SntB Affects Growth to Regulate Infecting Potential in Penicillium italicum
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Plasmids
2.2. Construction and Verifification of Knockout Mutant
2.3. Physiological Analysis in P. italicum
2.3.1. Radial Growth, Spore Production, and Spore Germination
2.3.2. Pathogenicity Assessment of P. italicum
2.3.3. Effects of Various Stress Environments on P. italicum
2.3.4. Effect of pH on Mycelium Growth of P. italicum
2.4. Observation of Hyphal Ultrastructure by Transmission Electron Microscopy (TEM)
2.5. Cell Death Assay
2.6. RNA Transcriptome Sequencing Analysis
2.7. Quantitative Real-Time PCR (RT-qPCR) Verification
2.8. Statistic Analysis
3. Results
3.1. Validation of Mutant Strains
3.2. Effect of SntB Knockout on the Growth and Pathogenicity of P. italicum
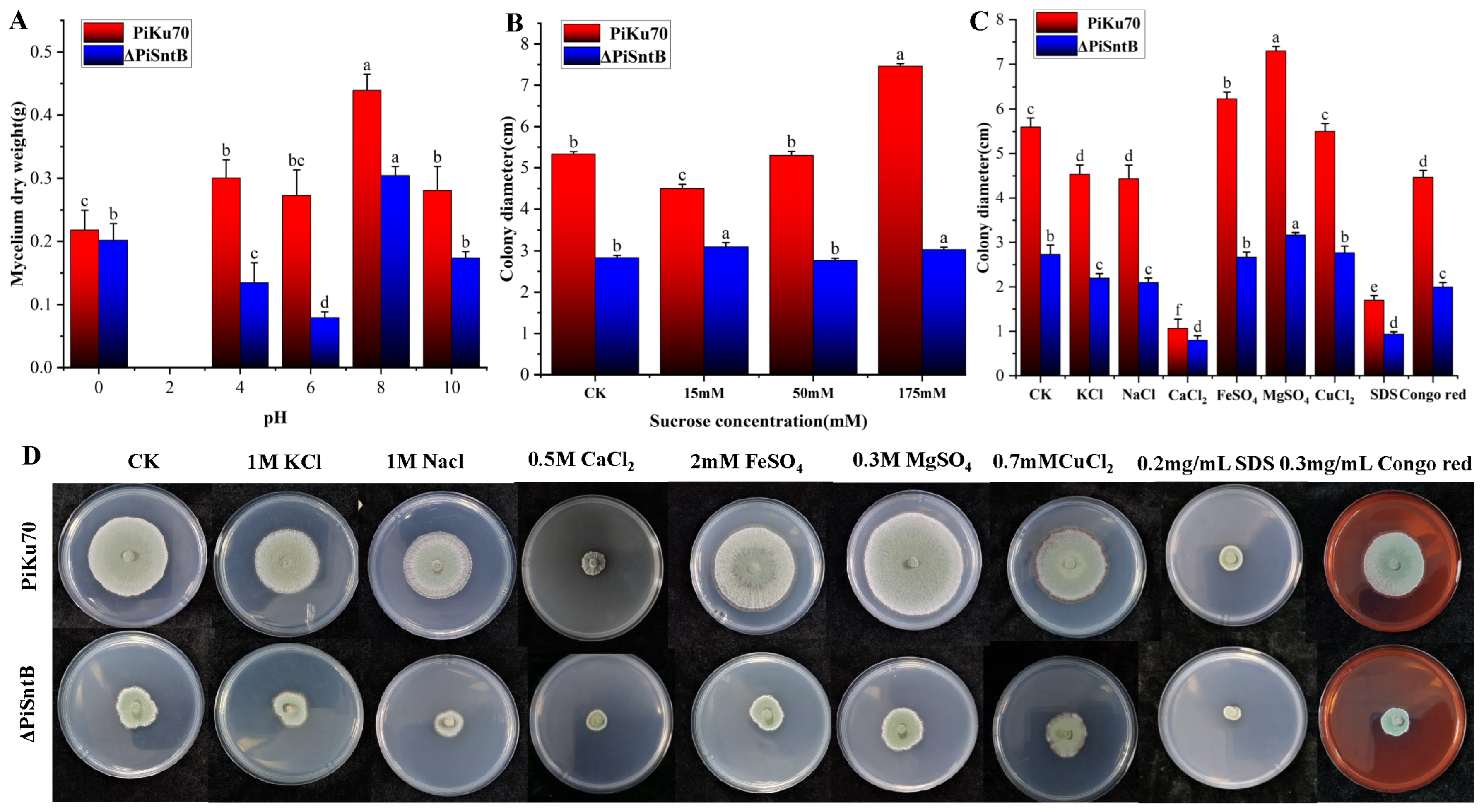
3.3. Effects of Stress Environments on the Growths of P. italicum
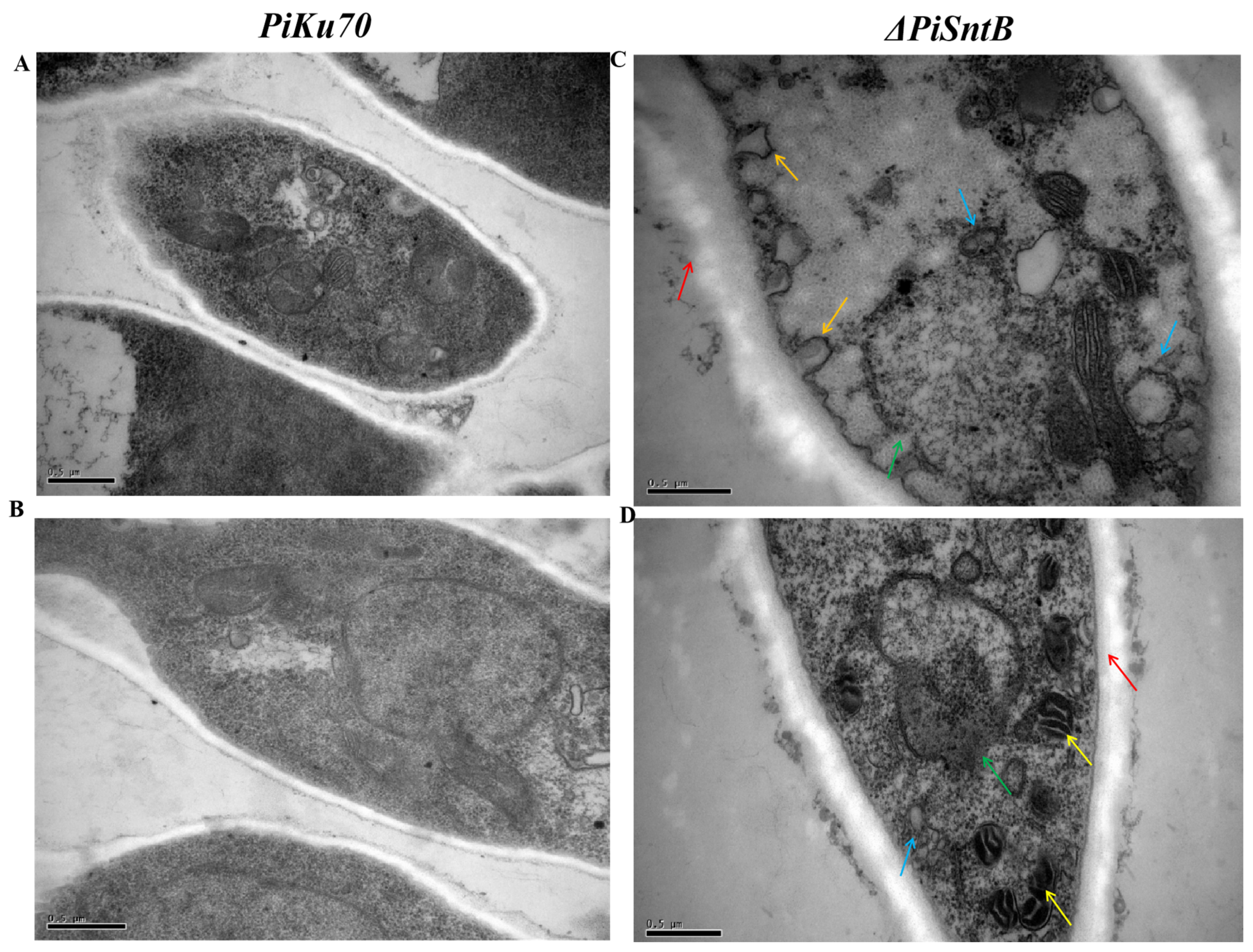
3.4. Effects of SntB Knockout on the Ultrastructure of Hypahe
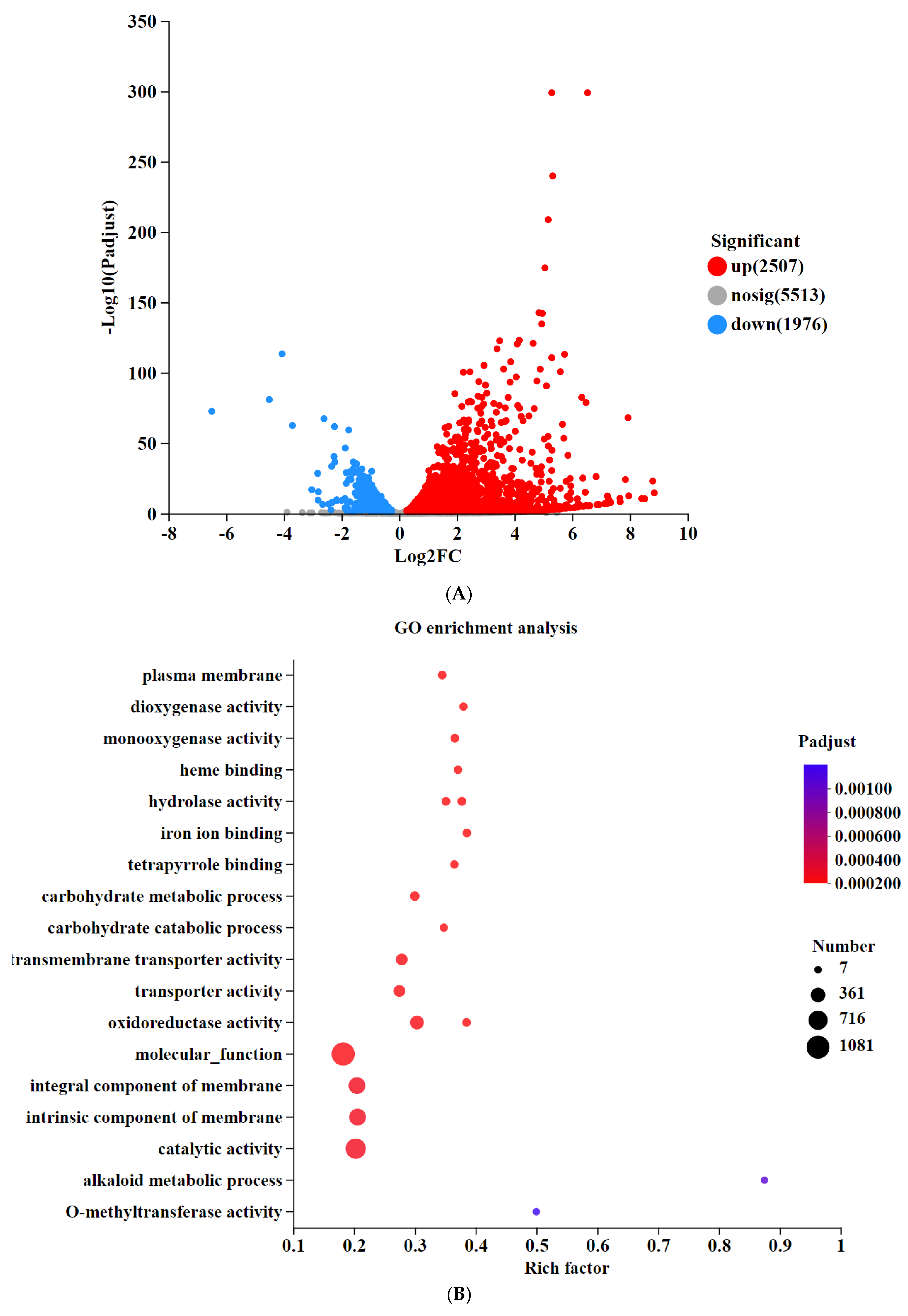
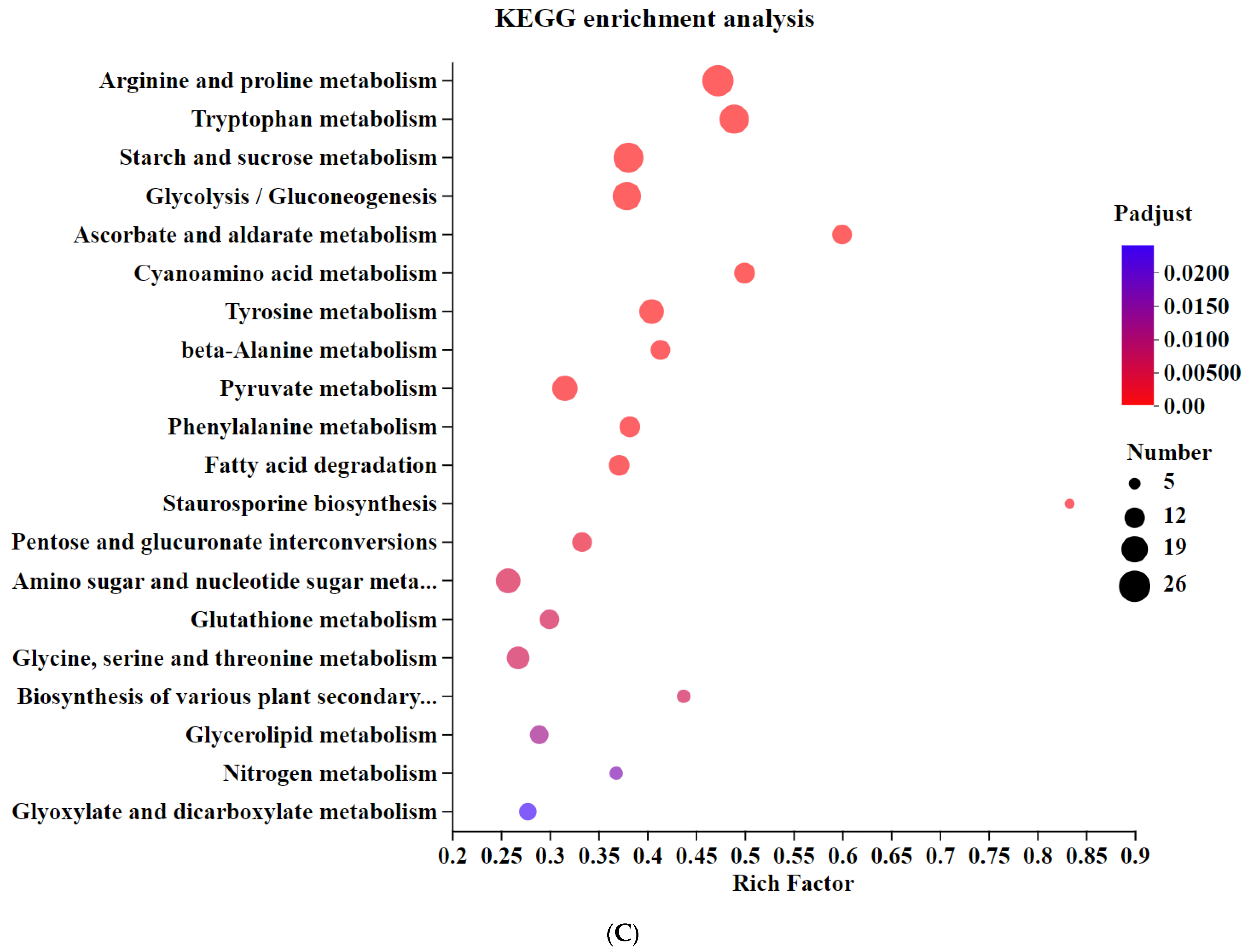
3.5. Transcriptomic Analysis of Differentially Expressed Genes in Knockout Strains
3.5.1. Deletion of SntB Leads to Differential Expressed Genes Related to Carbohydrate Metabolism
3.5.2. Deletion of SntB Leads to Expression Changes in Lipid Metabolism-Related Genes
3.5.3. Deletion of SntB Leads to Higher Demand for Energy Metabolism
3.5.4. Deletion of SntB Altered the Expressions of Genes Involved in Cellular Structure and Material Transport
3.5.5. Deletion of SntB Resulted in Significant Changes in Amino Acid/Protein Metabolism
3.5.6. Deletion of the SntB Gene Triggers a Series of Oxidative Stress and Immune Response
3.5.7. Deletion of the SntB Gene Downregulated Pathogenicity-Related Genes
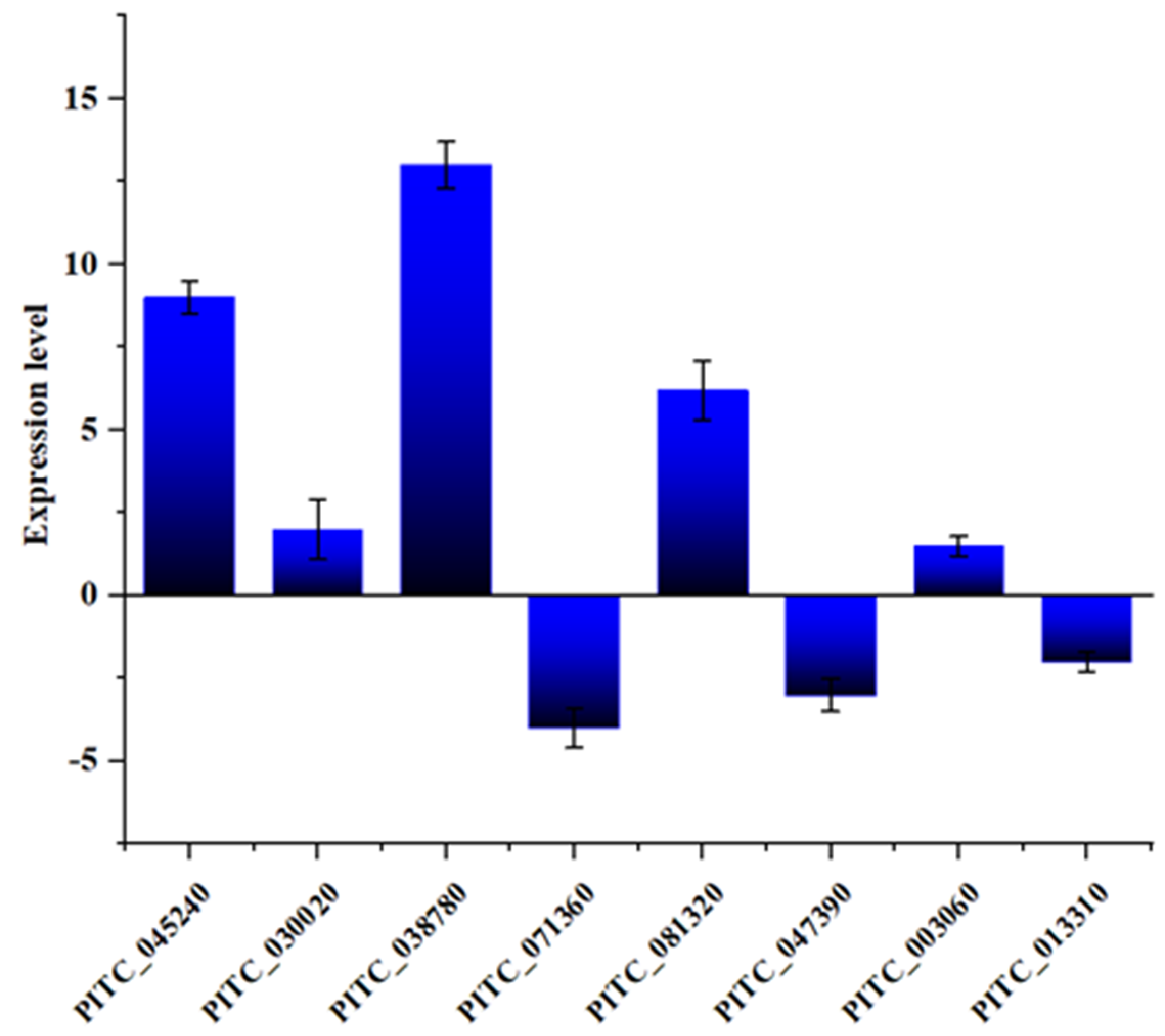
3.6. Gene Expression Measurements
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aboutorabi, M. A Review on the Biological Control of Plant Diseases using Various Microorganisms. J. Res. Med. Dent. Sci. 2018, 6, 30–35. [Google Scholar]
