Influence of Zinc on Histoplasma capsulatum Planktonic and Biofilm Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. TPEN Chelator Inhibition Profile
2.3. Planktonic Assay
2.3.1. Optical Density (OD)
2.3.2. Measurement of Metabolic Activity
2.4. Biofilm Assay
2.4.1. Measurement of Metabolic Activity
2.4.2. Measurement of Biofilm Biomass
2.4.3. Measurement of Biofilm Extracellular Matrix
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. Confocal Fluorescence Microscopy (CLSM)
2.5. Congo Red Assay
2.6. Statistical Analysis
3. Results
3.1. Fungal Growth Inhibition Profile with TPEN
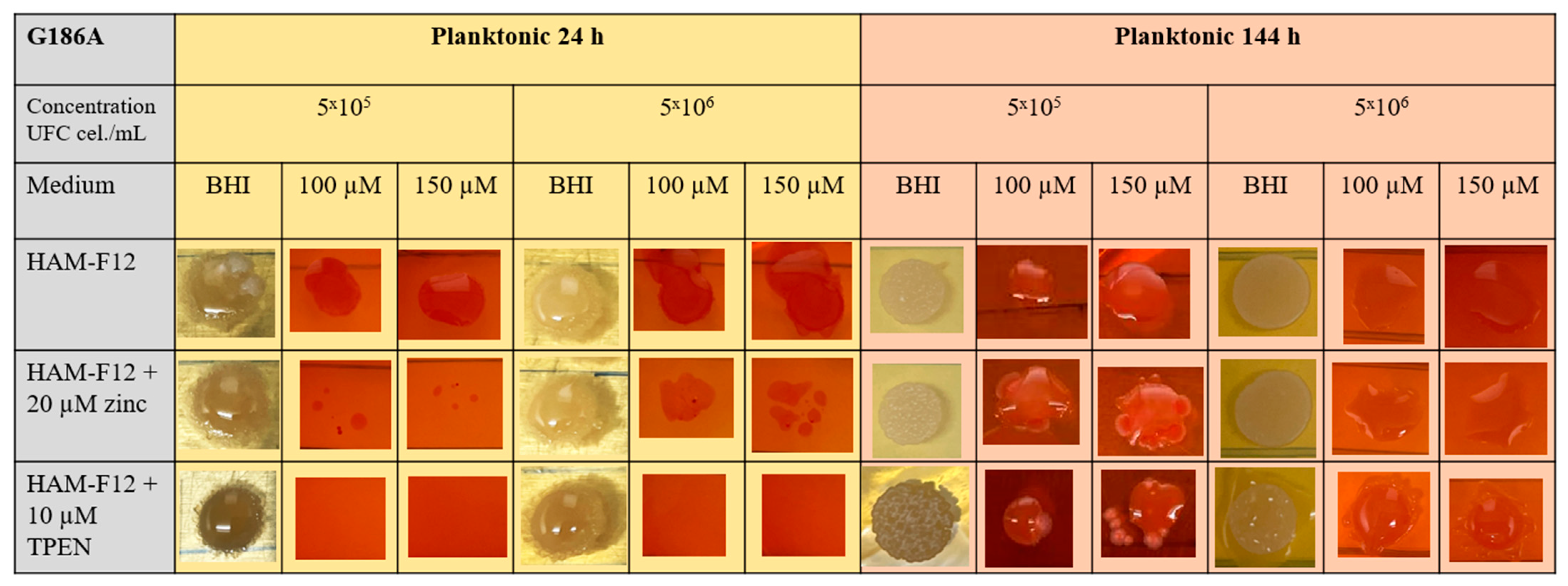
3.2. Influence of Zinc and TPEN on Histoplasma Growth in Planktonic Form
Characterization of Planktonic Growth with Metabolic Activity and Optical Density
3.3. Influence of Zinc and TPEN on Histoplasma Growth in Biofilm Form
3.3.1. Characterization of Biofilm with Metabolic Activity, Quantification of Biomass, and Quantification of Extracellular Matrix
3.3.2. Scanning Electron Microscopy (SEM) and Confocal Microscopy (CSLM) of Histoplasma Biofilm
3.3.3. Susceptibility to Congo Red (CR) Dye to Determine the Interference of TPEN and Zinc in the Cell Wall
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Ponce, M.G.; Gendlina, I.; Nosanchuk, J.D. Histoplasma Capsulatum: Mechanisms for Pathogenesis. Curr. Top. Microbiol. Immunol. 2019, 422, 157–191. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M.; Leitao, T.S.; Gómez, B.L.; Couppié, P.; Adenis, A.; Damasceno, L.; Demar, M.; Samayoa, B.; Cáceres, D.H.; Pradinaud, R.; et al. The Fight against HIV-Associated Disseminated Histoplasmosis in the Americas: Unfolding the Different Stories of Four Centers. J. Fungi 2019, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Alvarez, R.O.; Pérez-Torres, A.; Taylor, M.L. Adherence patterns of Histoplasma capsulatum yeasts to bat tissue sections. Mycopathologia 2010, 170, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, S.A.; Voorhies, M.; Gebhart, D.; Sil, A. Genome-Wide Reprogramming of Transcript Architecture by Temperature Specifies the Developmental States of the Human Pathogen Histoplasma. PLoS Genet. 2015, 11, e1005395. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Terwilliger, A.; Maresso, A.W. Iron and zinc exploitation during bacterial pathogenesis. Met. Integr. Biometal Sci. 2015, 7, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Garfoot, A.L.; Rappleye, C.A. Histoplasma capsulatum surmounts obstacles to intracellular pathogenesis. FEBS J. 2016, 283, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Brechting, P.J.; Rappleye, C.A. Histoplasma Responses to Nutritional Immunity Imposed by Macrophage Activation. J. Fungi 2019, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Caruso, J.A.; Deepe, G.S., Jr. Zinc sequestration: Arming phagocyte defense against fungal attack. PLoS Pathog. 2013, 9, e1003815. [Google Scholar] [CrossRef]
- Guimarães, A.J.; Nakayasu, E.S.; Sobreira, T.J.; Cordero, R.J.; Nimrichter, L.; Almeida, I.C.; Nosanchuk, J.D. Histoplasma capsulatum heat-shock 60 orchestrates the adaptation of the fungus to temperature stress. PLoS ONE 2011, 6, e14660. [Google Scholar] [CrossRef] [PubMed]
