Will the Real Immunogens Please Stand Up: Exploiting the Immunogenic Potential of Cryptococcal Cell Antigens in Fungal Vaccine Development
Abstract
1. Introduction
2. Cryptococcal Capsule
3. Cryptococcal Glucans
4. Cryptococcal Chitin and Chitosan
5. Mannoproteins
6. Extracellular Vesicles as Fungal Vaccine Platform
7. Immunological Responses of Current Whole-Cell Cryptococcal Vaccine Candidates
8. Adaptive Immunity
9. Innate Immunity and Trained Innate Immunity Responses
10. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Dao, A.; Kim, H.Y.; Garnham, K.; Kidd, S.; Sati, H.; Perfect, J.; Sorrell, T.C.; Harrison, T.; Rickerts, V.; Gigante, V.; et al. Cryptococcosis—A systematic review to inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae043. [Google Scholar] [CrossRef] [PubMed]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022.
- Hagen, F.; Khayhan, K.; Theelen, B.; Kolecka, A.; Polacheck, I.; Sionov, E.; Falk, R.; Parnmen, S.; Lumbsch, H.T.; Boekhout, T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015, 78, 16–48. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Lodge, J.; Xue, C. Harnessing the immune response to fungal pathogens for vaccine development. Annu. Rev. Microbiol. 2022, 76, 703–726. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Liu, Y.; Qian, C.; Huang, X.; Wang, L.; Whitfield, C.; Zhang, J. Chapter 5—Bacterial capsules. In Molecular Medical Microbiology, 3rd ed.; Tang, Y., Hindiyeh, M.Y., Liu, D., Sails, A., Spearman, P., Zhang, J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 69–96. [Google Scholar]
- Boodwa-Ko, D.; Doering, T.L. A quick reCAP: Discovering Cryptococcus neoformans capsule mutants. J. Fungi 2024, 10, 114. [Google Scholar] [CrossRef]
- Cherniak, R.; Reiss, E.; Slodki, M.E.; Plattner, R.D.; Blumer, S.O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Mol. Immunol. 1980, 17, 1025–1032. [Google Scholar] [CrossRef]
- Merrifield, E.H.; Stephen, A.M. Structural investigations of two capsular polysaccharides from Cryptococcus neoformans. Carbohydr. Res. 1980, 86, 69–76. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Alspaugh, J.A. The Cryptococcus neoformans capsule: A sword and a shield. Clin. Microbiol. Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef]
- Fonseca, F.L.; Reis, F.C.G.; Sena, B.A.G.; Jozefowicz, L.J.; Kmetzsch, L.; Rodrigues, M.L. The overlooked glycan components of the Cryptococcus capsule. Fungal Physiol. Immunopathogenes. 2019, 422, 31–43. [Google Scholar]
- Bouklas, T.; Jain, N.; Fries, B.C. Modulation of replicative lifespan in Cryptococcus neoformans: Implications for virulence. Front. Microbiol. 2017, 8, 98. [Google Scholar] [CrossRef]
- Bouklas, T.; Pechuan, X.; Goldman, D.L.; Edelman, B.; Bergman, A.; Fries, B.C. Old Cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis. mBio 2013, 4, e00455-13. [Google Scholar] [CrossRef] [PubMed]
- Al-Huthaifi, A.M.; Radman, B.A.; Al-Alawi, A.A.; Mahmood, F.; Liu, T.B. Mechanisms and virulence factors of Cryptococcus neoformans dissemination to the central nervous system. J. Fungi 2024, 10, 586. [Google Scholar] [CrossRef]
- Coelho, C.; Camacho, E.; Salas, A.; Alanio, A.; Casadevall, A. Intranasal inoculation of Cryptococcus neoformans in mice produces nasal infection with rapid brain dissemination. mSphere 2019, 4, e00483-19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhao, X.; Lu, W.; Zhong, Y.; Fu, Y.V. Antifungal peptide SP1 damages polysaccharide capsule of Cryptococcus neoformans and enhances phagocytosis of macrophages. Microbiol. Spectr. 2023, 11, 4562. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Chrisman, C.J.; Castelli, M.V.; Frases, S.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L.; Casadevall, A. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008, 10, 2043–2057. [Google Scholar] [CrossRef]
