Diversity, Prevalence and Virulence of Colletotrichum Species Causing Anthracnose on Cassava Leaves in the Northern Region of Brazil
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling, Isolation and Collection
2.2. DNA Extraction, PCR Amplification and DNA Sequencing
2.3. Phylogenetic Analysis and Species Recognition
2.4. Prevalence of Colletotrichum Species
2.5. Pathogenicity, Aggressiveness and Virulence
3. Results
3.1. Collection and Isolation
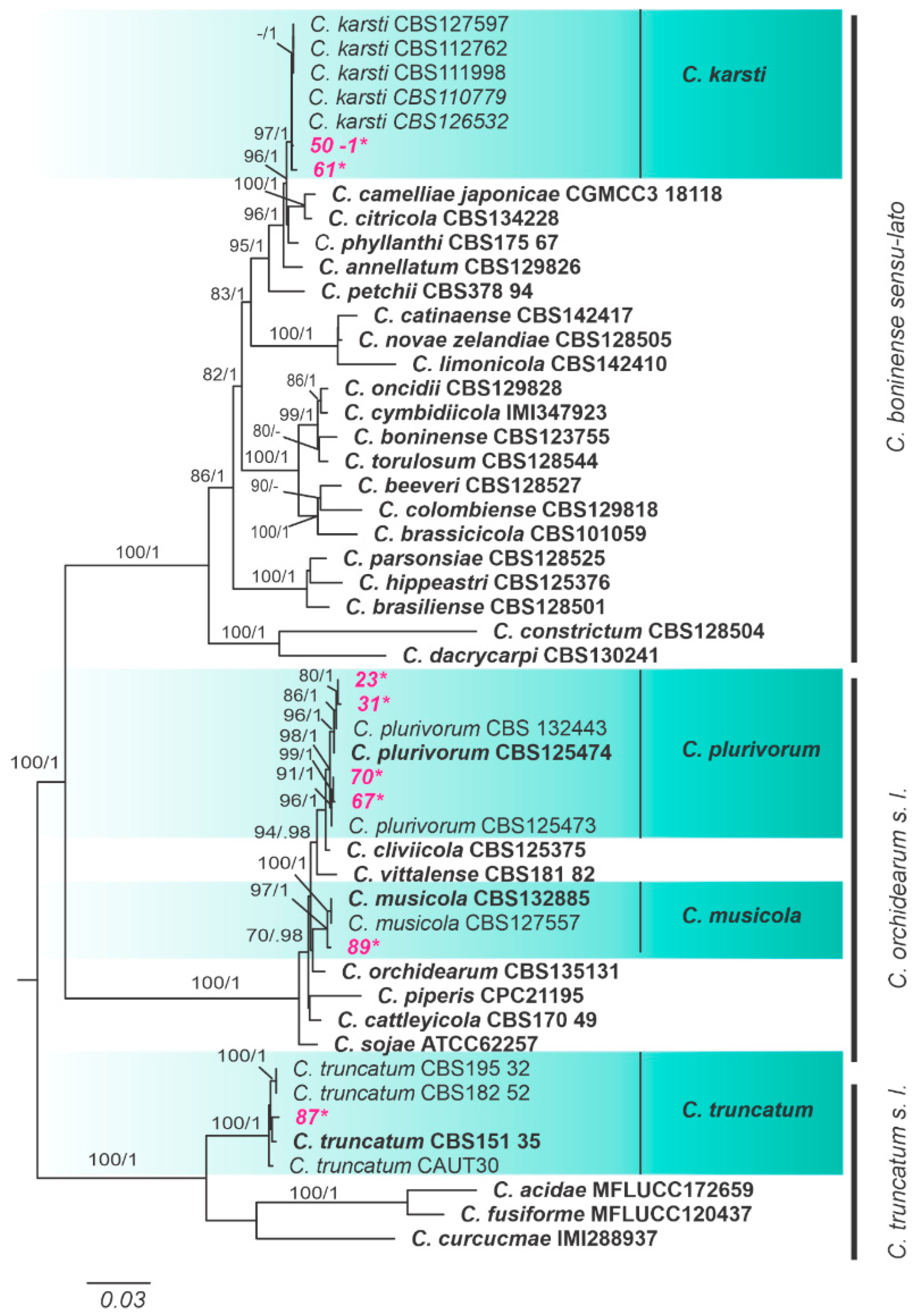
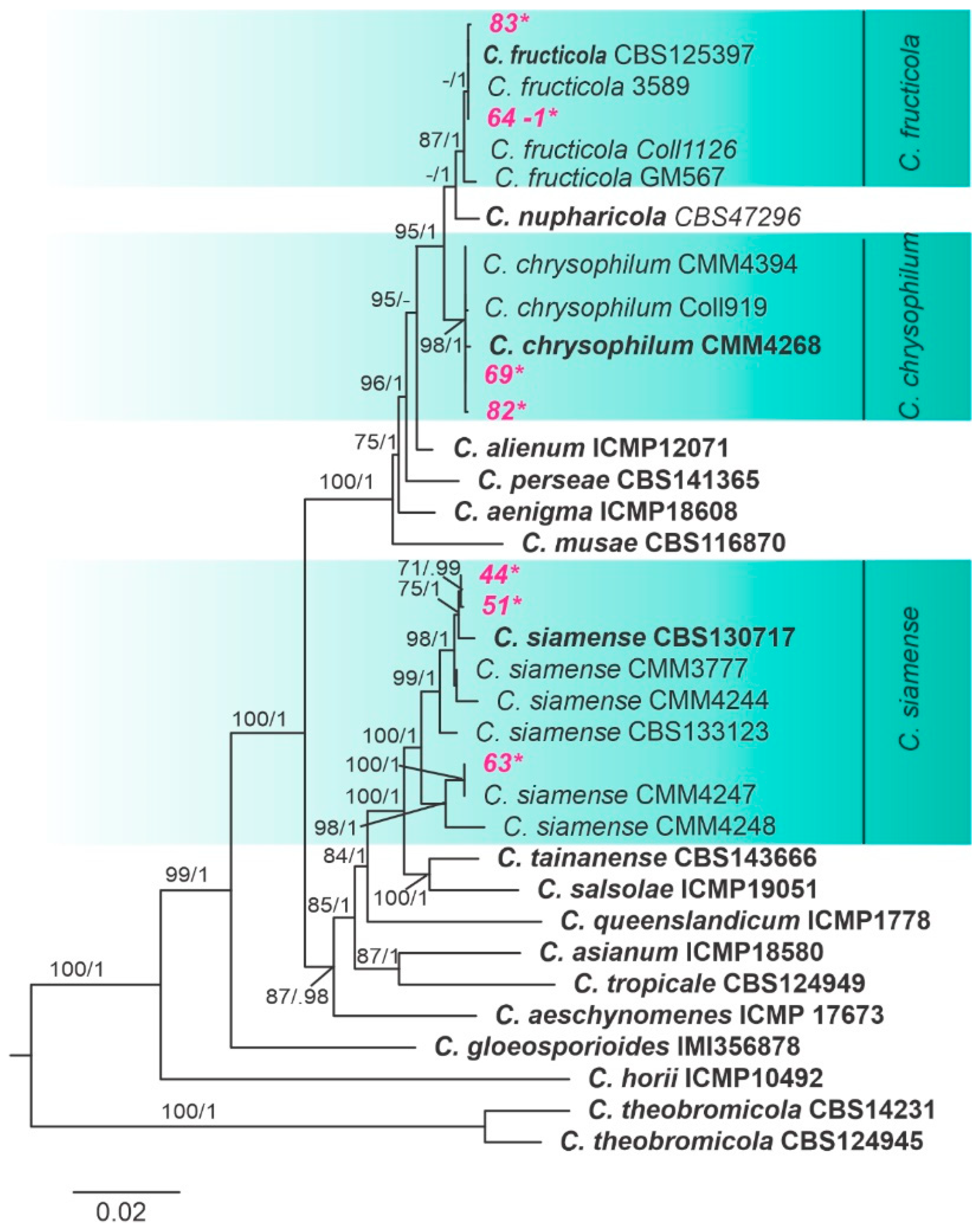
3.2. Phylogenetic Analyses and Species Assignment
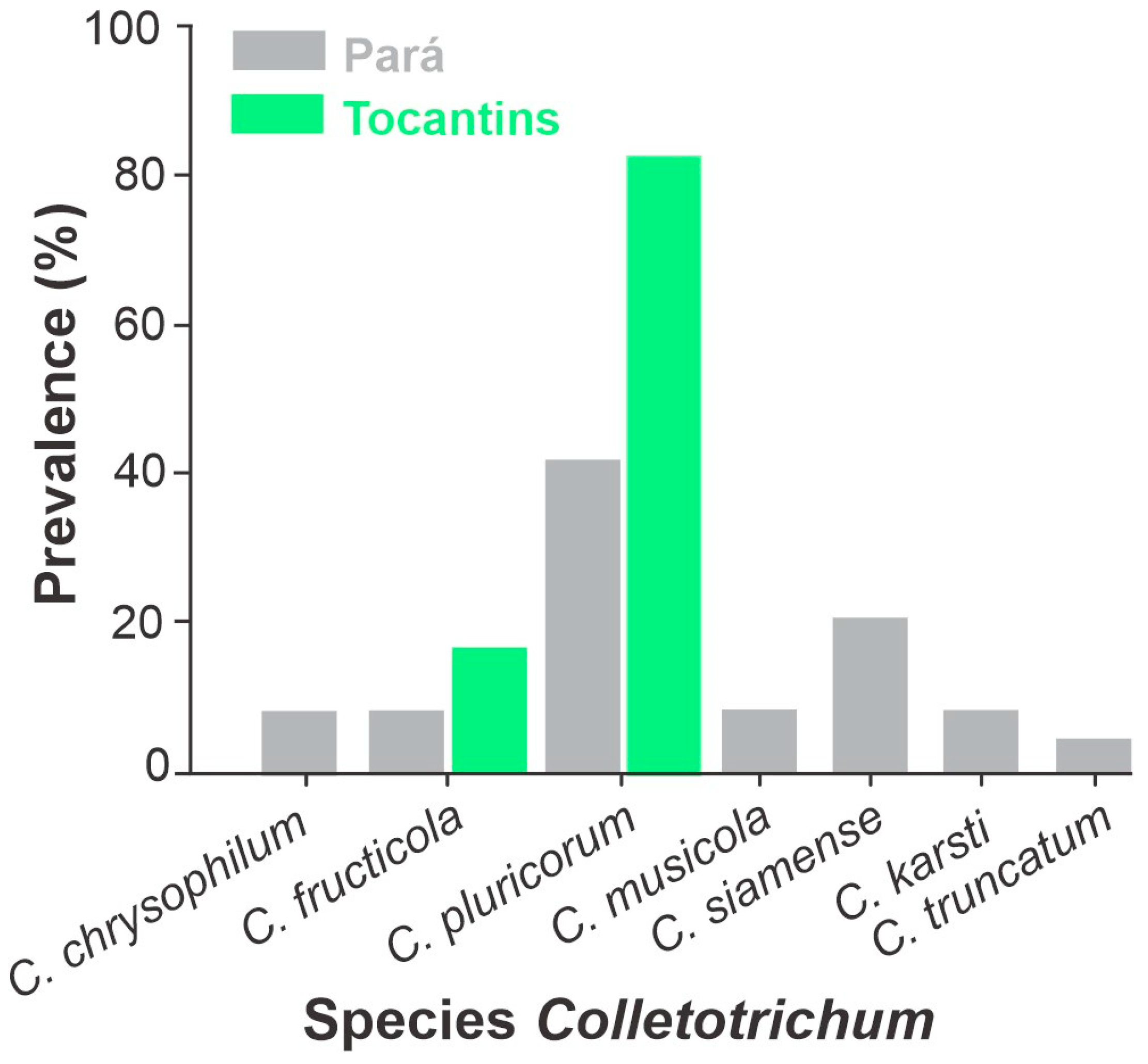
3.3. Species Prevalence of Colletotrichum Isolates by Locality