- Kanetis, L.; Förster, H.; Adaskaveg, J.E. Comparative efficacy of the new postharvest fungicides azoxystrobin, fludioxonil, and pyrimethanil for managing citrus green mold. Plant Dis. 2007, 91, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Talibi, I.; Boubaker, H.; Boudyach, E.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Plaza, P.; Usall, J.; Teixidó, N.; Vinas, I. Effect of water activity and temperature on germination and growth of Penicillium digitatum, P. italicum and Geotrichum candidum. J. Appl. Microbiol. 2003, 94, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Singh, Z.; Khangura, R.; Ahmad, S. Management of citrus blue and green moulds through application of organic elicitors. Australas. Plant Pathol. 2012, 41, 69–77. [Google Scholar] [CrossRef]
- Bhatta, U.K. Alternative management approaches of citrus diseases caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Front. Plant Sci. 2022, 12, 833328. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qi, W.; Peng, X.; Chen, J.; Wan, C. Inhibitory effect of 7-demethoxytylophorine on Penicillium italicum and its possible mechanism. Microorganisms 2019, 7, 36. [Google Scholar] [CrossRef]
- Yang, Q.; Qian, X.; Dhanasekaran, S.; Boateng, N.A.S.; Yan, X.; Zhu, H.; He, F.; Zhang, H. Study on the infection mechanism of Penicillium digitatum on postharvest citrus (Citrus reticulata Blanco) based on transcriptomics. Microorganisms 2019, 7, 672. [Google Scholar] [CrossRef]
- Kamoun, S. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu. Rev. Phytopathol. 2006, 44, 41–60. [Google Scholar] [CrossRef]
- Huitema, E.; Bos, J.I.; Tian, M.; Win, J.; Waugh, M.E.; Kamoun, S. Linking sequence to phenotype in Phytophthora–plant interactions. Trends Microbiol. 2004, 12, 193–200. [Google Scholar] [CrossRef]
