Effects of Orange Peel Extract on Laccase Activity and Gene Expression in Trametes versicolor
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culturing Conditions
2.2. Enzymatic Assays
2.3. Quantification of Colonization
2.4. Nucleic Acid Extraction
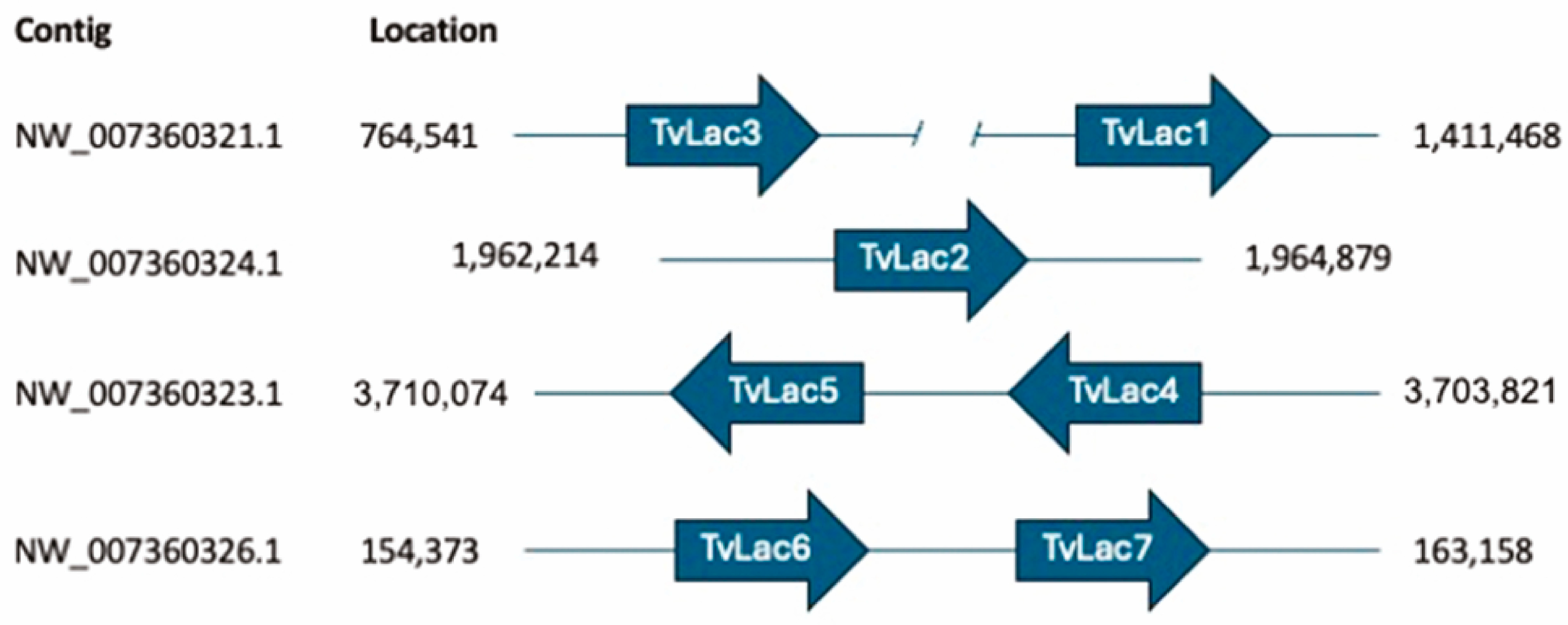
2.5. Bioinformatic Analysis
2.6. Quantitative Reverse Transcriptase PCR
3. Results and Discussion
3.1. Growth and Oxidative Potential of Four Different T. versicolor Strains in Liquid Cultures
3.2. Impact of the Addition of Orange Peel Extract and Veratryl Alcohol on the Oxidative Potential
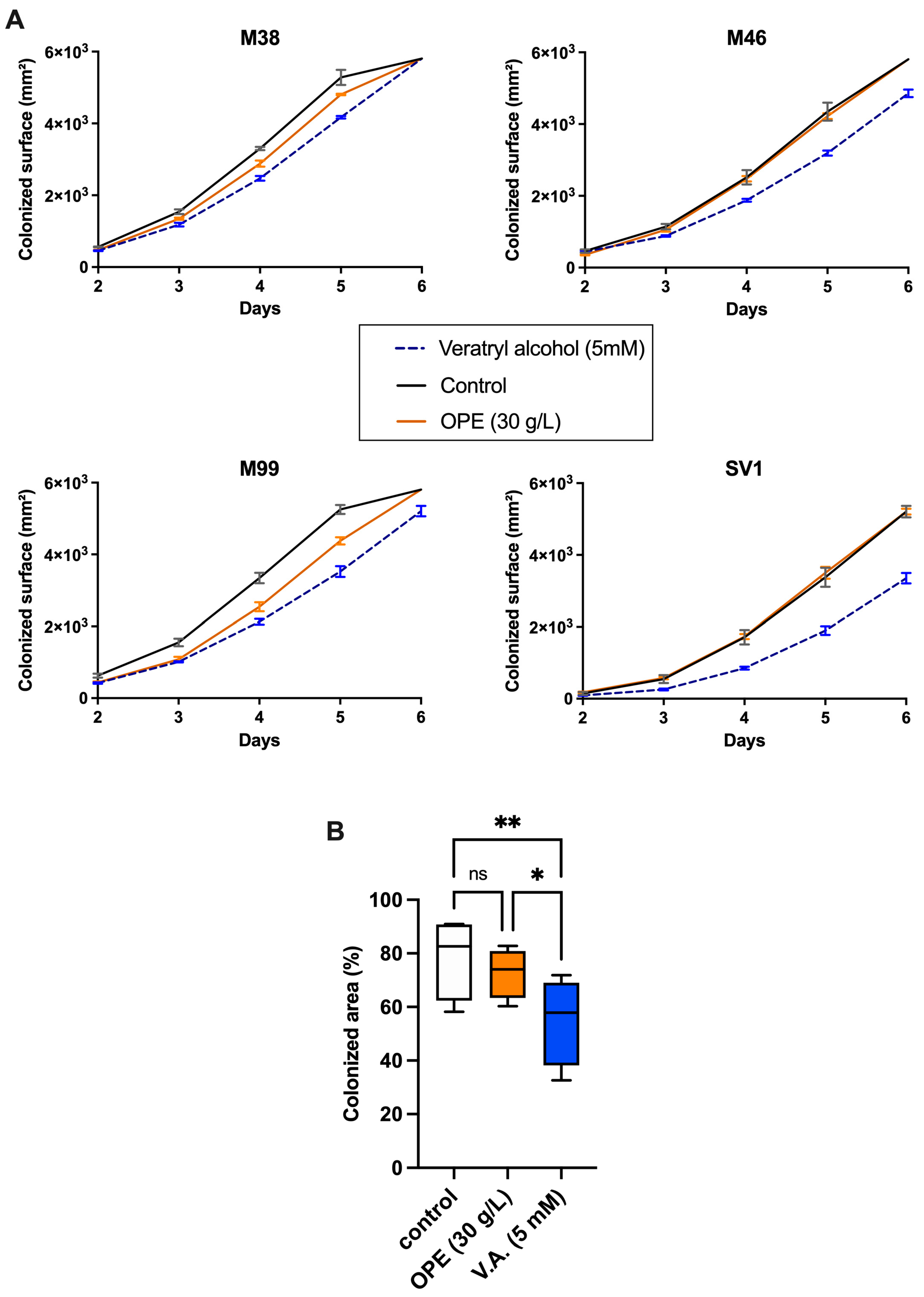
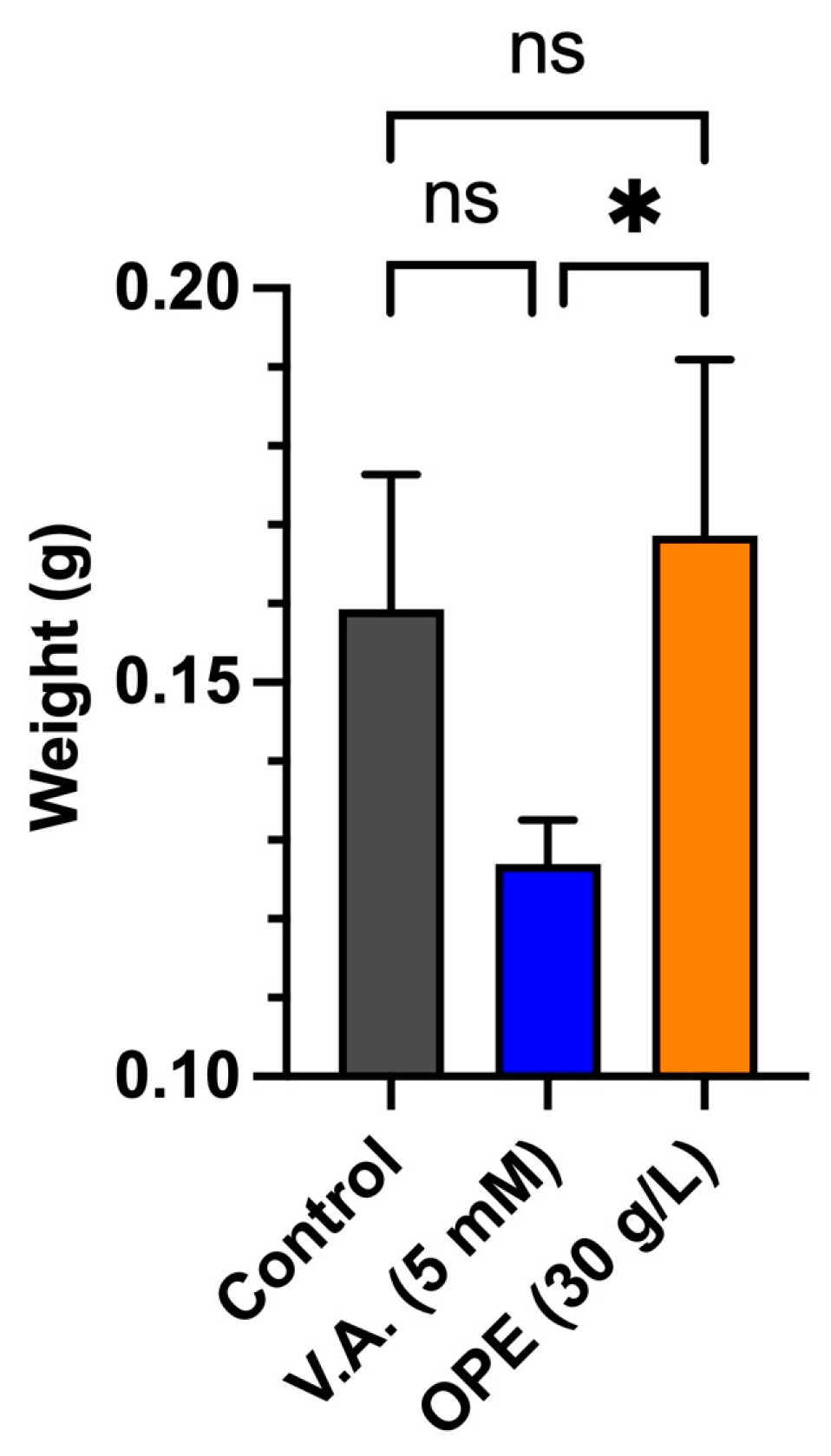
3.3. Impact of Orange Peel Extract and Veratryl Alcohol on Surface Colonization and Biomass Yield
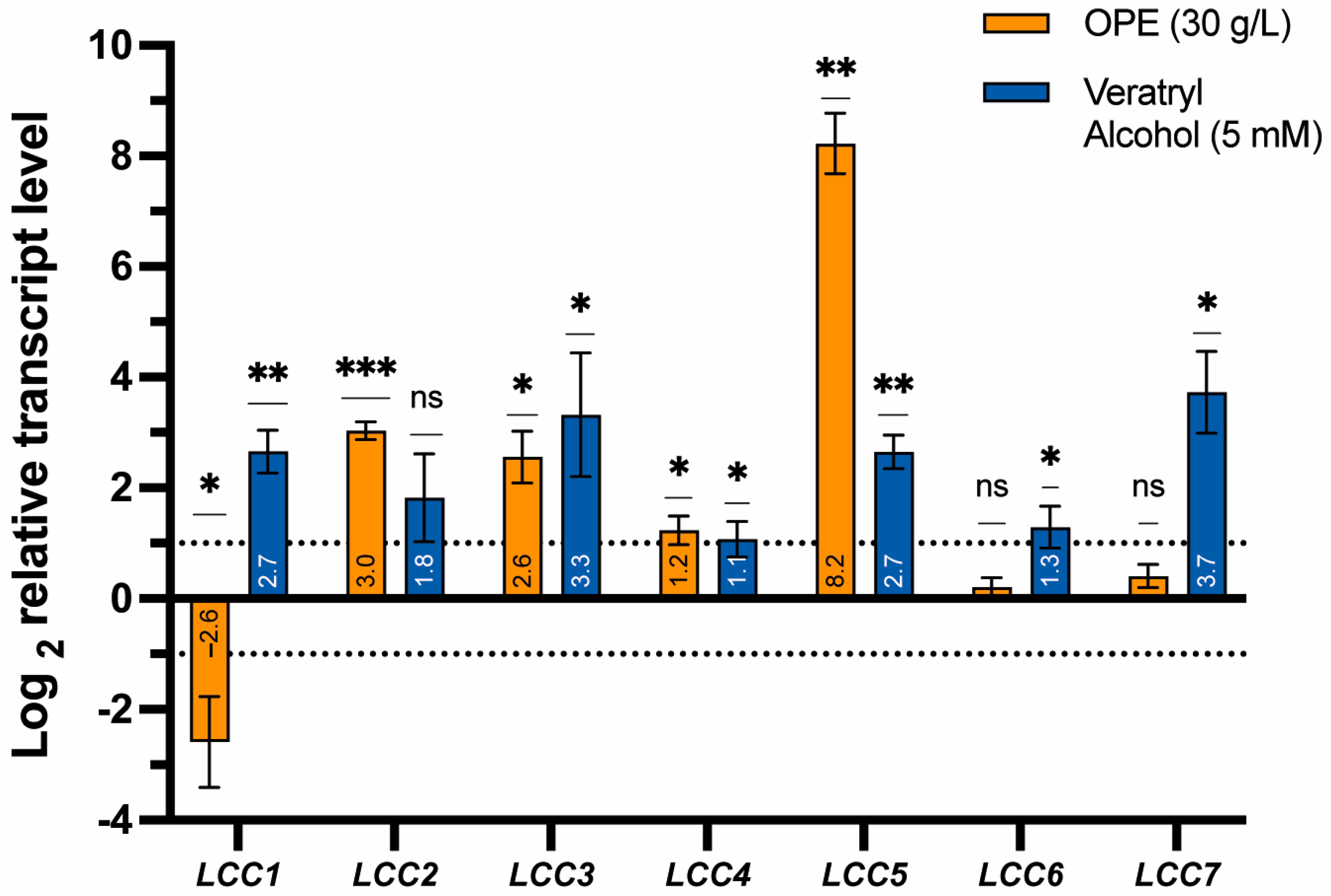
3.4. Effects of Orange Peel Extract and Veratryl Alcohol on the Transcriptional Expression of Laccase Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Zhu, M.; Guo, X.; Yang, B.; Zhuo, R. Coupling of Fenton reaction and white rot fungi for the degradation of organic pollutants. Ecotoxicol. Environ. Saf. 2023, 254, 114697. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wei, M.; Zhang, X.; Xun, Y.; Duan, M.; Yang, Z.; Zhu, M.; Zhu, Y.; Zhuo, R. Copper removal from aqueous solutions by white rot fungus Pleurotus ostreatus GEMB-PO1 and its potential in co-remediation of copper and organic pollutants. Bioresour. Technol. 2024, 395, 130337. [Google Scholar] [CrossRef] [PubMed]
- Malkin, R.; Malmström, B.G. The state and function of copper in biological systems. Adv. Enzymol. Relat. Areas Mol. Biol. 1970, 33, 177–244. [Google Scholar] [CrossRef] [PubMed]
- Aza, P.; Camarero, S. Fungal laccases: Fundamentals, engineering and classification update. Biomolecules 2023, 13, 1716. [Google Scholar] [CrossRef]