- Jesus, M.D.; Nicola, A.M.; Chow, S.K.; Lee, I.R.; Nong, S.; Specht, C.A.; Levitz, S.M.; Casadevall, A. Glucuronoxylomannan, galactoxylomannan, and mannoprotein occupy spatially separate and discrete regions in the capsule of Cryptococcus neoformans. Virulence 2010, 1, 500–508. [Google Scholar] [CrossRef]
- Reuwsaat, J.C.V.; Motta, H.; Garcia, A.W.A.; Vasconcelos, C.B.; Marques, B.M.; Oliveira, N.K.; Rodrigues, J.; Ferrareze, P.A.G.; Frases, S.; Lopes, W.; et al. A predicted mannoprotein participates in Cryptococcus gattii capsular structure. mSphere 2018, 3, e00023-18. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Cherniak, R.; Kozel, T.R.; Granger, D.L.; Morris, L.C.; Weinhold, L.C.; Kwon-Chung, K.J. Structure and biological activities of acapsular Cryptococcus neoformans 602 complemented with the CAP64 gene. Infect. Immun. 1997, 65, 1584–1592. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kwon-Chung, K.J. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J. Bacteriol. 1999, 181, 5636–5643. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kwon-Chung, K.J. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun. 1998, 66, 2230–2236. [Google Scholar] [CrossRef]
- Chang, Y.C.; Penoyer, L.A.; Kwon-Chung, K.J. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun. 1996, 64, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Kwon-Chung, K.J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell Biol. 1994, 14, 4912–4919. [Google Scholar] [PubMed]
- Kozel, T.R.; Gotschlich, E.C. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 1982, 129, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Pham, T.; Hipsher, K.; Glueck, N.; Fan, Y.; Lin, X. Immunoprotection against cryptococcosis offered by Znf2 depends on capsule and the hyphal morphology. mBio 2022, 13, 2785. [Google Scholar] [CrossRef]
- Normile, T.G.; Chu, T.H.; Sheridan, B.S.; Del Poeta, M. Vaccine protection by Cryptococcus neoformans Δsgl1 is mediated by γδ T cells via TLR2 signaling. Mucosal Immunol. 2022, 15, 1416–1430. [Google Scholar] [CrossRef]
- Datta, K.; Pirofski, L.A. Towards a vaccine for Cryptococcus neoformans: Principles and caveats. FEMS Yeast Res. 2006, 6, 525–536. [Google Scholar] [CrossRef][Green Version]
- Maitta, R.W.; Datta, K.; Chang, Q.; Luo, R.X.; Witover, B.; Subramaniam, K.; Pirofski, L.A. Protective and nonprotective human immunoglobulin M monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan manifest different specificities and gene use profiles. Infect. Immun. 2004, 72, 4810–4818. [Google Scholar] [CrossRef]
- Casadevall, A.; Cleare, W.; Feldmesser, M.; Glatman-Freedman, A.; Goldman, D.L.; Kozel, T.R.; Lendvai, N.; Mukherjee, J.; Pirofski, L.A.; Rivera, J.; et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 1998, 42, 1437–1446. [Google Scholar] [CrossRef]
- Larsen, R.A.; Pappas, P.G.; Perfect, J.; Aberg, J.A.; Casadevall, A.; Cloud, G.A.; James, R.; Filler, S.; Dismukes, W.E. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated Cryptococcal meningitis. Antimicrob. Agents Chemother. 2005, 49, 952–958. [Google Scholar] [CrossRef]
- Casadevall, A.; Mukherjee, J.; Devi, S.J.; Schneerson, R.; Robbins, J.B.; Scharff, M.D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 1992, 165, 1086–1093. [Google Scholar] [CrossRef]
- Devi, S.J. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine 1996, 14, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Fleuridor, R.; Lees, A.; Pirofski, L. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans Infection1. J. Immunol. 2001, 166, 1087–1096. [Google Scholar] [CrossRef]
- Crawford, C.J.; Liporagi-Lopes, L.; Coelho, C.; Santos Junior, S.R.; Moraes Nicola, A.; Wear, M.P.; Vij, R.; Oscarson, S.; Casadevall, A. Semisynthetic glycoconjugate vaccine candidates against Cryptococcus neoformans. ACS Infect. Dis. 2024, 10, 2089–2100. [Google Scholar] [CrossRef]
- Chow, S.K.; Casadevall, A. Evaluation of Cryptococcus neoformans galactoxylomannan–protein conjugate as vaccine candidate against murine cryptococcosis. Vaccine 2011, 29, 1891–1898. [Google Scholar] [CrossRef]