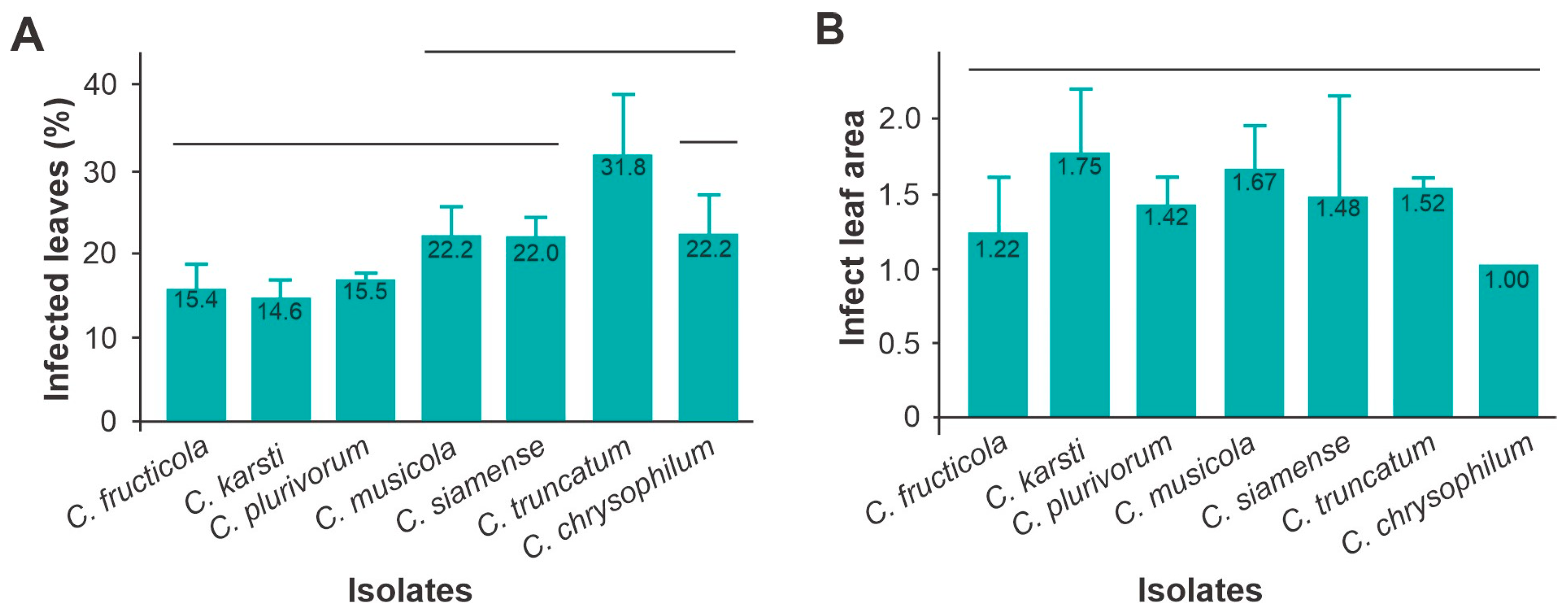
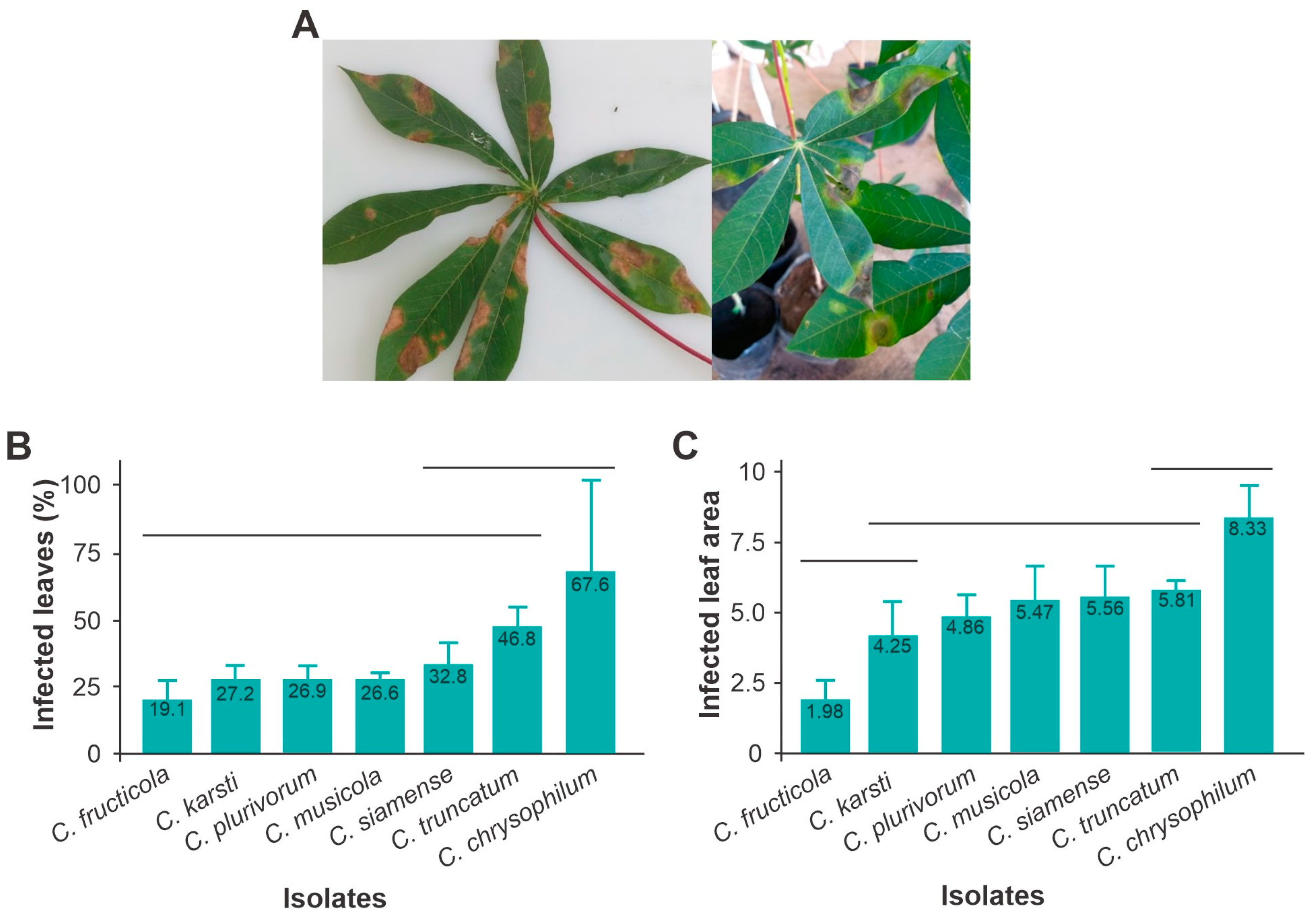
3.4. Aggressiveness and Virulence of Colletotrichum Manihot esculenta
- (a)
- Aggressiveness
- (b) Virulence in cassava leaves
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pushpalatha, R.; Gangadharan, B. Is Cassava (Manihot esculenta Crantz) a climate “Smart” crop? A review in the context of bridging future food demand gap. Trop. Plant Biol. 2020, 13, 201–211. [Google Scholar] [CrossRef]
- Krishnakumar, T.; Sajeev, M.; Raju, S.; Giri, N.; Pradeepika, C.; Bansode, V. Studies on the development of cassava based reconstituted dry starch sago with modified starch as binder and characterization of its physico-functional properties. J. Environ. Biol. 2020, 41, 29–34. [Google Scholar] [CrossRef]