- Pfannenstiel, B.T.; Greco, C.; Sukowaty, A.T.; Keller, N.P. The epigenetic reader SntB regulates secondary metabolism, development and global histone modifications in Aspergillus flavus. Fungal Genet. Biol. 2018, 120, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Denisov, Y.; Freeman, S.; Yarden, O. Inactivation of Snt2, a BAH/PHD-containing transcription factor, impairs pathogenicity and increases autophagosome abundance in Fusarium oxysporum. Mol. Plant Pathol. 2011, 12, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Tannous, J.; Barda, O.; Luciano-Rosario, D.; Prusky, D.B.; Sionov, E.; Keller, N.P. New insight into pathogenicity and secondary metabolism of the plant pathogen Penicillium expansum through deletion of the epigenetic reader SntB. Front. Microbiol. 2020, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.A.; Ueberheide, B.M.; Dewell, S.; Chait, B.T.; Zheng, D.; Allis, C.D. The yeast Snt2 protein coordinates the transcriptional response to hydrogen peroxide-mediated oxidative stress. Mol. Cell. Biol. 2013, 33, 3735–3748. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xu, Y.; Chen, J.; Luo, Y.; Lv, Y.; Su, J.; Kershaw, M.J.; Li, W.; Wang, J.; Yin, J. MoSnt2-dependent deacetylation of histone H3 mediates MoTor-dependent autophagy and plant infection by the rice blast fungus Magnaporthe oryzae. Autophagy 2018, 14, 1543–1561. [Google Scholar] [CrossRef]
- Bok, J.W.; Keller, N.P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell 2004, 3, 527–535. [Google Scholar] [CrossRef]
- Chang, P.-K.; Scharfenstein, L.L.; Ehrlich, K.C.; Wei, Q.; Bhatnagar, D.; Ingber, B.F. Effects of laeA deletion on Aspergillus flavus conidial development and hydrophobicity may contribute to loss of aflatoxin production. Fungal Biol. 2012, 116, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Tannous, J.; Sionov, E.; Keller, N.; Prusky, D. Apple intrinsic factors modulating the global regulator, LaeA, the patulin gene cluster and patulin accumulation during fruit colonization by Penicillium expansum. Front. Plant Sci. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Kumar, D.; Barad, S.; Chen, Y.; Luo, X.; Tannous, J.; Dubey, A.; Glam Matana, N.; Tian, S.; Li, B.; Keller, N. LaeA regulation of secondary metabolism modulates virulence in Penicillium expansum and is mediated by sucrose. Mol. Plant Pathol. 2017, 18, 1150–1163. [Google Scholar] [CrossRef]
- Selvig, K.; Alspaugh, J.A. pH response pathways in fungi: Adapting to host-derived and environmental signals. Mycobiology 2011, 39, 249–256. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Tian, S. Function of pH-dependent transcription factor PacC in regulating development, pathogenicity, and mycotoxin biosynthesis of phytopathogenic fungi. FEBS J. 2022, 289, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, B.; Xu, X.; Zhang, Z.; Tian, S. The pH-responsive PacC transcription factor plays pivotal roles in virulence and patulin biosynthesis in Penicillium expansum. Environ. Microbiol. 2018, 20, 4063–4078. [Google Scholar] [CrossRef] [PubMed]
- Barda, O.; Maor, U.; Sadhasivam, S.; Bi, Y.; Zakin, V.; Prusky, D.; Sionov, E. The pH-responsive transcription factor PacC governs pathogenicity and ochratoxin A biosynthesis in Aspergillus carbonarius. Front. Microbiol. 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.-M.; von Bargen, K.W.; Espino, J.J.; Pfannmüller, A.; Humpf, H.-U.; Tudzynski, B. Characterization of the fusaric acid gene cluster in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2014, 98, 1749–1762. [Google Scholar] [CrossRef]
- López-Díaz, C.; Rahjoo, V.; Sulyok, M.; Ghionna, V.; Martín-Vicente, A.; Capilla, J.; Di Pietro, A.; López-Berges, M.S. Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Plant Pathol. 2018, 19, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Zheng, W.; Islam, W.; Arif, M.; Abubakar, Y.S.; Wang, Z.; Lu, G. Carbon catabolite repression in filamentous fungi. Int. J. Mol. Sci. 2017, 19, 48. [Google Scholar] [CrossRef]