- Pitangui, N.S.; Sardi, J.C.O.; Silva, J.F.; Benaducci, T.; Moraes da Silva, R.A.; Rodríguez-Arellanes, G.; Taylor, M.L.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Adhesion of Histoplasma capsulatum to pneumocytes and biofilm formation on an abiotic surface. Biofouling 2012, 28, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.N.C.; Costa-Orlandi, C.B.; Bila, N.M.; Vaso, C.O.; Da Silva, R.A.M.; Mendes-Giannini, M.J.S.; Taylor, M.L.; Fusco-Almeida, A.M. Biofilm Formation by Histoplasma capsulatum in Different Culture Media and Oxygen Atmospheres. Front. Microbiol. 2020, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Fregonezi, N.F.; Oliveira, L.T.; Singulani, J.d.L.; Marcos, C.M.; dos Santos, C.T.; Taylor, M.L.; Mendes-Giannini, M.J.S.; de Oliveira, H.C.; Fusco-Almeida, A.M. Heat Shock Protein 60, Insights to Its Importance in Histoplasma capsulatum: From Biofilm Formation to Host-Interaction. Front. Cell. Infect. Microbiol. 2021, 10, 591950. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, J.; d’Enfert, C. Candida albicans biofilms: Building a heterogeneous, drug-tolerant environment. Curr. Opin. Microbiol. 2013, 16, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Zarnowski, R.; Sanchez, H.; Edward, J.A.; Reinicke, E.L.; Nett, J.E.; Mitchell, A.P.; Andes, D.R. Community participation in biofilm matrix assembly and function. Proc. Natl. Acad. Sci. USA 2015, 112, 4092–4097. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; de Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Casadevall, A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agentes in vitro. Antimicrob. Agents Chemother. 2006, 50, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Liu, N.; Dong, G.; Sheng, C. Tackling Fungal Resistance by Biofilm Inhibitors. J. Med. Chem. 2017, 60, 2193–2211. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.F.; Griffith, M.; Alarcon, E. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef]
- Jung, W.H. The Zinc Transport Systems and Their Regulation in Pathogenic Fungi. Mycobiology 2015, 43, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in fungal virulence. FEMS Microbiol. Rev. 2018, 42, fux050. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Lu, C.; Gong, Y.; Li, M.; Sun, S. Promising Antifungal Targets Against Candida albicans Based on Ion Homeostasis. Front. Cell. Infect. Microbiol. 2018, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Ceri, H.; Yerly, J.; Rabiei, M.; Hu, Y.; Martinuzzi, R.; Turner, R.J. Metal ions may suppress or enhance cellular differentiation in Candida albicans and Candida tropicalis biofilms. Appl. Environ. Microbiol. 2007, 73, 4940–4949. [Google Scholar] [CrossRef] [PubMed]
- Shafeeq, S.; Pannanusorn, S.; Elsharabasy, Y.; Ramírez-Zavala, B.; Morschhäuser, J.; Römling, U. Impact of manganese on biofilm formation and cell morphology of Candida parapsilosis clinical isolates with different biofilm forming abilities. FEMS Yeast Res. 2019, 19, foz057. [Google Scholar] [CrossRef] [PubMed]