- Rui, Z.; Fangfang, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-R.; Baldrian, P.; Murugesan, K.; Chang, Y.-S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Ihssen, J.; Reiss, R.; Luchsinger, R.; Thöny-Meyer, L.; Richter, M. Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci. Rep. 2015, 5, 10465. [Google Scholar] [CrossRef]
- Schuetz, M.; Benske, A.; Smith, R.A.; Watanabe, Y.; Tobimatsu, Y.; Ralph, J.; Demura, T.; Ellis, B.; Samuels, A.L. Laccases direct lignification in the discrete secondary cell wall domains of protoxylem. Plant Physiol. 2014, 166, 798–807. [Google Scholar] [CrossRef]
- Forootanfar, H.; Faramarzi, M.A. Insights into laccase producing organisms, fermentation states, purification strategies, and biotechnological applications. Biotechnol. Prog. 2015, 31, 1443–1463. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, S.; Afreen, S.; Bhalla, A.; Khurana, J.; Chandel, S.; Aggarwal, A.; Arya, S.K. Laccase mediated delignification of wasted and non-food agricultural biomass: Recent developments and challenges. Int. J. Biol. Macromol. 2023, 235, 123840. [Google Scholar] [CrossRef] [PubMed]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of lignocellulose using ligninolytic enzymes from white-rot fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef] [PubMed]
- Euring, M.; Rühl, M.; Ritter, N.; Kües, U.; Kharazipour, A. Laccase mediator systems for eco-friendly production of medium-density fiberboard (MDF) on a pilot scale: Physicochemical analysis of the reaction mechanism. Biotechnol. J. 2011, 6, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Mate, D.M.; Alcalde, M. Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Liang, H.; Li, M.; Liu, Y.; Wang, L.; Lai, W.; Tang, T.; Diao, Y.; Bai, Y.; et al. Distinct laccase expression and activity profiles of Trametes versicolor facilitate degradation of benzo(a)pyrene. Front. Bioeng. Biotechnol. 