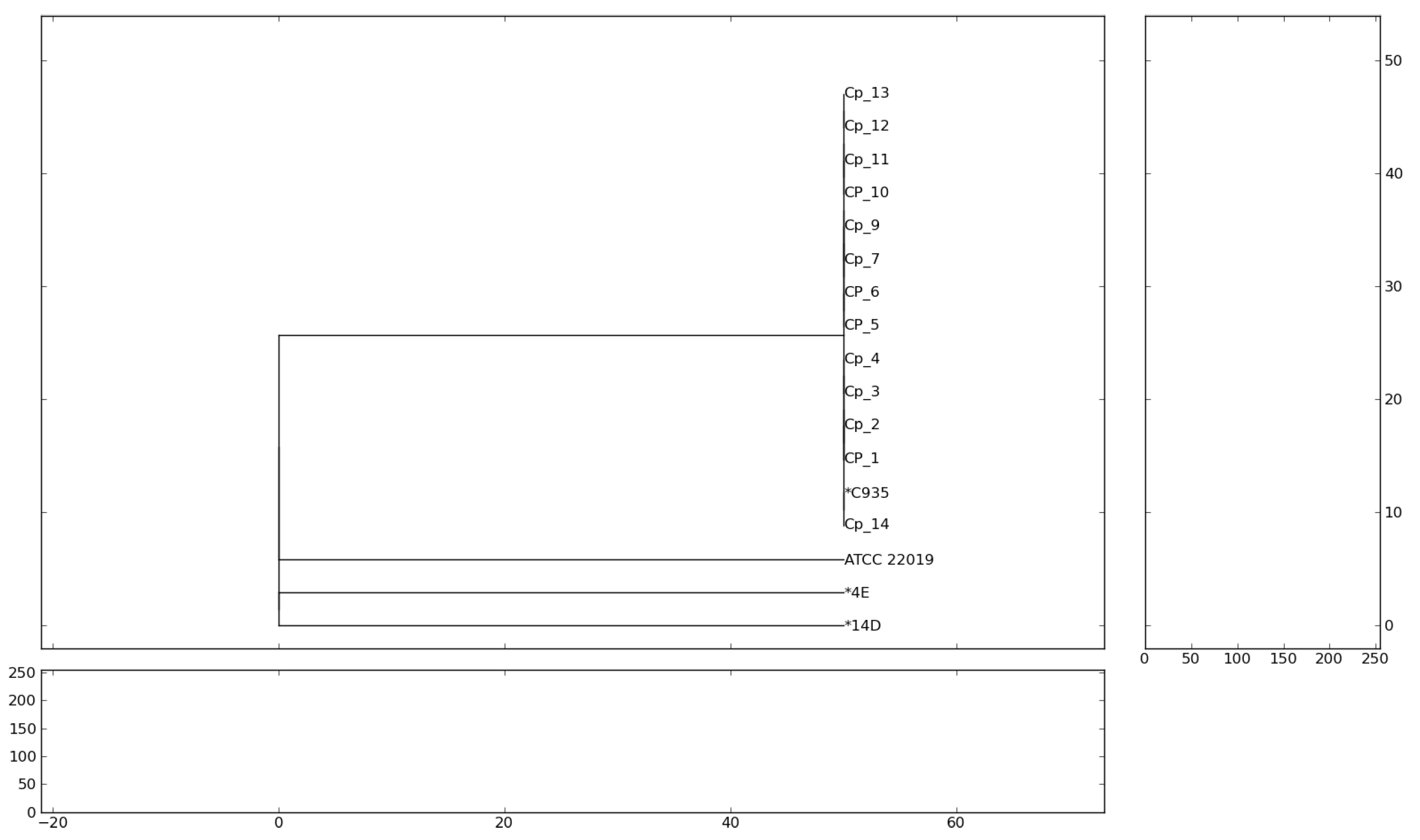
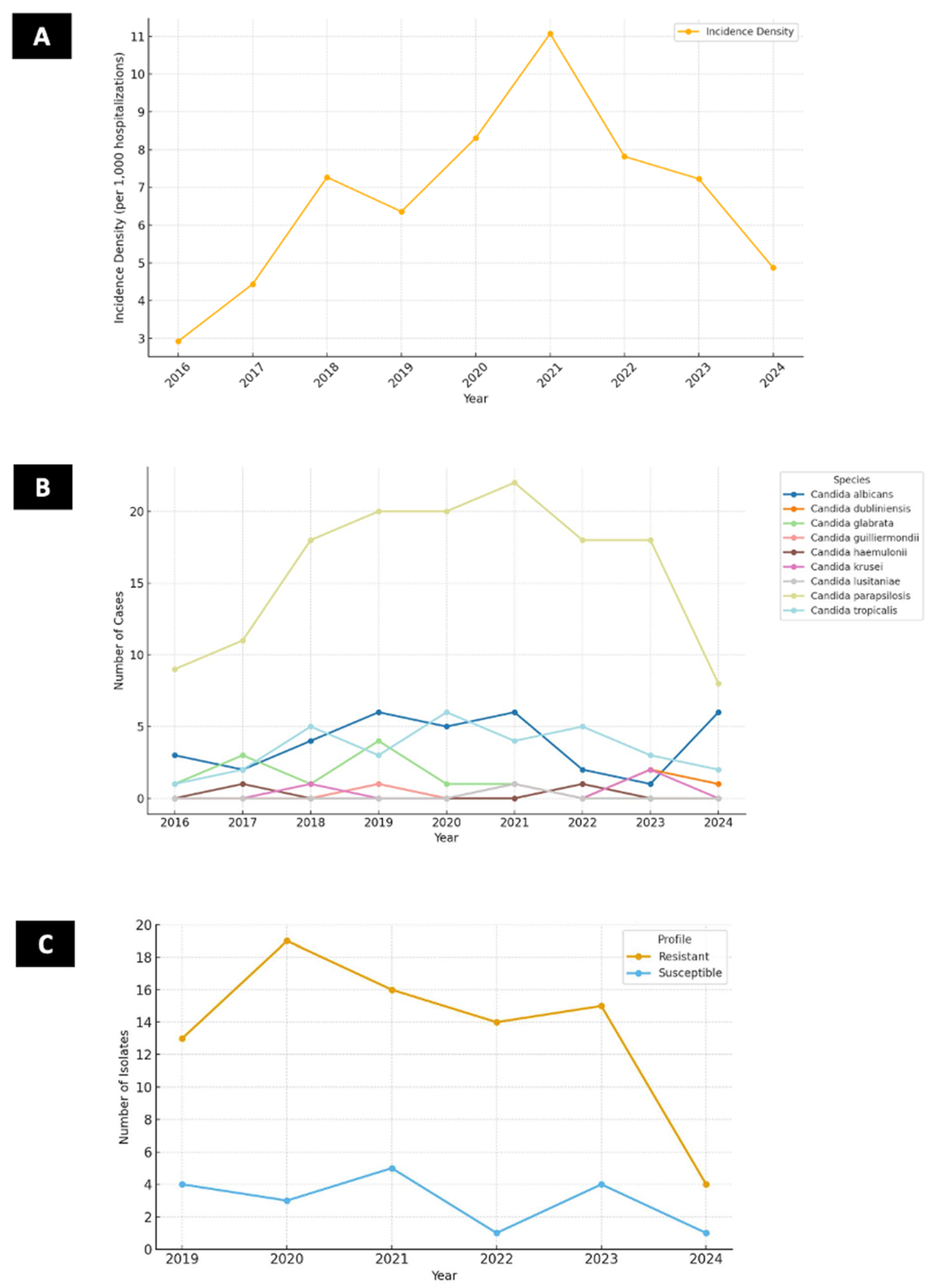
| Variable | All Patients (144) | Alive (64) | Dead (80) | p | OR (IC 99%) |
|---|---|---|---|---|---|
| Age (mean, ST; years) | 51.1 ± 22.6 | 58.1 ± 19.3 | 63.1 ± 16.8 | 0.360 | 1.02 (1.003–1.03) |
| Male | 78 (54.1%) | 33 (30%) | 45 (70%) | 0.575 | 0.91 (0.68–1.23) |
| Intensive Care Unit Admission | 144 (100%) | 64 (100%) | 80 (100%) | - | - |
| Length of Stay | 89 ± 62.8 | 91 | 52 | <0.01 | - |
| Comorbidities | |||||
| Cardiac disease | 109 (75%) | 43 (67%) | 66 (82%) | 0.003 | 2.30 (1.05–5.01) |
| Imunossupression | 31 (21%) | 14 (21%) | 17 (21%) | 0.928 | 0.96 (0.43–2.14) |
| Lung disease | 17 (11%) | 8 (12%) | 9 (11%) | 0.817 | 0.88 (0.32–2.44) |
| Diabetes mellitus | 38 (26%) | 15 (23%) | 23 (28%) | 0.472 | 1.31 (0.62–2.80) |
| Chronic Kidney disease | 19 (13%) | 6 (9%) | 13 (16%) | 0.226 | 1.87 (0.67–5.25) |
| COVID-19 | 13 (9%) | 5 (7%) | 8 (10%) | 0.649 | 1.31 (0.40–4.22) |
| Surgery | 132 (91%) | 59 (92%) | 73 (91%) | 0.840 | 0.88 (0.26–2.92) |
| Complications | |||||
| Deep Site Infection (n=141) | 31 (22%) | 15 (23%) | 16 (20%) | 0.638 | 1.09 (0.74–1.59) |
| Endophthalmitis (n = 24) | 1 (4%) | 0 | 1 (4.3) | - | - |
| Endocarditis | 8 (5%) | 5 (7%) | 3 (3%) | 0.290 | 0.46 (0.10–2.00) |
| Sternal osteomyelitis | 15 (10%) | 6 (9%) | 9 (11%) | 0.714 | 1.22 (0.41–3.64) |
| Intra-abdominal infection | 2 (1%) | 0 | 2 (2.5%) | 0.203 | 0.54 (0.47–0.63) |