- Jin, J.; Diao, Y.; Xiong, X.; Yu, C.; Tian, Y.; Li, C.; Liu, H. The regulation of the growth and pathogenicity of valsa mali by the carbon metabolism repressor CreA. Int. J. Mol. Sci. 2023, 24, 9252. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Cai, R.; Guo, J.; Zhong, Z.; Bao, J.; Wang, Z.; Chen, X.; Zhou, J.; Lu, G.-d. Carbon catabolite repressor MoCreA is required for the asexual development and pathogenicity of the rice blast fungus. Fungal Genet. Biol. 2021, 146, 103496. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, L.; Li, D.; Xia, H.; Su, X.; Peng, L.; Pan, S. Use of active extracts of poplar buds against Penicillium italicum and possible modes of action. Food Chem. 2016, 196, 610–618. [Google Scholar] [CrossRef]
- Li, L.; He, L.; Lai, Y.; Shao, Y.; Chen, F. Cloning and functional analysis of the Gβ gene Mgb1 and the Gγ gene Mgg1 in Monascus ruber. J. Microbiol. 2014, 52, 35–43. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, D.; Liu, J.; Yang, S.; Li, D.; Peng, L. Vacuolar ATPase subunit H regulates growth development and pathogenicity of Penicillium digitatum. Postharvest Biol. Technol. 2023, 199, 112295. [Google Scholar] [CrossRef]
- Wang, F.; Lin, Y.; Yin, W.-X.; Peng, Y.-L.; Schnabel, G.; Huang, J.-B.; Luo, C.-X. The Y137H mutation of VvCYP51 gene confers the reduced sensitivity to tebuconazole in Villosiclava virens. Sci. Rep. 2015, 5, 17575. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, S.; Li, D.; Peng, L.; Fan, G.; Pan, S. The plasma membrane H+-ATPase is critical for cell growth and pathogenicity in Penicillium digitatum. Appl. Microbiol. Biotechnol. 2022, 106, 5123–5136. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Li, F.; Hu, X.; Liao, X.; Zhang, Y. Quality comparison of “Laba” garlic processed by high hydrostatic pressure and high pressure carbon dioxide. Sci. Rep. 2020, 10, 3719. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P. Acyl-lipid metabolism. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2013; Volume 11. [Google Scholar]