- Besold, A.N.; Gilston, B.A.; Radin, J.N.; Ramsoomair, C.; Culbertson, E.M.; Li, C.X.; Cormack, B.P.; Chazin, W.J.; Kehl-Fie, T.E.; Culotta, V.C. Role of Calprotectin in Withholding Zinc and Copper from Candida albicans. Infect. Immun. 2018, 86, e00779-17. [Google Scholar] [CrossRef] [PubMed]
- Petito, G.; de Curcio, J.S.; Pereira, M.; Bailão, A.M.; Paccez, J.D.; Tristão, G.B.; de Morais, C.O.B.; de Souza, M.V.; de Castro Moreira Santos, A.; Fontes, W.; et al. Metabolic Adaptation of Paracoccidioides brasiliensis in Response to in vitro Copper Deprivation. Front. Microbiol. 2020, 11, 1834. [Google Scholar] [CrossRef]
- Ollig, J.; Kloubert, V.; Weßels, I.; Haase, H.; Rink, L. Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro. Metals 2016, 6, 71. [Google Scholar] [CrossRef]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Hilty, J.; George Smulian, A.; Newman, S.L. Histoplasma capsulatum utilizes siderophores for intracellular iron acquisition in macrophages. Med. Mycol. 2011, 49, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Winters, M.S.; Chan, Q.; Caruso, J.A.; Deepe, G.S., Jr. Metallomic analysis of macrophages infected with Histoplasma capsulatum reveals a fundamental role for zinc in host defenses. J. Infect. Dis. 2010, 202, 1136–1145. [Google Scholar] [CrossRef]
- Dade, J.; DuBois, J.C.; Pasula, R.; Donnell, A.M.; Caruso, J.A.; Smulian, A.G.; Deepe, G.S., Jr. HcZrt2, a zinc responsive gene, is indispensable for the survival of Histoplasma capsulatum in vivo. Med. Mycol. 2016, 54, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Assunção, L.d.P.; Moraes, D.; Soares, L.W.; Silva-Bailão, M.G.; de Siqueira, J.G.; Baeza, L.C.; Báo, S.N.; Soares, C.M.d.A.; Bailão, A.M. Insights into Histoplasma capsulatum Behavior on Zinc Deprivation. Front. Cell. Infect. Microbiol. 2020, 10, 573097. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: Comparison of biomass production and metabolic activity and development of cut-off points. Int. J. Med. Microbiol 2014, 304, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, L.M.; Zamith-Miranda, D.; Burnet, M.C.; Choi, H.; Nimrichter, L.; Nakayasu, E.S.; Nosanchuk, J.D. Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Sci. Rep. 2018, 8, 8065. [Google Scholar] [CrossRef]
- Vaso, C.O.; Bila, N.M.; Pandolfi, F.; De Vita, D.; Bortolami, M.; Bonatti, J.L.; De Moraes Silva, R.A.; Gonçalves, L.N.; Tudino, V.; Costi, R.; et al. Evaluation of the Anti-Histoplasma capsulatum Activity of Indole and Nitrofuran Derivatives and Their Pharmacological Safety in Three-Dimensional Cell Cultures. Pharmaceutics 2022, 14, 1043. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Santos, C.T.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. In vitro Characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling 2014, 30, 719–727. [Google Scholar] [CrossRef]
- Morris, C.E.; Monier, J.; Jacques, M. Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms. Appl. Environ. Microbiol. 1997, 63, 1570–1576. [Google Scholar] [CrossRef]
- Ram, A.F.J.; Klis, F.M. Identification of funga cell wall mutants using susceptibility assays based on calcofluor white and congo red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef]