| Surgical Site infection | 18 (12%) | 10 (15%) | 8 (10%) | 0.310 | 0.60 (0.22–1.62) |
| Persistent Candidemia (n = 80) | 26 (32%) | 14 (21%) | 12 (15%) | 0.536 | 0.84 (0.49–1.43) |
| Clinical characteristics | |||||
| Fever | 29 (20%) | 15 (23%) | 14 (17.5%) | 0.68 | 0.82 (0.28–2.41) |
| Source control (n = 36) | 36 (25%) | 18 (50%) | 18 (50%) | 0.14 | 2.12 (1.48–3.03) |
| Dialysis | 83 (57%) | 30 (46%) | 53 (66%) | 0.019 | 2.22 (1.13–4.37) |
| Vasoactive drug | 106 (73%) | 37 (57%) | 69 (86%) | <0.01 | 4.57 (2.04–10.25) |
| SOFA (n = 110) | 7.6 ± 4.2 | 4.83 ± 3.3 | 9.59 ± 3.72 | <0.01 | - |
| Mean arterial pression (n = 128) | 70.15 ± 18.7 | 70.4 ± 20.7 | 69.9 ±17.2 | 0.54 | - |
| Mechanical Ventilation | 83 (57%) | 28 (43%) | 55 (68%) | 0.003 | 2.82 (1.42–5.60) |
| Bacteremia | 38 (26%) | 18 (28%) | 20 (25%) | 0.179 | 1.07 (0.76–1.51) |
| Parenteral Nutrition | 25 (17%) | 5 (7%) | 20 (25%) | 0.007 | 3.93 (1.38–11.17) |
| Variable | All Patients (144) | Alive (64) | Dead (80) | p | OR (IC 99%) |
|---|---|---|---|---|---|
| Prior drug exposure | |||||
| Prior Antibiotics | 140 (97%) | 62 (96%) | 78 (97.5%) | 0.821 | 1.25 (0.17–9.18) |
| Prior antifungal | 71 (49%) | 27 (42%) | 44 (55%) | 0.126 | 1.67 (0.86–3.25) |
| Microbiology | |||||
| C.parapsilosis Fluconazole-Resistant (n = 99) | 81 (81%) | 35 (54%) | 46 (57%) | 0.113 | 1.07 (0.71–1.62) |
| >1 Candida on the same blood culture | 3 (2%) | 1 (1,5%) | 2 (2.5%) | 0.684 | 1.35 (0.31–5.90) |