- Doering, T.L. How sweet it is! cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu. Rev. Microbiol. 2009, 63, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Mukaremera, L. The Cryptococcus wall: A different wall for a unique lifestyle. PLoS Pathog. 2023, 19, e1011141. [Google Scholar] [CrossRef]
- Wang, Y.; Aisen, P.; Casadevall, A. Cryptococcus neoformans melanin and virulence: Mechanism of action. Infect. Immun. 1995, 63, 3131–3136. [Google Scholar] [CrossRef] [PubMed]
- James, P.G.; Cherniak, R.; Jones, R.G.; Stortz, C.A.; Reiss, E. Cell-wall glucans of Cryptococcus neoformans cap 67. Carbohydr. Res. 1990, 198, 23–38. [Google Scholar] [CrossRef]
- Reese, A.J.; Doering, T.L. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol. Microbiol. 2003, 50, 1401–1409. [Google Scholar] [CrossRef]
- Thompson, J.R.; Douglas, C.M.; Li, W.; Jue, C.K.; Pramanik, B.; Yuan, X.; Rude, T.H.; Toffaletti, D.L.; Perfect, J.R.; Kurtz, M. A glucan synthase FKS1 homolog in Cryptococcus neoformans is single copy and encodes an essential function. J. Bacteriol. 1999, 181, 444–453. [Google Scholar] [CrossRef]
- Reese, A.J.; Yoneda, A.; Breger, J.A.; Beauvais, A.; Liu, H.; Griffith, C.L.; Bose, I.; Kim, M.J.; Skau, C.; Yang, S.; et al. Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol. Microbiol. 2007, 63, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.M.; Donlin, M.J.; Gerik, K.J.; Specht, C.A.; Djordjevic, J.T.; Wilson, C.F.; Sorrell, T.C.; Lodge, J.K. KRE genes are required for β-1, 6-glucan synthesis, maintenance of capsule architecture and cell wall protein anchoring in Cryptococcus neoformans. Mol. Microbiol. 2010, 76, 517–534. [Google Scholar] [CrossRef]
- Basso, A.M.M.; De Castro, R.J.A.; de Castro, T.B.; Guimarães, H.I.; Polez, V.L.P.; Carbonero, E.R.; Pomin, V.H.; Hoffmann, C.; Grossi-de-Sa, M.F.; Tavares, A.H.; et al. Immunomodulatory activity of β-glucan-containing exopolysaccharides from auricularia auricular in phagocytes and mice infected with Cryptococcus neoformans. Med. Mycol. 2019, 58, 227–239. [Google Scholar] [CrossRef]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Agarwal, S.; Ram, S.; Rice, P.A.; Specht, C.A.; Levitz, S.M. Relative contributions of dectin-1 and complement to immune responses to particulate β-glucans. J. Immunol. 2012, 189, 312–317. [Google Scholar] [CrossRef]
- Specht, C.A.; Homan, E.J.; Lee, C.K.; Mou, Z.; Gomez, C.L.; Hester, M.M.; Abraham, A.; Rus, F.; Ostroff, G.R.; Levitz, S.M. Protection of mice against experimental cryptococcosis by synthesized peptides delivered in glucan particles. mBio 2021, 13, e0336721. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio 2010, 1, e00164-10. [Google Scholar] [CrossRef]
- Specht, C.A.; Lee, C.K.; Huang, H.; Hester, M.M.; Liu, J.; Luckie, B.A.; Torres Santana, M.A.; Mirza, Z.; Khoshkenar, P.; Abraham, A.; et al. Vaccination with recombinant Cryptococcus proteins in glucan particles protects mice against cryptococcosis in a manner dependent upon mouse strain and cryptococcal species. mBio 2017, 8, e01872-17. [Google Scholar] [CrossRef]
- Wang, R.; Oliveira, L.V.N.; Lourenco, D.; Gomez, C.L.; Lee, C.K.; Hester, M.M.; Mou, Z.; Ostroff, G.R.; Specht, C.A.; Levitz, S.M. Immunological correlates of protection following vaccination with glucan particles containing Cryptococcus neoformans chitin deacetylases. npj Vaccines 2023, 8, 6. [Google Scholar] [CrossRef]
- Banks, I.R.; Specht, C.A.; Donlin, M.J.; Gerik, K.J.; Levitz, S.M.; Lodge, J.K. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell. 2005, 4, 1902–1912. [Google Scholar] [CrossRef]
- Bacon, J.S.; Jones, D.; Farmer, V.C.; Webley, D.M. The occurrence of α (1–3) glucan in Cryptococcus, schizosaccharomyces and polyporus species, and its hydrolysis by a streptomyces culture filtrate lysing cell walls of Cryptococcus. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1968, 158, 313–315. [Google Scholar] [CrossRef]