2023, 11, 1264135. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhang, Z.X.; Xie, S.W.; Xing, H.; Zhu, Y.N.; Li, H.Y.; Yi, Z.S. Study on transformation and degradation of bisphenol A by Trametes versicolor laccase and simulation of molecular docking. Chemosphere 2019, 224, 743–750. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Collins, P.J.; Dobson, A. Regulation of laccase gene transcription in Trametes versicolor. Appl. Environ. Microbiol. 1997, 63, 3444–3450. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.G.M.; Tychanowicz, G.K.; de Souza, D.F.; Peralta, R.M. Production of laccase isoforms by Pleurotus pulmonarius in response to presence of phenolic and aromatic compounds. J. Basic Microbiol. 2004, 44, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Faraco, V.; Giardina, P.; Sannia, G. Metal-responsive elements in Pleurotus ostreatus laccase gene promoters. Microbiol. Read. Engl. 2003, 149, 2155–2162. [Google Scholar] [CrossRef]
- Piscitelli, A.; Giardina, P.; Lettera, V.; Pezzella, C.; Sannia, G.; Faraco, V. Induction and Transcriptional Regulation of Laccases in Fungi. Curr. Genom. 2011, 12, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.; Yuan, P.; Yang, Y.; Zhang, S.; Ma, F.; Zhang, X. Induction of laccase by metal ions and aromatic compounds in Pleurotus ostreatus HAUCC 162 and decolorization of different synthetic dyes by the extracellular laccase. Biochem. Eng. J. 2017, 117, 62–72. [Google Scholar] [CrossRef]
- Vasconcelos, A.F.D.; Barbosa, A.M.; Dekker, R.F.H.; Scarminio, I.S.; Rezende, M.I. Optimization of laccase production by Botryosphaeria sp. in the presence of veratryl alcohol by the response-surface method. Process Biochem. 2000, 35, 1131–1138. [Google Scholar] [CrossRef]