| >1 Candida during CP candiemia | 8 (5%) | 3 (4%) | 5 (6%) | 0.684 | 0.88 (0.19–7.45) |
| C.albicans | 5 (3%) | 2 (3%) | 3 (3%) | 0.839 | 1.20 (0.44–1.91) |
| C.tropicalis | 5 (3%) | 1 (1.5%) | 4 (5%) | 0.263 | 3.31 (0.36–30.42) |
| C.glabrata | 1 (0.6%) | 0 | 1 (1.25%) | 0.369 | 0.55 (0.47–0.64) |
| Prior C.parapsilosis colonization | 42 (29%) | 17 (26%) | 25 (31%) | 0.378 | 0.90 (0.66–1.23) |
| Treatment | |||||
| Antifungal prescribed | 124 (86%) | 55 (85%) | 69 (86%) | 1.000 | 1.03 (0.20 -3.58) |
| Effective therapy | 109 (75%) | 52 (94%) | 57 (82%) | 0.043 | 1.53 (1.12–2.08) |
| Fluconazole (n = 124) | 28 (22%) | 13 (23%) | 15 (21%) | 0.063 | 1.05 (0.71–1.54) |
| Echinocandin (n = 124) | 91 (73%) | 39 (71%) | 52 (75%) | 0.311 | 0.90 (0.61–1.31) |
| Amphotericin (n = 124) | 8 (6%) | 3 (5.5%) | 5 (7.2%) | 0.163 | 0.83 (0.50 –1.54) |
| Optimal Therapy | 33 (22%) | 22 (14%) | 11 (18%) | 0.003 | 1.53 (1.12–2.08) |
| Catheter removal (n = 140) | 77 (55%) | 48 (62%) | 29 (37%) | <0.01 | 2.06 (1.50–2.83) |
| Early catheter removal (n = 81) | 37 (45%) | 22 (59%) | 14 (40%) | 0.700 | 0.89 (0.51–1.55) |
| Laboratory Findings | |||||
| Platelets (×103/mm3) | 159.770 ± 117.469 | 210.265 ± 119.541 | 119.375 ± 99.317 | <0.01 | - |
| Creatinine (mg/dL) | 1.85 ± 1.24 | 1.68 ± 1.24 | 1.98 ± 1.24 | 0.038 | - |
| Bilirrubins (n = 111) | 2.54 ± 4.3 | 0.77 ± 0.54 | 3.75 ± 5.24 | <0.01 | - |
| Leucocytes | 13.571 ± 8319 | 13.10 ± 7035 | 13.948 ± 9246 | 0.887 | - |