- Upadhya, R.; Lam, W.C.; Maybruck, B.; Specht, C.A.; Levitz, S.M.; Lodge, J.K. Induction of protective immunity to cryptococcal infection in mice by a heat-killed, chitosan-deficient strain of Cryptococcus neoformans. mBio 2016, 7, e00547-16. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell. 2007, 6, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M.; Huang, H.; Ostroff, G.R.; Specht, C.A. Exploiting fungal cell wall components in vaccines. Semin. Immunopathol. 2015, 37, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.G.; Specht, C.A.; Lodge, J.K. Cell wall chitosan is necessary for virulence in the opportunistic pathogen Cryptococcus neoformans. Eukaryot. Cell. 2011, 10, 1264–1268. [Google Scholar] [CrossRef]
- Upadhya, R.; Baker, L.G.; Lam, W.C.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Cryptococcus neoformans Cda1 and Its Chitin Deacetylase Activity Are Required for Fungal Pathogenesis. mBio. 2018, 9, e02087-18. [Google Scholar] [CrossRef]
- Lam, W.C.; Upadhya, R.; Specht, C.A.; Ragsdale, A.E.; Hole, C.R.; Levitz, S.M.; Lodge, J.K. Chitosan biosynthesis and virulence in the human fungal pathogen Cryptococcus gattii. mSphere 2019, 4, e00644-19. [Google Scholar] [CrossRef]
- Upadhya, R.; Lam, W.C.; Hole, C.R.; Vasselli, J.G.; Lodge, J.K. Cell wall composition in Cryptococcus neoformans is media dependent and alters host response, inducing protective immunity. Front. Fungal Biol. 2023, 4, 1183291. [Google Scholar] [CrossRef]
- Hole, C.R.; Lam, W.C.; Upadhya, R.; Lodge, J.K. Cryptococcus neoformans chitin synthase 3 plays a critical role in dampening host inflammatory responses. mBio 2020, 11, e03373-19. [Google Scholar] [CrossRef]
- Hester, M.M.; Oliveira, L.V.N.; Wang, R.; Mou, Z.; Lourenco, D.; Ostroff, G.R.; Specht, C.A.; Levitz, S.M. Cross-reactivity between vaccine antigens from the chitin deacetylase protein family improves survival in a mouse model of cryptococcosis. Front. Immunol. 2022, 13, 1015586. [Google Scholar] [CrossRef]
- Zaman, M.; Chandrudu, S.; Toth, I. Strategies for intranasal delivery of vaccines. Drug Deliv. Transl. Res. 2013, 3, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Specht, C.A.; Levitz, S.M. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 2006, 6, 513–524. [Google Scholar]
- Specht, C.A.; Nong, S.; Dan, J.M.; Lee, C.K.; Levitz, S.M. Contribution of glycosylation to T cell responses stimulated by recombinant Cryptococcus neoformans mannoprotein. J. Infect. Dis. 2007, 196, 796–800. [Google Scholar] [CrossRef]
- Levitz, S.M.; Nong, S.; Mansour, M.K.; Huang, C.; Specht, C.A. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc. Natl. Acad. Sci. USA 2001, 98, 10422–10427. [Google Scholar] [CrossRef]
- Viudes, A.; Lazzell, A.; Perea, S.; Kirkpatrick, W.R.; Peman, J.; Patterson, T.F.; Martinez, J.P.; López-Ribot, J.L. The C-terminal antibody binding domain of Candida albicans mp58 represents a protective epitope during candidiasis. FEMS Microbiol. Lett. 2004, 232, 133–138. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lau, C.C.; Tung, E.T.; Chong, K.T.; Yang, F.; Zhang, H.; Lo, R.K.; Cai, J.P.; Au-Yeung, R.K.; et al. Mp1p is a virulence factor in Talaromyces (penicillium) marneffei. PLoS Neglected Trop. Dis. 2016, 10, e0004907. [Google Scholar] [CrossRef]
- Cao, L.; Chan, C.M.; Lee, C.; Wong, S.S.; Yuen, K.Y. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus penicillium marneffei. Infect. Immun. 1998, 66, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Pietrella, D.; Corbucci, C.; Perito, S.; Bistoni, G.; Vecchiarelli, A. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect. Immun. 2005, 73, 820–827. [Google Scholar] [CrossRef]
- Mansour, M.K.; Schlesinger, L.S.; Levitz, S.M. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J. Immunol. 2002, 168, 2872–2879. [Google Scholar] [CrossRef]
