1. Introduction
Fusarium sulphureum is an important postharvest pathogenic fungus and can cause postharvest diseases of muskmelon and potato tuber [
1,
2]. Postharvest diseases caused by
F. sulphureum not only deteriorate the postharvest quality of fruit and vegetables but also lead to diacetoxyscirpenol (DAS) contamination in fruit and vegetables. DAS is a highly toxic type A trichothecene that can contaminate food and animal feed, thus causing serious harm to human and animal health [
3].
F. sulphureum invades mainly through wounds or natural openings, resulting in the colonization and spread of pathogenic fungi in plants, ultimately leading to the occurrence of disease. When the pathogens infect host plants, ambient pH plays an important role in the colonization and expansion of pathogens.
pH is one of the main environmental factors affecting the pathogenicity of fungi to some certain extent, and can affect the growth and development of pathogenic fungi [
4]. Spore germination and sporulation are important indexes to measure the reproductive ability and spore survival ability of filamentous fungi. The central developmental pathway composed of three key developmental activators,
BrlA,
AbaA, and
WetA, regulates asexual development and sporulation through the transduction of developmental signals.
BrlA is a zinc-finger domain-containing transcription factor, and is also the hub of the central pathway and upstream regulatory factor signaling.
BrlA is independent and necessary in developmental regulation. Activation of
BrlA is an important marker of the initiation of asexual development and occurs in the early stage of conidiophore development [
5]. The important role of
BrlA after activation is to immediately activate
AbaA, which is expressed in the middle stage of conidiophore development.
WetA is an important regulator of spore maturation. In the late stage of conidiophore development, activated
AbaA activates the expression of
WetA [
6]. The downstream central pathway of
VosA exists in the nucleus of mature spores and is also an important factor regulating spore maturation, which is mainly responsible for the accumulation of trehalose in spores [
7]. At the end of sporulation, the expression of
BrlA is inhibited by their feedback, thus completing the whole asexual development process. Zhang et al. [
8] found that different ambient pH had a significant effect on the mycelial growth and sporulation of
Ustilaginoidea virens. The mycelium grew better at pH 4.5–7.0, while the mycelial growth was inhibited at pH ≤ 4.0 and pH ≥ 7.5. When the ambient pH was 5.0, sporulation was at its highest. Similar results were found by Jimdjio et al. [
9], who suggested that colony growth of
Penicillium expansum was significantly inhibited in too acidic (2.5) or too basic (8.5) an environment.
In addition to influencing the growth and development of pathogenic fungi, ambient pH also affects the secretion and expression of pathogenic factors. Pathogenic fungi interfere with the normal physiological metabolism of host cells and destroy cells by secreting metabolites that are harmful to the host, such as cell wall-degrading enzymes (CWDEs), mycotoxins, and pathogenic hormones, which play crucial roles in pathogenicity [
10]. CWDEs secreted by pathogenic fungi are enzymes that can degrade the host cell wall when infecting the host plant. CWDEs are considered to be the most important pathogenic factors in the pathogenesis of most postharvest diseases [
11,
12]. CWDEs are mainly composed of keratinase, pectinase, and cellulase. Pectin is an important CWDE, and is composed of polygalacturonase (PG), pectin methylase (PME), pectin methyl polygalacturonase (PMG), pectin lyase (PL), polygalacturonic acid trans-eliminase (PGTE), and pectin methyl-trans-eliminase (PMTE). PME first catalyzes the demethylation of the main chain of pectin in the cell wall and the middle layer of the fruit and produces a free carboxyl group while releasing methanol. The release of methanol leads to a decrease in the pH value of the cell wall, which is more conducive to the role of PG. PG can hydrolyze the α-1,4 glycosidic bond in the polygalacturonic acid in the cell wall and the middle glue layer, resulting in the destruction of the middle glue layer and the loosening of the cell wall structure, resulting in softening and decay of the fruit tissue. PMG is a hydrolase that specifically hydrolyzes the glycosidic bond of the substrate. It has a high selectivity for the degree of esterification of the substrate and can hydrolyze the α-1,4 glycosidic bond of highly esterified pectin esters. PL can catalyze the degradation of galacturonic acid and produce unsaturated oligosaccharides with 4-deoxy-α-D-galactose-4-enoic acid groups at its non-reducing end through β-elimination [
13,
14]. PGTE and PMTE are two important lyases and use pectin acid and pectin as substrates, respectively [
15]. Cellulase is mainly composed of cellulase (Cx) and β-glucosidase (β-Glu). Cx is an important cell wall enzyme in the process of fruit softening, and can degrade cellulose and soften fruit. The increase in Cx activity is one of the main reasons for fruit softening. β-Glu mainly hydrolyzes β-D-glycosidic bonds at non-reducing ends to produce β-D-glucose [
14]. Studies have shown that ambient pH affects the pathogenicity of fungi to fruit by regulating the secretion of CWDEs such as PG, PME, PMG, and PL [
16,
17]. In addition, ambient pH also plays a crucial role in regulating the accumulation of mycotoxins. Maor et al. [
18] found that ambient pH regulated ochratoxin A biosynthesis in
Aspergillus carbonarius. Similarly, Jimdjio et al. [
9] found that ambient pH influenced the accumulation of patulin in
P. expansum by influencing patulin’s biosynthesis. Therefore, for pathogens, the appropriate ambient pH value can activate the activity of CWDEs and promote the accumulation of mycotoxins, enhancing their capability to infect host plants.