- Sohnle, P.G.; Hahn, B.L.; Karmarkar, R. Effect of metals on Candida albicans growth in the presence of chemical chelators and human abscess fluid. J. Lab. Clin. Med. 2001, 137, 284–289. [Google Scholar] [CrossRef]
- Hashemi, M.; Ghavami, S.; Eshraghi, M.; Booy, E.P.; Los, M. Cytotoxic effects of intra and extracellular zinc chelation on human breast cancer cells. Eur. J. Pharmacol. 2007, 557, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.J.; Sohn, J.H.; Ha, D.W.; Ahn, Y.H.; Koh, J.Y.; Yoon, Y.H. Depletion of intracellular zinc and copper with TPEN results in apoptosis of cultured human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 460–465. [Google Scholar] [PubMed]
- Laskaris, P.; Atrouni, A.; Calera, J.A.; d’Enfert, C.; Munier-Lehmann, H.; Cavaillon, J.-M.; Latgé, J.-P.; Ibrahim-Granet, O. Administration of Zinc Chelators Improves Survival of Mice Infected with Aspergillus fumigatus both in Monotherapy and in Combination with Caspofungin. Antimicrob. Agents Chemother. 2016, 60, 5631–5639. [Google Scholar] [CrossRef] [PubMed]
- Kurakado, S.; Arai, R.; Sugita, T. Association of the hypha-related protein Pra1 and zinc transporter Zrt1 with biofilm formation by the pathogenic yeast Candida albicans. Microbiol. Immunol. 2018, 62, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Staats, C.; Kmetzsch, L.; Schrank, A.; Vainstein, M. Fungal zinc metabolism and its connections to virulence. Front. Cell. Infect. Microbiol. 2013, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Kurakado, S.; Chiba, R.; Sato, C.; Matsumoto, Y.; Sugita, T. N,N,N’,N’-tetrakis(2-pyridylmethyl)ethylenediamine, a zinc chelator, inhibits biofilm and hyphal formation in Trichosporon asahii. BMC Res. Notes 2020, 13, 142. [Google Scholar] [CrossRef]
- Valdez, A.F.; Miranda, D.Z.; Guimarães, A.J.; Nimrichter, L.; Nosanchuk, J.D. Pathogenicity & virulence of Histoplasma capsulatum—A multifaceted organism adapted to intracellular environments. Virulence 2022, 13, 1900–1919. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. In vitro growth and analysis of Candida biofilms. Nat. Protoc. 2008, 3, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liang, Y.; Lin, S.; Chen, D.; Li, B.; Li, L.; Deng, Y. Crystal violet and XTT assays on Staphylococcus aureus biofilm quantification. Curr. Microbiol. 2016, 73, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Yang, H.C.; Homma, T. Use of Congo red as a microscopic fluorescence indicator of hyphal growth. Appl. Microbiol. Biotechnol. 1995, 43, 102–108. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Ghaemi, E.; Koohsar, F. Influence of ZnO nanoparticles on Candida albicans isolates biofilm formed on the urinary catheter. Iran J. Microbiol. 2018, 10, 424–432. [Google Scholar]