- Biondo, C.; Messina, L.; Bombaci, M.; Mancuso, G.; Midiri, A.; Beninati, C.; Cusumano, V.; Gerace, E.; Papasergi, S.; Teti, G. Characterization of two novel cryptococcal mannoproteins recognized by immune sera. Infect. Immun. 2005, 73, 7348–7355. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Mota, C.; Thak, E.J.; Kim, J.; Son, Y.J.; Oh, D.B.; Kang, H.A. Effects of altered N-glycan structures of Cryptococcus neoformans mannoproteins, MP98 (Cda2) and MP84 (Cda3), on interaction with host cells. Sci. Rep. 2023, 13, 1175. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Lam, W.C.; Hole, C.R.; Parchment, D.; Lee, C.K.; Specht, C.A.; Levitz, S.M.; Lodge, J.K. Cryptococcus neoformans Cda1 and Cda2 coordinate deacetylation of chitin during infection to control fungal virulence. Cell Surf. 2021, 7, 100066. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.S.; Miah, M.I.; Rahman, S.R. A comprehensive immunoinformatic analysis of chitin deacetylase’s and MP88 for designing multi-epitope vaccines against Cryptococcus neoformans. J. Biomol. Struct. Dyn. 2023, 42, 10711–10726. [Google Scholar] [CrossRef]
- Wang, R.; Oliveira, L.V.N.; Hester, M.M.; Carlson, D.; Christensen, D.; Specht, C.A.; Levitz, S.M. Protection against experimental cryptococcosis elicited by cationic adjuvant formulation 01-adjuvanted subunit vaccines. PLoS Pathog. 2024, 20, e1012220. [Google Scholar] [CrossRef]
- Biondo, C.; Mancuso, G.; Midiri, A.; Bombaci, M.; Messina, L.; Beninati, C.; Teti, G. Identification of major proteins secreted by Cryptococcus neoformans. FEMS Yeast Res. 2006, 6, 645–651. [Google Scholar] [CrossRef]
- Cadieux, B.; Lian, T.; Hu, G.; Wang, J.; Biondo, C.; Teti, G.; Liu, V.; Murphy, M.E.; Creagh, A.L.; Kronstad, J.W. The mannoprotein Cig1 supports iron acquisition from heme and virulence in the pathogenic fungus Cryptococcus neoformans. J. Infect. Dis. 2013, 207, 1339–1347. [Google Scholar] [CrossRef]
- Yu, C.H.; Sephton-Clark, P.; Tenor, J.L.; Toffaletti, D.L.; Giamberardino, C.; Haverkamp, M.; Cuomo, C.A.; Perfect, J.R. Gene expression of diverse Cryptococcus isolates during infection of the human central nervous system. mBio 2021, 12, 2313. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Norton, D.; Price, M.S.; Hay, C.; Clements, M.F.; Nichols, C.B.; Alspaugh, J.A. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog. 2010, 6, e1000776. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Holmer, S.M.; Selvig, K.; Dietrich, F.; Alspaugh, J.A. Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. mBio 2013, 4, e00522-12. [Google Scholar] [CrossRef]
- Geddes, J.M.; Croll, D.; Caza, M.; Stoynov, N.; Foster, L.J.; Kronstad, J.W. Secretome profiling of Cryptococcus neoformans reveals regulation of a subset of virulence-associated proteins and potential biomarkers by protein kinase A. BMC Microbiol. 2015, 15, 206. [Google Scholar] [CrossRef] [PubMed]
- Geddes, J.M.; Caza, M.; Croll, D.; Stoynov, N.; Foster, L.J.; Kronstad, J.W. Analysis of the Protein Kinase A-Regulated Proteome of Cryptococcus neoformans Identifies a Role for the Ubiquitin-Proteasome Pathway in Capsule Formation. mBio 2016, 7, e01862-15. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, K.; Wang, Y.; Liu, T.B.; Rivera, A.; Xue, C. Ubiquitin proteolysis of a CDK-related kinase regulates titan cell formation and virulence in the fungal pathogen Cryptococcus neoformans. Nat. Commun. 2022, 13, 6397. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of extracellular vesicles in immune response and immunity. Immunity 2024, 57, 1752–1768. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, K.; Hu, H.; Xing, X.; Huang, X.; Gao, H. Extracellular vesicles: Their functions in plant–pathogen interactions. Mol. Plant Pathol. 2022, 23, 760–771. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; Castelli, R.F.; Reis, F.C.G.; Rizzo, J.; Rodrigues, M.L. Pathogenic delivery: The biological roles of cryptococcal extracellular vesicles. Pathogens 2020, 9, 754. [Google Scholar] [CrossRef]