- Boukhers, I.; Boudard, F.; Morel, S.; Servent, A.; Portet, K.; Guzman, C.; Vitou, M.; Kongolo, J.; Michel, A.; Poucheret, P. Nutrition, healthcare benefits and phytochemical properties of cassava (Manihot esculenta) leaves sourced from three countries (Reunion, Guinea, and Costa Rica). Foods 2022, 11, 2027. [Google Scholar] [CrossRef]
- Fathima, A.A.; Sanitha, M.; Tripathi, L.; Muiruri, S. Cassava (Manihot esculenta) dual use for food and bioenergy: A review. Food Energy Secur. 2023, 12, e380. [Google Scholar] [CrossRef]
- Abotbina, W.; Sapuan, S.M.; Ilyas, R.A.; Sultan, M.T.H.; Alkbir, M.F.M.; Sulaiman, S.; Harussani, M.M.; Bayraktar, E. Recent developments in cassava (Manihot esculenta) based biocomposites and their potential industrial applications: A comprehensive Review. Materials 2022, 15, 6992. [Google Scholar] [CrossRef]
- da Silva Pereira, G.V.; da Silva Pereira, G.V.; Xavier Neves, E.M.P.; Albuquerque, G.A.; de Arimatéia Rodrigues do Rêgo, J.; Cardoso, D.N.P.; do Socorro Barros Brasil, D.; Joele, M.R.S.P. Effect of the mixture of polymers on the rheological and technological properties of composite films of acoupa weakfish (Cynoscion acoupa) and cassava starch (Manihot esculenta C.). Food Bioprocess. Technol. 2021, 14, 1199–1215. [Google Scholar] [CrossRef]
- FAO. Food Supply: Crops Primary Equivalent; FAO: Quebec City, QC, Canada, 2020; Volume 2022. [Google Scholar]
- Ono, L.T.; Taniwaki, M.H. Fungi and mycotoxins in cassava (Manihot esculenta Crantz) and its products. Braz. J. Food Technol. 2021, 24, e2020240. [Google Scholar] [CrossRef]
- Ono, L.T.; Silva, J.J.; Doná, S.; Martins, L.M.; Iamanaka, B.T.; Fungaro, M.H.P.; Pitt, J.I.; Taniwaki, M.H. Aspergillus section Flavi and aflatoxins in Brazilian cassava (Manihot esculenta Crantz) and products. Mycotoxin Res. 2021, 37, 221–228. [Google Scholar] [CrossRef]
- EMBRAPA. Mandioca em Números, Embrapa Amazônia Oriental. Available online: https://www.embrapa.br/congresso-de-mandioca-2018/mandioca-em-numeros (accessed on 7 October 2021).