- Dekker, R.F.H.; Barbosa, A.M.; Sargent, K. The effect of lignin-related compounds on the growth and production of laccases by the ascomycete, Botryosphaeria sp. Enzym. Microb. Technol. 2002, 30, 374–380. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, F.; Zhuo, R.; Fan, F.; Liu, H.; Zhang, C.; Ma, L.; Jiang, M.; Zhang, X. Enhancing the Laccase Production and Laccase Gene Expression in the White-Rot Fungus Trametes velutina 5930 with Great Potential for Biotechnological Applications by Different Metal Ions and Aromatic Compounds. PLoS ONE 2013, 8, e79307. [Google Scholar] [CrossRef] [PubMed]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R.A.; Henrissat, B.; Martínez, A.T.; Otillar, R.; Spatafora, J.W.; Yadav, J.S.; et al. The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Sciences 2012, 336, 1715–1719. [Google Scholar] [CrossRef]
- Moiseenko, K.V.; Maloshenok, L.G.; Vasina, D.V.; Bruskin, S.A.; Tyazhelova, T.V.; Koroleva, O.V. Laccase multigene families in Agaricomycetes. J. Basic. Microbiol. 2016, 56, 1392–1397. [Google Scholar] [CrossRef]
- Tišma, M.; Žnidaršič-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes versicolor in lignocellulose-based bioeconomy: State of the art, challenges and opportunities. Bioresour. Technol. 2021, 330, 124997. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, V.E.; Michelin, M.; Vici, A.C.; de Almeida, P.Z.; Teixeira de Moraes Polizeli, M.L. Trametes versicolor laccase production using agricultural wastes: A comparative study in erlenmeyer flasks, bioreactor and tray. Bioprocess. Biosyst. Eng. 2020, 43, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, L.; Zhao, L.T.; Ding, Z.Y.; Ma, H.I.; Terry, N. Fungal laccase production from lignocellulosic agricultural wastes by solid-state fermentation: A review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, K.; Wang, F.; Zhao, L.; Hu, J.; Ma, H.; Ding, Z. Laccase production by Trametes versicolor in solid-state fermentation using tea residues as substrate and its application in dye decolorization. J. Environ. Manag. 