- Rizzo, J.; Wong, S.S.W.; Gazi, A.D.; Moyrand, F.; Chaze, T.; Commere, P.H.; Novault, S.; Matondo, M.; Péhau-Arnaudet, G.; Reis, F.C.G.; et al. Cryptococcus extracellular vesicles properties and their use as vaccine platforms. J. Extracell. Vesicles 2021, 10, e12129. [Google Scholar] [CrossRef]
- Colombo, A.C.; Rella, A.; Normile, T.; Joffe, L.S.; Tavares, P.M.; de SAraújo, G.R.; Frases, S.; Orner, E.P.; Farnoud, A.M.; Fries, B.C.; et al. Cryptococcus neoformans Glucuronoxylomannan and Sterylglucoside Are Required for Host Protection in an Animal Vaccination Model. mBio 2019, 10, e02909-18. [Google Scholar] [CrossRef]
- Rella, A.; Mor, V.; Farnoud, A.M.; Singh, A.; Shamseddine, A.A.; Ivanova, E.; Carpino, N.; Montagna, M.T.; Luberto, C.; Del Poeta, M. Role of Sterylglucosidase 1 (Sgl1) on the pathogenicity of Cryptococcus neoformans: Potential applications for vaccine development. Front Microbiol. 2015, 6, 836. [Google Scholar] [CrossRef]
- Del Poeta, M.; Wormley, F.L., Jr.; Lin, X. Host populations, challenges, and commercialization of cryptococcal vaccines. PLoS Pathog. 2023, 19, e1011115. [Google Scholar] [CrossRef] [PubMed]
- Wormley, F.L., Jr.; Perfect, J.R.; Steele, C.; Cox, G.M. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect. Immun. 2007, 75, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Leopold Wager, C.M.; Hole, C.R.; Campuzano, A.; Castro-Lopez, N.; Cai, H.; Caballero Van Dyke, M.C.; Wozniak, K.L.; Wang, Y.; Wormley, F.L., Jr. IFN-γ immune priming of macrophages in vivo induces prolonged STAT1 binding and protection against Cryptococcus neoformans. PLoS Pathog. 2018, 14, e1007358. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, M.C.C.; Chaturvedi, A.K.; Hardison, S.E.; Leopold Wager, C.M.; Castro-Lopez, N.; Hole, C.R.; Wozniak, K.L.; Wormley, F.L., Jr. Induction of broad-spectrum protective immunity against disparate Cyptococcus serotypes. Front. Immunol. 2017, 8, 1359. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Wozniak, K.L.; Masso-Silva, J.; Upadhyay, S.; Hole, C.; Rivera, A.; Wormley, F.L., Jr.; Lin, X. Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. mBio 2015, 6, 1433. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, Y.; Ferraro, A.R.; Yang, E.; Lewis, Z.A.; Lin, X. Transcription factor Znf2 coordinates with the chromatin remodeling SWI/SNF complex to regulate cryptococcal cellular differentiation. Commun. Biol. 2019, 2, 412. [Google Scholar] [CrossRef]
- Pham, T.; Li, Y.; Watford, W.; Lin, X. Vaccination with a ZNF2oe strain of Cryptococcus provides long-lasting protection against cryptococcosis and is effective in immunocompromised hosts. Infect. Immun. 2023, 91, 198. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Masso-Silva, J.; Rivera, A.; Xue, C. A heat-killed Cryptococcus mutant strain induces host protection against multiple invasive mycoses in a murine vaccine model. mBio 2019, 10, e02145-19. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Rivera, A.; Xue, C. Development of a heat-killed fbp1 mutant strain as a therapeutic agent to treat invasive Cryptococcus infection. Microbiol Spectr. 2023, 11, e0495522. [Google Scholar] [CrossRef]
- Wang, K.; Espinosa, V.; Wang, Y.; Lemenze, A.; Kumamoto, Y.; Xue, C.; Rivera, A. Innate cells and STAT1-dependent signals orchestrate vaccine-induced protection against invasive Cryptococcus infection. mBio 2024, 15, e01944-24. [Google Scholar] [CrossRef]
- Masso-Silva, J.; Espinosa, V.; Liu, T.B.; Wang, Y.; Xue, C.; Rivera, A. The f-box protein fbp1 shapes the immunogenic potential of Cryptococcus neoformans. mBio 2018, 9, e01828-17. [Google Scholar] [CrossRef] [PubMed]
- Hester, M.M.; Carlson, D.; Lodge, J.K.; Levitz, S.M.; Specht, C.A. Immune evasion by Cryptococcus gattii in vaccinated mice coinfected with C. neoformans. Front. Immunol. 2024, 15, 1356651. [Google Scholar] [CrossRef] [PubMed]