- Wokocha, R.; Nneke, N.; Umechuruba, C. Screening Colletotrichum gloeosporioides f. sp Manihotis isolates for virulence on cassava in Akwa Ibom state of Nigeria. Agro-Science 2010, 9, 56–63. [Google Scholar] [CrossRef]
- Liu, X.; Shi, T.; Li, B.; Cai, J.; Li, C.; Huang, G. Colletotrichum species associated with cassava anthracnose in China. J. Phytopathol. 2019, 167, 12765. [Google Scholar] [CrossRef]
- Oliveira, S.A.S.; Silva, L.L.; Diamantino, M.S.A.S.; Ferreira, C.F. First Report of Colletotrichum theobromicola and C. siamense causing anthracnose on cultivated and wild cassava species in Brazil. Plant Dis. 2018, 102, 819. [Google Scholar] [CrossRef]
- de Oliveira, S.A.S.; da Silva, L.L.; Nascimento, D.d.S.; Diamantino, M.S.A.S.; Ferreira, C.F.; de Oliveira, T.A.S. Colletotrichum species causing cassava (Manihot esculenta Crantz) anthracnose in different eco-zones within the Recôncavo Region of Bahia, Brazil. J. Plant Dis. Prot. 2020, 127, 411–416. [Google Scholar] [CrossRef]
- Morais, M.d.S.; Nascimento, L.C.d.; Moreira, K.A.; Cavalcanti, M.d.S.; Oliveira, N.T.d. Levantamento e avaliação da incidência das doenças da mandioca no estado da Paraíba. Summa Phytopathol. 2013, 39, 204–206. [Google Scholar] [CrossRef]
- Kunkeaw, S.; Worapong, J.; Smith, D.R.; Triwitayakorn, K. An in vitro detached leaf assay for pre-screening resistance to anthracnose disease in cassava (Manihot esculenta Crantz). Australas. Plant Pathol. 2010, 39, 547–550. [Google Scholar] [CrossRef]
- Onyeka, T.; Owolade, O.; Ogunjobi, A.; Dixon, A.G.; Okechukwu, R.; Bamkefa, B. Prevalence and severity of bacterial blight and anthracnose diseases of cassava in different agroecological zones of Nigeria. Afr. J. Agric. Res. 2008, 3, 049–059. [Google Scholar]
- Sangpueak, R.; Phansak, P.; Buensanteai, N. Morphological and molecular identification of Colletotrichum species associated with cassava anthracnose in Thailand. J. Phytopathol. 2018, 166, 129–142. [Google Scholar] [CrossRef]
- Fokunang, C.; Dixon, A. Postharvest evaluation of Colletotrichum gloeosporiodes f. sp. manihotis on cassava genotypes. Plant Pathol. J. 2006, 5, 60–66. [Google Scholar] [CrossRef][Green Version]
- Vieira, W.A.d.S.; Bezerra, P.A.; Silva, A.C.d.; Veloso, J.S.; Câmara, M.P.S.; Doyle, V.P. Optimal markers for the identification of Colletotrichum species. Mol. Phylogenet. Evol. 2020, 143, 106694. [Google Scholar] [CrossRef]
- Hyde, K.; Cai, L.; Cannon, P.; Crouch, J.; Crous, P.; Damm, U.; Goodwin, P.; Chen, H.; Johnston, P.; Jones, E. Colletotrichum names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar]
- Bragança, C.; Silva, L.; Haddad, F.; Oliveira, S. First report of Colletotrichum fructicola causing anthracnose in cassava (Manihot esculenta Crantz) in Brazil. Plant Dis. 2016, 100, 857. [Google Scholar] [CrossRef]
- Machado, S.d.C.; Veloso, J.S.; Câmara, M.P.; Campos, F.; Sarmento, R.d.A.; Giongo, M.V.; dos Santos, G.R. First report of Colletotrichum chrysophillum causing cassava Anthracnose in Brazil. Plant Dis. 2021, 105, 1196. [Google Scholar] [CrossRef]
- Sutton, B.C. The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute: Kew, UK, 1980; pp. 1–696. [Google Scholar]
- Castellani, A. Maintenance and cultivation of common pathogenic fungi of man in sterile distilled water. Further researches. Am. J. Trop. Med. Hyg. 1967, 70, 181–184. [Google Scholar]
- Doyle, J.J. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Rozas, J.; Sánchez-DelBarrio, J.C.; Messeguer, X.; Rozas, R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003, 19, 2496–2497. [Google Scholar] [CrossRef]
- Staden, R.; Beal, K.F.; Bonfield, J.K. The Staden Package, 1998. In Bioinformatics Methods and Protocols; Misener, S., Krawetz, S.A., Eds.; Humana Press: Totowa, NJ, USA, 1999; pp. 115–130. [Google Scholar]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic. Acids. Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nylander, J. MrModeltest v25; Program distributed by the author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Drummond, A.; Rambaut, A. Tracer v1. 5. 2007. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 14 June 2023).