2020, 270, 110904. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, X.; Yu, Z.; Cui, Y.; Xu, L.; Huo, S.; Ding, Z.; Zhao, L.; Du, L.; Qiu, Y. Improved laccase production by Trametes versicolor using copper-glycyl-L-histidyl-L-lysine as a novel and high-efficient inducer. Front. Bioeng. Biotechnol. 2023, 11, 1176352. [Google Scholar] [CrossRef] [PubMed]
- Rosales, E.; Rodríguez Couto, S.; Sanromán, M.A. Increased laccase production by Trametes hirsuta grown on ground orange peelings. Enzym. Microb. Technol. 2007, 40, 1286–1290. [Google Scholar] [CrossRef]
- Borràs, E.; Blánquez, P.; Sarrà, M.; Caminal, G.; Vicent, T. Trametes versicolor pellets production: Low-cost medium and scale-up. Biochem. Eng. J. 2008, 42, 61–66. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Raaska, L.; Itävaara, M. Detection of white-rot fungi by a non-toxic stain. Mycol. Res. 1990, 94, 27–31. [Google Scholar] [CrossRef]
- Srinivasan, C.; Dsouza, T.M.; Boominathan, K.; Reddy, C.A. Demonstration of Laccase in the White Rot Basidiomycete Phanerochaete chrysosporium BKM-F1767. Appl. Environ. Microbiol. 1995, 61, 4274–4277. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1989, 38, 315–322. [Google Scholar] [CrossRef]
- Champagne, P.-P.; Ramsay, J.A. Contribution of manganese peroxidase and laccase to dye decoloration by Trametes versicolor. Appl. Microbiol. Biotechnol. 2005, 69, 276–285. [Google Scholar] [CrossRef]
- Couto, S.R. Decolouration of industrial azo dyes by crude laccase from Trametes hirsuta. J. Hazard. Mater. 2007, 148, 768–770. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules 2021, 26, 1348. [Google Scholar] [CrossRef]