| Neutrophils | 10.668 ± 7095 | 10.474 ± 6475 | 10.823 ± 7593 | 0.963 | - |
| PCR [(mg/L (n = 142)] | 127 ± 75.8 | 103.6 ± 80.69 | 147.45 ±93.98 | 0.156 | - |
| Time frames | |||||
| Days between candidemia and death | 31.2 ± 32.1 | 56.7 ± 32.7 | 10.9 ± 8.14 | <0.01 | - |
| Days between hospitalization and candidemia | 57.8 ± 49.3 | 55.08 ± 49.7 | 59.9 ± 49.2 | 0.604 | - |
| Days between surgery and candidemia (n = 133) | 28.7 ± 102.3 | 30.6 ± 49.2 | 27.2 ± 130.4 | 0.013 | - |
| Days between candidemia and treatment (n = 86) | 11.69 ± 78.56 | 2.32 ± 1.77 | 14.34 ± 88.97 | 0.371 | - |
| Days between candidemia and first negative blood culture (n = 72) | 18.7 ± 62 | 25.6 ± 74.4 | 4.17 ± 2.61 | 0.004 | - |
| Days between candidemia and catheter removal (n = 79) | 5.4 ± 34 | 1.5 ± 13.6 | 11.7 ± 52.9 | 0.406 | - |
| Total treatment duration (n = 123) | 22 ± 20.7 | 33 ± 25.4 | 13.3 ± 9.7 | <0.01 | - |
| 30-Day crude mortality | 80 (55%) | - | - | - | - |
| Variable | p | OR | CI 95% |
|---|---|---|---|
| Cardiac disease | 0.006 | 19.356 | 2.293–163.3 |
| SOFA | 0.003 | 1.54 | 1.158–2.049 |
| Parenteral nutrition | 0.013 | 29.767 | 2.032–436.0 |
| Dialysis | 0.043 | 6.59 | 1.059–41.162 |
| Total treatment duration | <0.001 | 0.807 | 0.717–0.907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambo, C.T.; de Almeida, B.L.; Fialkovitz, G.; Cocio, T.A.; da Silva Junior, A.R.; Siqueira, L.P.M.; Silva, I.C.O.; Rossi, F.; Guimarães, T.; Siciliano, R.F.; et al. Nine-Year Surveillance of Candida parapsilosis Candidemia in a Cardiothoracic ICU: Insights into Mortality and Resistance. J. Fungi 2025, 11, 692. https://doi.org/10.3390/jof11100692