- Rambaut, A. FigTree-Version 1.4. 3, a Graphical Viewer of Phylogenetic Trees. 2017. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 14 June 2023).
- Dettman, J.R.; Jacobson, D.J.; Taylor, J.W. A multilocus genealogical approach to phylogenetic species recognition in the model Eukaryote Neurospora. Evolution 2003, 57, 2703–2720. [Google Scholar] [CrossRef] [PubMed]
- Doyle, V.P.; Oudemans, P.V.; Rehner, S.A.; Litt, A. Habitat and host indicate lineage identity in Colletotrichum gloeosporioides s.l. from wild and agricultural landscapes in North America. PLoS ONE 2013, 8, e62394. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M.C. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 2000, 31, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.R.S.; Barguil, B.M.; Vieira, W.A.S.; Lima, W.G.; Michereff, S.J.; Doyle, V.P.; Câmara, M.P.S. Diversity, prevalence, and virulence of Colletotrichum species associated with lima bean in Brazil. Plant Dis. 2019, 103, 1961–1966. [Google Scholar] [CrossRef]
- Ferreira, D.F. SISVAR: A computer statistical analysis system. Ciênc. Agrotec. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Santos, G.R.d.; Café-Filho, A.C.; Leão, F.F.; César, M.; Fernandes, L.E. Progresso do crestamento gomoso e perdas na cultura da melancia. Hortic. Bras. 2005, 23, 228–232. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Damm, U.; Sato, T.; Alizadeh, A.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud. Mycol. 2018, 90, 71–118. [Google Scholar] [CrossRef] [PubMed]
- de Silva, D.D.; Groenewald, J.Z.; Crous, P.W.; Ades, P.K.; Nasruddin, A.; Mongkolporn, O.; Taylor, P.W.J. Identification, prevalence and pathogenicity of Colletotrichum species causing anthracnose of Capsicum annuum in Asia. IMA Fungus 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Batista, I.C.A.; Boari, A.d.J.; Kauffmann, C.M.; Nechet, K.d.L. Colletotrichum plurivorum causes anthracnose on okra in Brazil. J. Plant Pathol. 2020, 102, 1331. [Google Scholar] [CrossRef]
- Costa, J.F.O.; Ramos-Sobrinho, R.; Chaves, T.P.; Silva, J.R.A.; Pinho, D.B.; Assunção, I.P.; Lima, G.S.A. First report of Colletotrichum fructicola causing anthracnose on Annona leaves in Brazil. Plant Dis. 2017, 101, 386. [Google Scholar] [CrossRef]
- Sharma, G.; Shenoy, B.D. Colletotrichum fructicola and C. siamense are involved in chilli anthracnose in India. Arch. Phytopathol. Plant Prot. 2014, 47, 1179–1194. [Google Scholar] [CrossRef]
- Lima, N.B.; Batista, M.V.d.A.; De Morais, M.A.; Barbosa, M.A.G.; Michereff, S.J.; Hyde, K.D.; Câmara, M.P.S. Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers. 2013, 61, 75–88. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, B.-R.; Park, I.-H.; Hahm, S.-S. First Report of two Colletotrichum species associated with bitter rot on apple fruit in Korea—C. fructicola and C. siamense. Mycobiology 2018, 46, 154–158. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.-A.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Pest categorisation of Colletotrichum fructicola. EFSA J. 2021, 19, e06803. [Google Scholar] [CrossRef] [PubMed]
- Soares, V.F.; Velho, A.C.; Stadnik, M.J. First Report of Colletotrichum chrysophilum Causing Anthracnose on Blueberry in Brazil. Plant Dis. 2022, 106, 322. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.R.; de Moraes, A.J.G.; Cavalcante, A.d.P.d.S.; da Silva, G.B. First report of leaf anthracnose caused by Colletotrichum chrysophilum on açaí palm (Euterpe oleracea Mart.) in Brazil. J. Gen. Plant Pathol. 2022, 88, 212–216. [Google Scholar] [CrossRef]
- Vieira, W.A.S.; Lima, W.G.; Nascimento, E.S.; Michereff, S.J.; Câmara, M.P.S.; Doyle, V.P. The impact of phenotypic and molecular data on the inference of Colletotrichum diversity associated with Musa. Mycologia 2017, 109, 912–934. [Google Scholar] [CrossRef] [PubMed]