- Saier Jr, M.H.; Reddy, V.S.; Tsu, B.V.; Ahmed, M.S.; Li, C.; Moreno-Hagelsieb, G. The transporter classification database (TCDB): Recent advances. Nucleic Acids Res. 2016, 44, D372–D379. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Singh, A.; Yadav, A.N. Fungal enzymes: Degradation and detoxification of organic and inorganic pollutants. In Recent Trends in Mycological Research: Volume 2: Environmental and Industrial Perspective; Springer: Berlin/Heidelberg, Germany, 2021; pp. 99–125. [Google Scholar]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef]
- Komalapriya, C.; Kaloriti, D.; Tillmann, A.T.; Yin, Z.; Herrero-de-Dios, C.; Jacobsen, M.D.; Belmonte, R.C.; Cameron, G.; Haynes, K.; Grebogi, C. Integrative model of oxidative stress adaptation in the fungal pathogen Candida albicans. PLoS ONE 2015, 10, e0137750. [Google Scholar] [CrossRef] [PubMed]
- Denisov, Y.; Yarden, O.; Freeman, S. The transcription factor SNT2 is involved in fungal respiration and reactive oxidative stress in Fusarium oxysporum and Neurospora crassa. Physiol. Mol. Plant Pathol. 2011, 76, 137–143. [Google Scholar] [CrossRef]
- Tannous, J.; Kumar, D.; Sela, N.; Sionov, E.; Prusky, D.; Keller, N.P. Fungal attack and host defence pathways unveiled in near-avirulent interactions of Penicillium expansum creA mutants on apples. Mol. Plant Pathol. 2018, 19, 2635–2650. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zong, Y.; Shang, Y.; Zhang, Z.; Xu, X.; Wang, X.; Long, M.; Tian, S. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ. Microbiol. 2019, 21, 1124–1139. [Google Scholar] [CrossRef]
- Pontes, J.o.G.d.M.; Fernandes, L.S.; dos Santos, R.V.; Tasic, L.; Fill, T.P. Virulence factors in the phytopathogen–host interactions: An overview. J. Agric. Food Chem. 2020, 68, 7555–7570. [Google Scholar] [CrossRef] [PubMed]