- Rachini, A.; Pietrella, D.; Lupo, P.; Torosantucci, A.; Chiani, P.; Bromuro, C.; Proietti, C.; Bistoni, F.; Cassone, A.; Vecchiarelli, A. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect Immun. 2007, 11, 5085–5094. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Shi, L.; Barreto-Bergter, E.; Nimrichter, L.; Farias, S.E.; Rodrigues, E.G.; Travassos, L.R.; Nosanchuk, J.D. Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin Vaccine Immunol. 2007, 10, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shao, J.; Dai, M.; Fang, W.; Yang, Y.L. Adaptive immunology of Cryptococcus neoformans infections—An update. Front. Immunol. 2023, 14, 1174967. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Young, M.L.; Wormley, F.L., Jr. Protective immunity against experimental pulmonary ryptococcosis in T cell-depleted mice. Clin. Vaccine Immunol. 2011, 18, 717–723. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Ravi, S.; Macias, S.; Young, M.L.; Olszewski, M.A.; Steele, C.; Wormley, F.L. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS ONE 2009, 4, e6854. [Google Scholar] [CrossRef] [PubMed]
- Normile, T.G.; Rella, A.; Del Poeta, M. Cryptococcus neoformans Δ sgl1 vaccination requires either CD4 or CD8 T cells for complete host protection. Front. Cell. Infect. Microbiol. 2021, 11, 739027. [Google Scholar] [CrossRef]
- Espinosa, V.; Dutta, O.; Heung, L.J.; Wang, K.; Chang, Y.J.; Soteropoulos, P.; Hohl, T.M.; Siracusa, M.C.; Rivera, A. Cutting edge: Neutrophils license the maturation of monocytes into effective antifungal effectors. J. Immunol. 2022, 209, 1827–1831. [Google Scholar] [CrossRef]
- Espinosa, V.; Dutta, O.; McElrath, C.; Du, P.; Chang, Y.J.; Cicciarelli, B.; Pitler, A.; Whitehead, I.; Obar, J.J.; Durbin, J.E.; et al. Type III interferon is a critical regulator of innate antifungal immunity. Sci. Immunol. 2017, 2, eaan5357. [Google Scholar] [CrossRef]
- Espinosa, V.; Jhingran, A.; Dutta, O.; Kasahara, S.; Donnelly, R.; Du, P.; Rosenfeld, J.; Leiner, I.; Chen, C.C.; Ron, Y.; et al. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 2014, 10, e1003940. [Google Scholar] [CrossRef] [PubMed]
- Mukaremera, L.; Nielsen, K. Adaptive immunity to Cryptococcus neoformans infections. J. Fungi 2017, 3, 64. [Google Scholar] [CrossRef]
- Specht, C.A.; Wang, R.; Oliveira, L.V.N.; Hester, M.M.; Gomez, C.; Mou, Z.; Carlson, D.; Lee, C.K.; Hole, C.R.; Lam, W.C.; et al. Immunological correlates of protection mediated by a whole organism, Cryptococcus neoformans, vaccine deficient in chitosan. mBio 2024, 15, 1746. [Google Scholar] [CrossRef]
- Hardison, S.E.; Brown, G.D. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 2012, 13, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Osorio, F.; Rosas, M.; Freitas, R.P.; Schweighoffer, E.; Gross, O.; Verbeek, J.S.; Ruland, J.; Tybulewicz, V.; Brown, G.D.; et al. Dectin-2 is a syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 2009, 206, 2037–2051. [Google Scholar] [CrossRef]
- Saijo, S.; Ikeda, S.; Yamabe, K.; Kakuta, S.; Ishigame, H.; Akitsu, A.; Fujikado, N.; Kusaka, T.; Kubo, S.; Chung, S.H.; et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010, 32, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van Het Hof, B.; van Kooyk, Y.; Geijtenbeek, T.B. C-type lectin DC-SIGN modulates toll-like receptor signaling via raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity 2007, 26, 605–616. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van der Vlist, M.; Wevers, B.; Bruijns, S.C.; Geijtenbeek, T.B. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through raf-1 and syk. Nat. Immunol. 2009, 10, 203–213. [Google Scholar] [CrossRef]
- Leibund Gut-Landmann, S.; Gross, O.; Robinson, M.J.; Osorio, F.; Slack, E.C.; Tsoni, S.V.; Schweighoffer, E.; Tybulewicz, V.; Brown, G.D.; Ruland, J.; et al. Syk-and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007, 8, 630–638. [Google Scholar] [CrossRef]
- Rivera, A.; Siracusa, M.C.; Yap, G.S.; Gause, W.C. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol. 2016, 17, 356–363. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef]