- Shen, Q.; Rappleye, C.A. Differentiation of the fungus Histoplasma capsulatum into a pathogen of phagocytes. Curr. Opin. Microbiol. 2017, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.W.; Loprete, D.M.; Momany, M.; Ha, Y.; Harsch, L.M.; Livesay, J.A.; Mirchandani, A.; Murdock, J.J.; Vaughan, J.; Watt, M.B. Isolation of cell wall mutants in Aspergillus nidulans by screening for hypersensitivity to calcofluor white. Mycologia 2006, 98, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Che, C.; Zhao, Z.; Wang, Y.; Li, M.; Zhang, X.; Zhao, X. Extracellular polymeric substance from Rahnella sp. LRP3 converts available Cu into (Cu5(PO4)2(OH)4) in soil trhough biomineralization process. Environ. Pollut. 2020, 260, 114051. [Google Scholar] [CrossRef]
- Ortiz-Ramirèz, J.A.; Cuèlla-Cruz, M.; López-Romero, E. Responses of Sporothrix globosa to the cell wall perturbing agentes congo red and calcofluor white. Antonie Van Leeuwenhoek 2021, 114, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Nodet, P.; Capellano, A.; Fèvre, M. Morphongenetic of congo red on hyphal growth and cell wall development of fungus Saprolegnia monoica. J. Gen. Microbiol. 1990, 136, 303–310. [Google Scholar] [CrossRef]
- Chakraborty, T.; Tóth, Z.; Tórh, R.; Vávölgyi, C.; Gácser, A. Iron metabolism, pseudohyphae production, and biofilm formation trhough a multicopper oxidase in the human-pathogenic fungus Candida parapsilosis. Am. Soc. Microbiol. Msphere 2020, 5, e00227-20. [Google Scholar] [CrossRef]

| G186A | |||
|---|---|---|---|
| Time | HAM-F12 Control | HAM-F12 + 20 µM Zinc | HAM-F12 + 10 µM TPEN |
| 24 h SEM | A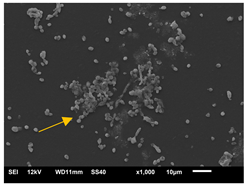 | B | C |
| 24 h CLSM 2D | D | E | F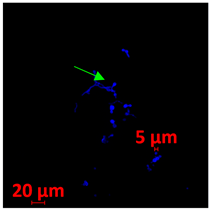 |
| 24 h CLSM 2.5D | G | H | I |
| 144 h SEM | J | K | L |
| 144 h CLSM 2D | M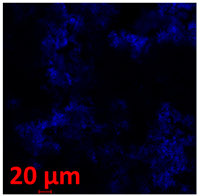 | N | O |
| 144 h CLSM 2.5 D | P | Q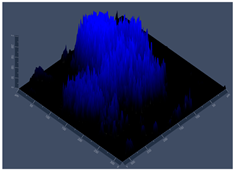 | R |
| EH-315 | |||
|---|---|---|---|
| Time | HAM-F12 Control | HAM-F12 + 20 µM Zinc | HAM-F12 + 10 µM TPEN |
| 24 h SEM | A | B | C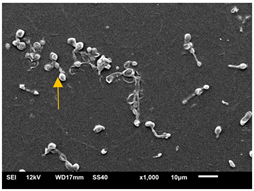 |
| 24 h CLSM 2D | D | E | F |
| 24 h CLSM 2.5D | G | H | I |
| 144 h SEM | J | K | L |
| 144 h CLSM 2D | M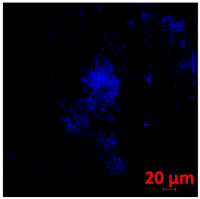 | N | O |
| 144 h CLSM 2.5 D | P | Q | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, A.C.M.d.S.; Carvalho, A.R.; Vaso, C.O.; Mendes-Giannini, M.J.S.; Singulani, J.d.L.; Fusco-Almeida, A.M. Influence of Zinc on Histoplasma capsulatum Planktonic and Biofilm Cells. J. Fungi 2024, 10, 361. https://doi.org/10.3390/jof10050361
Pires ACMdS, Carvalho AR, Vaso CO, Mendes-Giannini MJS, Singulani JdL, Fusco-Almeida AM. Influence of Zinc on Histoplasma capsulatum Planktonic and Biofilm Cells. Journal of Fungi. 2024; 10(5):361. https://doi.org/10.3390/jof10050361
Chicago/Turabian StylePires, Ana Carolina Moreira da Silva, Angélica Romão Carvalho, Carolina Orlando Vaso, Maria José Soares Mendes-Giannini, Junya de Lacorte Singulani, and Ana Marisa Fusco-Almeida. 2024. "Influence of Zinc on Histoplasma capsulatum Planktonic and Biofilm Cells" Journal of Fungi 10, no. 5: 361. https://doi.org/10.3390/jof10050361
APA StylePires, A. C. M. d. S., Carvalho, A. R., Vaso, C. O., Mendes-Giannini, M. J. S., Singulani, J. d. L., & Fusco-Almeida, A. M. (2024). Influence of Zinc on Histoplasma capsulatum Planktonic and Biofilm Cells. Journal of Fungi, 10(5), 361. https://doi.org/10.3390/jof10050361