- Soares, V.F.; Velho, A.C.; Carachenski, A.; Astolfi, P.; Stadnik, M.J. First Report of Colletotrichum karstii causing Anthracnose on strawberry in Brazil. Plant Dis. 2021, 105, 3295. [Google Scholar] [CrossRef]
- Sousa, E.S.; Carvalho, G.d.S.; Barguil, B.M.; Matos, K.d.S.; Beserra, J.E.A. First report of anthracnose on Spigelia anthelmia caused by Colletotrichum karstii and Colletotrichum siamense in Brazil. J. Plant Dis. Prot. 2021, 128, 875–880. [Google Scholar] [CrossRef]
- Nascimento, M.B.; Bellé, C.; Azambuja, R.M.; Maich, S.L.P.; Neves, C.G.; Souza-Junior, I.T.; Jacobsen, C.R.F.; Barros, D.R. First report of Colletotrichum karstii Causing anthracnose spot on pitaya (Hylocereus undatus) in Brazil. Plant Dis. 2019, 103, 2137. [Google Scholar] [CrossRef]
- Boufleur, T.R.; Castro, R.R.L.; Rogério, F.; Ciampi-Guillardi, M.; Baroncelli, R.; Massola Júnior, N.S. First report of Colletotrichum musicola causing soybean anthracnose in Brazil. Plant Dis. 2020, 104, 1858. [Google Scholar] [CrossRef]
- Capobiango, N.P.; Pinho, D.B.; Zambolim, L.; Pereira, O.L.; Lopes, U.P. Anthracnose on strawberry fruits caused by Colletotrichum siamense in Brazil. Plant Dis. 2016, 100, 859. [Google Scholar] [CrossRef]
- Soares, M.G.O.; Alves, E.; Silveira, A.L.; Pereira, F.D.; Guimarães, S.S.C. Colletotrichum siamense is the main aetiological agent of anthracnose of avocado in south-eastern Brazil. Plant Pathol. 2021, 70, 154–166. [Google Scholar] [CrossRef]
- Fantinel, V.S.; Muniz, M.F.B.; Blume, E.; Araújo, M.M.; Poletto, T.; da Silva, T.T.; Dutra, A.F.; Maciel, C.G.; Harakava, R. First Report of Colletotrichum siamense causing anthracnose on Acca sellowiana fruits in Brazil. Plant Dis. 2017, 101, 1035. [Google Scholar] [CrossRef]
- Rogério, F.; Ciampi-Guillardi, M.; Barbieri, M.C.G.; Bragança, C.A.D.; Seixas, C.D.S.; Almeida, A.M.R.; Massola, N.S., Jr. Phylogeny and variability of Colletotrichum truncatum associated with soybean anthracnose in Brazil. J. Appl. Microbiol. 2017, 122, 402–415. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, S.d.C.S.; Veloso, J.S.; Câmara, M.P.S.; Vieira, W.A.S.; Jumbo, L.O.V.; Aguiar, R.W.S.; Cangussu, A.S.R.; Giongo, M.V.; Moraes, C.B.; Campos, F.S.; et al. Diversity, Prevalence and Virulence of Colletotrichum Species Causing Anthracnose on Cassava Leaves in the Northern Region of Brazil. J. Fungi 2024, 10, 367. https://doi.org/10.3390/jof10060367
Machado SdCS, Veloso JS, Câmara MPS, Vieira WAS, Jumbo LOV, Aguiar RWS, Cangussu ASR, Giongo MV, Moraes CB, Campos FS, et al. Diversity, Prevalence and Virulence of Colletotrichum Species Causing Anthracnose on Cassava Leaves in the Northern Region of Brazil. Journal of Fungi. 2024; 10(6):367. https://doi.org/10.3390/jof10060367
Chicago/Turabian StyleMachado, Stella de C. S., Josiene S. Veloso, Marcos P. S. Câmara, Willie A. S. Vieira, Luis O. Viteri Jumbo, Raimundo Wagner S. Aguiar, Alex Sander R. Cangussu, Marcos V. Giongo, Cristiano B. Moraes, Fabricio S. Campos, and et al. 2024. "Diversity, Prevalence and Virulence of Colletotrichum Species Causing Anthracnose on Cassava Leaves in the Northern Region of Brazil" Journal of Fungi 10, no. 6: 367. https://doi.org/10.3390/jof10060367
APA StyleMachado, S. d. C. S., Veloso, J. S., Câmara, M. P. S., Vieira, W. A. S., Jumbo, L. O. V., Aguiar, R. W. S., Cangussu, A. S. R., Giongo, M. V., Moraes, C. B., Campos, F. S., Araújo, S. H. C., Oliveira, E. E., & Santos, G. R. d. (2024). Diversity, Prevalence and Virulence of Colletotrichum Species Causing Anthracnose on Cassava Leaves in the Northern Region of Brazil. Journal of Fungi, 10(6), 367. https://doi.org/10.3390/jof10060367