- Tarang, S.; Kesherwani, V.; LaTendresse, B.; Lindgren, L.; Rocha-Sanchez, S.M.; Weston, M.D. In silico design of a multivalent vaccine against Candida albicans. Sci. Rep. 2020, 10, 1066. [Google Scholar] [CrossRef]
- Tarcha Eric, J.; Venkatesha, B.; Hung, C.Y.; Gardner Malcolm, J.; Cole Garry, T. Multivalent recombinant protein vaccine against coccidioidomycosis. Infect. Immun. 2006, 74, 5802–5813. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, Q.J.; Ambati, S.; Link, C.D.; Lin, X.; Lewis, Z.A.; Meagher, R.B. Dectin-3-targeted antifungal liposomes efficiently bind and kill diverse fungal pathogens. Mol. Microbiol. 2023, 120, 723–739. [Google Scholar] [CrossRef]
- Pham, T.; Shi, R.; Ambati, S.; Meagher, R.; Lin, X. All hands on dec: Treating cryptococcosis with dectin decorated liposomes loaded with antifungals. iScience. 2024, 27, 110349. [Google Scholar] [CrossRef]


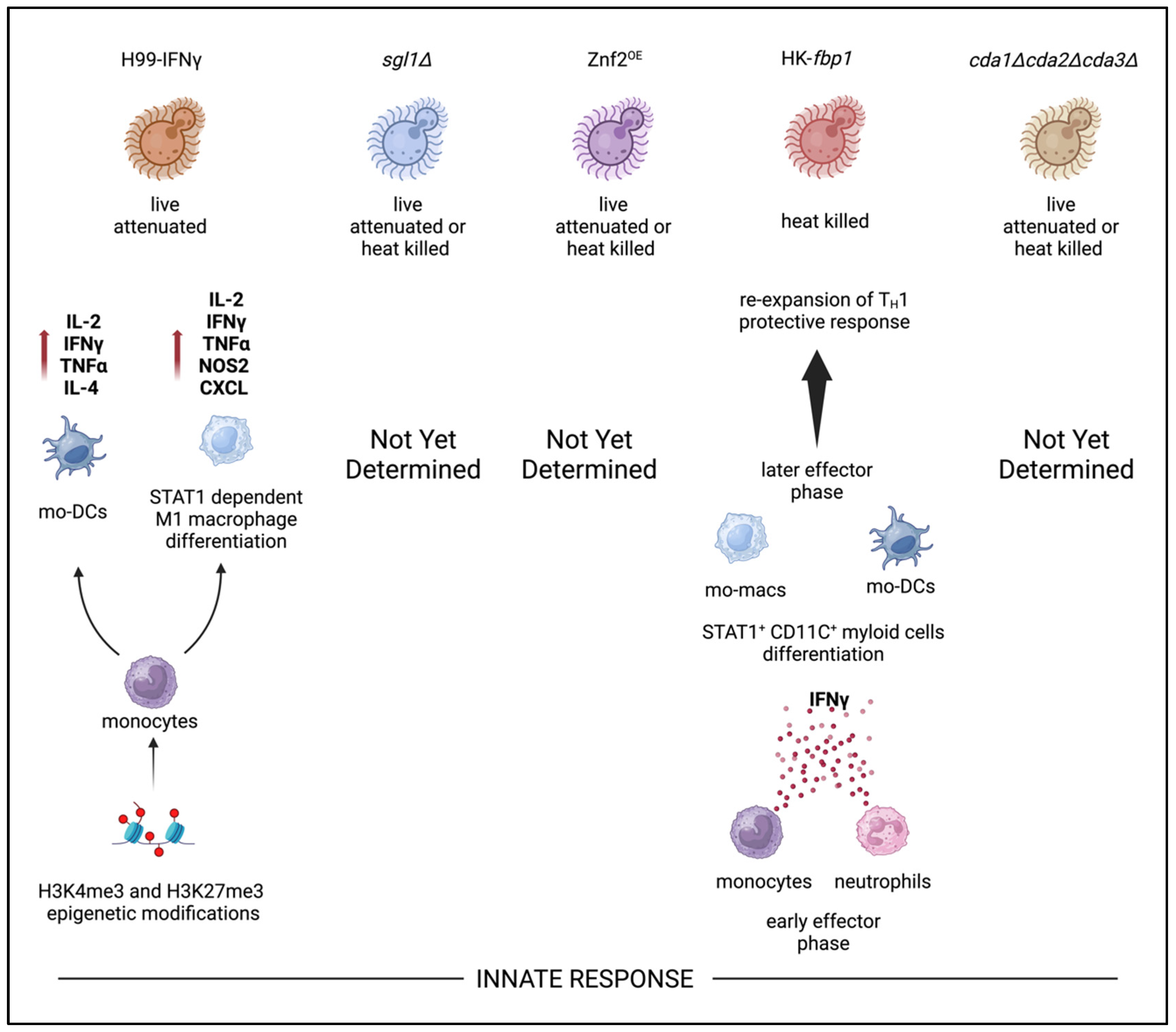
| Vaccine Candidate | Vaccination Method | Background | Vaccine Route Administration | Mechanism | Reference |
|---|---|---|---|---|---|
| sgl1Δ | Whole-cell, live-attenuated, and heat-killed | C. neoformans sterylglucosidase deficient strain | Intranasal | IFNγ and IL-17A produced by γδT CD4+, and CD8+cells | [27] |
| H99γ | Whole-cell, live-attenuated | Mouse IFNγ producing C. neoformans H99 strain | Intranasal | TH-1/proinflammatory cell response | [94] |
| Znf2OE | Whole-cell, live-attenuated, and heat-killed | C. neoformans zinc finger transcription factor 2 overexpressed | Intranasal | TH-1/TH-17 | [97] |
| HK-fbp1 | Whole-cell, heat-killed | Disruption of SCF E3 ligase complex via deletion of F-box protein 1 in C. neoformans | Intranasal | TH-1/TH-17 response | [100,103] |
| cda1∆2∆3∆ | Whole-cell, live-attenuated, and heat-killed | Deletion of 3 chitin deacetylases in C. neoformans | Intranasal | CD4+ T-cell response; proinflammatory cytokines IL-1β, IL-6, and IL-23 | [54] |
| Glucan Particles (GP) | Protein subunit vaccine | Synthesized subunit protein | Intranasal | Antibody and T-cell response | [48,49,51] |
| β-Glucan antibody | Antibody-based | Monoclonal antibody | Intraperitoneal | Antibody response | [105] |
| Glucosylceramide antibody | Antibody-based | Monoclonal antibody | Intraperitoneal | Antibody response | [106] |
| P13-TT | Antibody-based | Peptide mimic of C. neoformans GXM conjugated to tetanus toxoid | Subcutaneous | Antibody response | [34] |
| GXM-TT | Subunit vaccine | C. neoformans GXM conjugated to tetanus toxoid | Subcutaneous | Antibody response | [34] |
| GalXM-BSA | Subunit vaccine | C. neoformans GalXM conjugated to BSA | Subcutaneous | Antibody response | [36] |
| GXM antibody 18B7 | Antibody-based | Monoclonal antibody | Intravenous | Antibody responseclinical trial phase 1 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avina, S.L.; Pawar, S.; Rivera, A.; Xue, C. Will the Real Immunogens Please Stand Up: Exploiting the Immunogenic Potential of Cryptococcal Cell Antigens in Fungal Vaccine Development. J. Fungi 2024, 10, 840. https://doi.org/10.3390/jof10120840
Avina SL, Pawar S, Rivera A, Xue C. Will the Real Immunogens Please Stand Up: Exploiting the Immunogenic Potential of Cryptococcal Cell Antigens in Fungal Vaccine Development. Journal of Fungi. 2024; 10(12):840. https://doi.org/10.3390/jof10120840
Chicago/Turabian StyleAvina, Samantha L., Siddhi Pawar, Amariliz Rivera, and Chaoyang Xue. 2024. "Will the Real Immunogens Please Stand Up: Exploiting the Immunogenic Potential of Cryptococcal Cell Antigens in Fungal Vaccine Development" Journal of Fungi 10, no. 12: 840. https://doi.org/10.3390/jof10120840
APA StyleAvina, S. L., Pawar, S., Rivera, A., & Xue, C. (2024). Will the Real Immunogens Please Stand Up: Exploiting the Immunogenic Potential of Cryptococcal Cell Antigens in Fungal Vaccine Development. Journal of Fungi, 10(12), 840. https://doi.org/10.3390/jof10120840