- De Assis, L.J.; Ries, L.N.A.; Savoldi, M.; Dos Reis, T.F.; Brown, N.A.; Goldman, G.H. Aspergillus nidulans protein kinase A plays an important role in cellulase production. Biotechnol. Biofuels 2015, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, M.; Kwon, N.J.; Bakti, F.; M-Hamvas, M.; Jámbrik, K.; Park, H.; Pócsi, I.; Yu, J.H.; Emri, T. Extracellular proteinase formation in carbon starving Aspergillus nidulans cultures–physiological function and regulation. J. Basic Microbiol. 2011, 51, 625–634. [Google Scholar] [CrossRef]
- Ries, L.N.; Beattie, S.R.; Espeso, E.A.; Cramer, R.A.; Goldman, G.H. Diverse regulation of the CreA carbon catabolite repressor in Aspergillus nidulans. Genetics 2016, 203, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Ohtani, K.; Yamamoto, H.; Akimitsu, K. Overexpression of a gene encoding a catabolite repression element in Alternaria citri causes severe symptoms of black rot in citrus fruit. Phytopathology 2007, 97, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, M.; Miskei, M.; Karányi, Z.; Lenkey, B.; Pócsi, I.; Emri, T. Transcriptome changes initiated by carbon starvation in Aspergillus nidulans. Microbiology 2013, 159, 176–190. [Google Scholar] [CrossRef]
- Ellström, M.; Shah, F.; Johansson, T.; Ahren, D.; Persson, P.; Tunlid, A. The carbon starvation response of the ectomycorrhizal fungus Paxillus involutus. FEMS Microbiol. Ecol. 2015, 91, fiv027. [Google Scholar] [CrossRef]
- White, S.; McIntyre, M.; Berry, D.R.; McNeil, B. The autolysis of industrial filamentous fungi. Crit. Rev. Biotechnol. 2002, 22, 1–14. [Google Scholar] [CrossRef]
- Emri, T.; Molnár, Z.; Pusztahelyi, T.; Pócsi, I. Physiological and morphological changes in autolyzing Aspergillus nidulans cultures. Folia Microbiol. 2004, 49, 277–284. [Google Scholar] [CrossRef]
- Emri, T.; Molnár, Z.; Pócsi, I. The appearances of autolytic and apoptotic markers are concomitant but differently regulated in carbon-starving Aspergillus nidulans cultures. FEMS Microbiol. Lett. 2005, 251, 297–303. [Google Scholar] [CrossRef]