- Yeoh, S.; Shi, J.; Langrish, T.A.G. Comparisons between different techniques for water-based extraction of pectin from orange peels. Desalination 2008, 218, 229–237. [Google Scholar] [CrossRef]
- Perez-Cacho, P.R.; Rouseff, R.L. Fresh squeezed orange juice odor: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 681–695. [Google Scholar] [CrossRef]
- Ferrer, V.; Paymal, N.; Quinton, C.; Tomi, F.; Luro, F. Investigations of the chemical composition and aromatic properties of peel essential oils throughout the complete phase of fruit development for two cultivars of sweet orange (Citrus sinensis (L.) Osb.). Plants 2022, 11, 2747. [Google Scholar] [CrossRef]
- Böhmer, U.; Frömmel, S.; Bley, T.; Müller, M.; Frankenfeld, K.; Miethe, P. Solid-state fermentation of lignocellulotic materials for the production of enzymes by the white-rot fungus Trametes hirsuta in a modular bioreactor. Eng. Life Sci. 2011, 11, 395–401. [Google Scholar] [CrossRef]
- Ottoni, C.; Simões, M.F.; Fernandes, S.; Santos, C.R.; Lima, N. High laccase expression by Trametes versicolor in a simulated textile effluent with different carbon sources and pHs. Int. J. Environ. Res. Public Health 2016, 13, 778. [Google Scholar] [CrossRef] [PubMed]


| Strain | Geographical Origin | Source | Conservation |
|---|---|---|---|
| M38 | Czech Republic | Belgian Co-ordinated Collections of Micro-organisms (BCCM) | 10% glycerol −130 °C since 12 September 2013 |
| M46 | South Korea | Belgian Co-ordinated Collections of Micro-organisms (BCCM) | 10% glycerol −130 °C since 16 June 2005 |
| M99 | Unknown | Mycelia bvba (reference: T. versicolor M9912) | Actively cultured |
| SV1 | Belgium | Isolated from a decaying tree trunk in Schaveys park Linkebeek (Belgium) | Actively cultured |