Sambo CT, de Almeida BL, Fialkovitz G, Cocio TA, da Silva Junior AR, Siqueira LPM, Silva ICO, Rossi F, Guimarães T, Siciliano RF, et al. Nine-Year Surveillance of Candida parapsilosis Candidemia in a Cardiothoracic ICU: Insights into Mortality and Resistance. Journal of Fungi. 2025; 11(10):692. https://doi.org/10.3390/jof11100692
Chicago/Turabian StyleSambo, Caio Trevelin, Bianca Leal de Almeida, Gabriel Fialkovitz, Tiago Alexandre Cocio, Afonso Rafael da Silva Junior, Lumena Pereira Machado Siqueira, Isabela Cristina Oliveira Silva, Flavia Rossi, Thaís Guimarães, Rinaldo Focaccia Siciliano, and et al. 2025. "Nine-Year Surveillance of Candida parapsilosis Candidemia in a Cardiothoracic ICU: Insights into Mortality and Resistance" Journal of Fungi 11, no. 10: 692. https://doi.org/10.3390/jof11100692
APA StyleSambo, C. T., de Almeida, B. L., Fialkovitz, G., Cocio, T. A., da Silva Junior, A. R., Siqueira, L. P. M., Silva, I. C. O., Rossi, F., Guimarães, T., Siciliano, R. F., de Araújo, E. d. M. P., Del Negro, G. M. B., Benard, G., Strabelli, T. M. V., & Magri, M. M. C. (2025). Nine-Year Surveillance of Candida parapsilosis Candidemia in a Cardiothoracic ICU: Insights into Mortality and Resistance. Journal of Fungi, 11(10), 692. https://doi.org/10.3390/jof11100692