- Bazil, J.N.; Pannala, V.R.; Dash, R.K.; Beard, D.A. Determining the origins of superoxide and hydrogen peroxide in the mammalian NADH: Ubiquinone oxidoreductase. Free Radic. Biol. Med. 2014, 77, 121–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.; Xie, X.; Liu, H.; Chen, F.; Du, J.; Wang, X.; Jiang, X.; Yu, F.; Fan, H. Pyridine nucleotide-disulphide oxidoreductase domain 2 (PYROXD2): Role in mitochondrial function. Mitochondrion 2019, 47, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.E.; Bernardo, S.M.; Cheetham, B.F. The interaction of induction, repression and starvation in the regulation of extracellular proteases in Aspergillus nidulans: Evidence for a role for CreA in the response to carbon starvation. Curr. Genet. 2008, 54, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, B.M.; Burggraaf-van Welzen, A.-M.; Lamers, G.; Meyer, V.; Ram, A.F. Autophagy promotes survival in aging submerged cultures of the filamentous fungus Aspergillus niger. Appl. Microbiol. Biotechnol. 2013, 97, 8205–8218. [Google Scholar] [CrossRef]
- Pollack, J.K.; Harris, S.D.; Marten, M.R. Autophagy in filamentous fungi. Fungal Genet. Biol. 2009, 46, 1–8. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yang, S.; Zhang, M.; Yang, Y.; Li, Z.; Peng, L. SntB Affects Growth to Regulate Infecting Potential in Penicillium italicum. J. Fungi 2024, 10, 368. https://doi.org/10.3390/jof10060368
Li C, Yang S, Zhang M, Yang Y, Li Z, Peng L. SntB Affects Growth to Regulate Infecting Potential in Penicillium italicum. Journal of Fungi. 2024; 10(6):368. https://doi.org/10.3390/jof10060368
Chicago/Turabian StyleLi, Chunyan, Shuzhen Yang, Meihong Zhang, Yanting Yang, Zhengzheng Li, and Litao Peng. 2024. "SntB Affects Growth to Regulate Infecting Potential in Penicillium italicum" Journal of Fungi 10, no. 6: 368. https://doi.org/10.3390/jof10060368
APA StyleLi, C., Yang, S., Zhang, M., Yang, Y., Li, Z., & Peng, L. (2024). SntB Affects Growth to Regulate Infecting Potential in Penicillium italicum. Journal of Fungi, 10(6), 368. https://doi.org/10.3390/jof10060368