| Gene Name | Accession Number (NCBI) | Gene Length (bp) | Coding Sequence Length (bp) | Exon # | Sequences of Forward/Reverse qPCR Primers (5′ -> 3′) |
|---|---|---|---|---|---|
| TvLac1 | XM_008034546 | 2222 | 1560 | 11 | CATCACGTTGACCGACTGG/GACGTTGATCACAGCAAGCG |
| TvLac2 | XM_008038707 | 2222 | 1575 | 11 | AAGGCTATCAACTTCGCTTT/CTCATGATTTGCAGCAGAAC |
| TvLac3 | XM_008034423 | 2074 | 1563 | 9 | ACTTCGGTAACGTCGGGTTC/TGCAGGTTGACCTCGTTGAG |
| TvLac4 | XM_008037774 | 2118 | 1563 | 11 | CAGATTCTTAGCGGCACCAC/ GAATGTGTGACCGTGCAAGT |
| TvLac5 | XM_008037775 | 2266 | 1584 | 12 | CCCGTCACCGACTTGACTAT/GGTCTCGTTGGTCAGGTTGT |
| TvLac6 | XM_008040042 | 2266 | 1539 | 13 | CACCAAGTCGACGGACTTCA/CCCGTAGTGTTAGCACGGTT |
| TvLac7 | XM_008040097 | 2210 | 1548 | 13 | TTGAACTGTCCATCCCTGGC/GTACCACGGAGAAGGTGTGG |
| β-tubulin 2 * | XM_008041613.1 | 2723 | 1401 | 17 | ACAGTACCAAGAAGCGACGG/GCCTTCTGGTTGGGATCGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandelook, S.; Bassleer, B.; Elsacker, E.; Peeters, E. Effects of Orange Peel Extract on Laccase Activity and Gene Expression in Trametes versicolor. J. Fungi 2024, 10, 370. https://doi.org/10.3390/jof10060370
Vandelook S, Bassleer B, Elsacker E, Peeters E. Effects of Orange Peel Extract on Laccase Activity and Gene Expression in Trametes versicolor. Journal of Fungi. 2024; 10(6):370. https://doi.org/10.3390/jof10060370
Chicago/Turabian StyleVandelook, Simon, Berend Bassleer, Elise Elsacker, and Eveline Peeters. 2024. "Effects of Orange Peel Extract on Laccase Activity and Gene Expression in Trametes versicolor" Journal of Fungi 10, no. 6: 370. https://doi.org/10.3390/jof10060370
APA StyleVandelook, S., Bassleer, B., Elsacker, E., & Peeters, E. (2024). Effects of Orange Peel Extract on Laccase Activity and Gene Expression in Trametes versicolor. Journal of Fungi, 10(6), 370. https://doi.org/10.3390/jof10060370