Our previous study indicated that ambient pH significantly affected the pathogenicity of F. sulphureum during infection of muskmelon fruit. However, how pH affects the pathogenicity of F. sulphureum by regulating the activity of CWDEs and whether pH has a regulatory effect on the biosynthesis of mycotoxins in F. sulphureum-inoculated muskmelon fruit have not been reported. Therefore, the purpose of this study was: (1) to clarify the effect of ambient pH on pathogenicity in F. sulphureum-infected muskmelon; (2) to investigate the effects of different ambient pH on spore germination and germ tube growth of F. sulphureum; and (3) to determine the extent of spore suspension with different pH values on the CWDE activity of muskmelon fruit. Finally, the impact of ambient pH on DAS accumulation and the relative expression of genes involved in the DAS biosynthetic pathway were analyzed.
2. Materials and Methods
2.1. Fungal Strain and Muskmelon Fruit
Fusarium sulphureum was provided by Gansu Agricultural University, Lanzhou, China.
Muskmelon fruit (cv. “honeydew melon”) of commercial maturity was collected from the plantation base of Qingbaishi Town, located in the north of the Yellow River in Lanzhou, Gansu Province, in July 2021. Muskmelon fruit of good quality and without mechanical damage was selected and transported to the laboratory on the same day and stored at 5–8 °C, 85–90% RH for use.
2.2. Preparation of Spore Suspensions with Different pH and Fruit Inoculation
The F. sulphureum strain was cultured for 7 days in a 25 °C incubator. Sterile distilled water was buffered with 0.2 M Na2HPO4·12H2O and 0.1 M C6H8O·7H2O, and the pH was adjusted to 3, 5, 6, 7, and 9 using pH meter to prepare a spore suspension with a concentration of 1 × 106 spores/mL. According to our previous research results (the most favorable pH for F. sulphureum was 6), a pH value of 6 was used as a control during the whole experiment.
The method of inoculating muskmelon with
F. sulphureum followed Jimdjio et al. [
9] with slight modification. The muskmelon fruit was immersed in 1% NaClO
3 solution for 5 min for surface disinfection after washing with water, then were allowed to air-dry. Subsequently, the fruit was surface-disinfected with 75% alcohol. Then, four inoculated holes were drilled at the equator of the fruit to a depth of 3 mm with a diameter of 3 mm with a sterilizing iron nail, and 20 μL spore suspensions with different pH were inserted into the inoculated holes. The inoculated fruit was kept at a temperature of 14–18 °C and humidity of 85–90%. From the beginning of inoculation, suspensions buffered with 0.2 M Na
2HPO
4·12H
2O and 0.1 M C
6H
8O·7H
2O (pH 3, 5, 6, 7, and 9) were injected into the inoculation holes of the fruit every 12 h to maintain its pH until the end of our sampling. A total of 20 fruit were employed for each treatment, and each group of experiments was repeated at least three times, so a total of 300 fruit were required.
2.3. Assay of the Lesion Diameter and DAS Accumulation
The diameter of lesions (r) was measured at 0, 1, 3, 5, and 7 days post inoculation (DPI), and the lesion area was calculated based on πr
2. Samples (4.0 g) from the junction of diseased and healthy tissue were collected, quickly frozen in liquid nitrogen, ground into powder, and stored for further use. The decaying tissue at the lesion was cut with a sterile steel knife at 7 DPI and at −80 °C for the determination of DAS content. The determination of DAS content was based on our previous publication [
2]. One gram of frozen muskmelon fruit tissue was taken, then extracted with ethyl acetate (1:2,
v/
v) twice. The organic phase was combined to remove the ethyl acetate and subsequently dried with nitrogen stream. The dried residues were redissolved in 2 mL acetonitrile for UPLC-MS/MS analysis.
DAS content was analyzed with UPLC-MS/MS (Waters Acquity Ultra Performance LC system, Waters, Milford, MA, USA). The column of a ZORBAX Eclipse Plus C18 was used, and the column was maintained at 40 °C. The linear gradient elution was performed by starting with 95% of mobile phase A (0.1% formic acid aqueous solution) and 5% of mobile phase B (acetonitrile), then changing to 38% of mobile phase A and 62% of mobile-phase B in 1.8 min, subsequently shifted to 95% of mobile phase B within 0.5 min and maintained, then the initial proportion was recovered for 1 min. The flow rate was 0.4 mL min−1, and the injection volume was 5.0 μL. In sum, 20 fruit were employed for each treatment, and each group of experiments was repeated at least three times, so a total of 300 fruit were required.
2.4. Effects of Ambient pH on Spore Germination and Germ Tube Length
The pH values of PDA medium were adjusted to 3, 5, 6, 7, and 9 with 0.2 M Na2HPO4·12H2O and 0.1 M C6H8O·7H2O buffer, respectively. Spore suspensions of F. sulphureum were prepared (1 × 106 spores/mL), and 2 μL of each suspension was inoculated on PDA medium with different pH values. The spore germination and germ tube length were observed and measured under a microscope (CX21FS1C, Olympus, Beijing, China) at 4, 6, and 8 h, respectively.
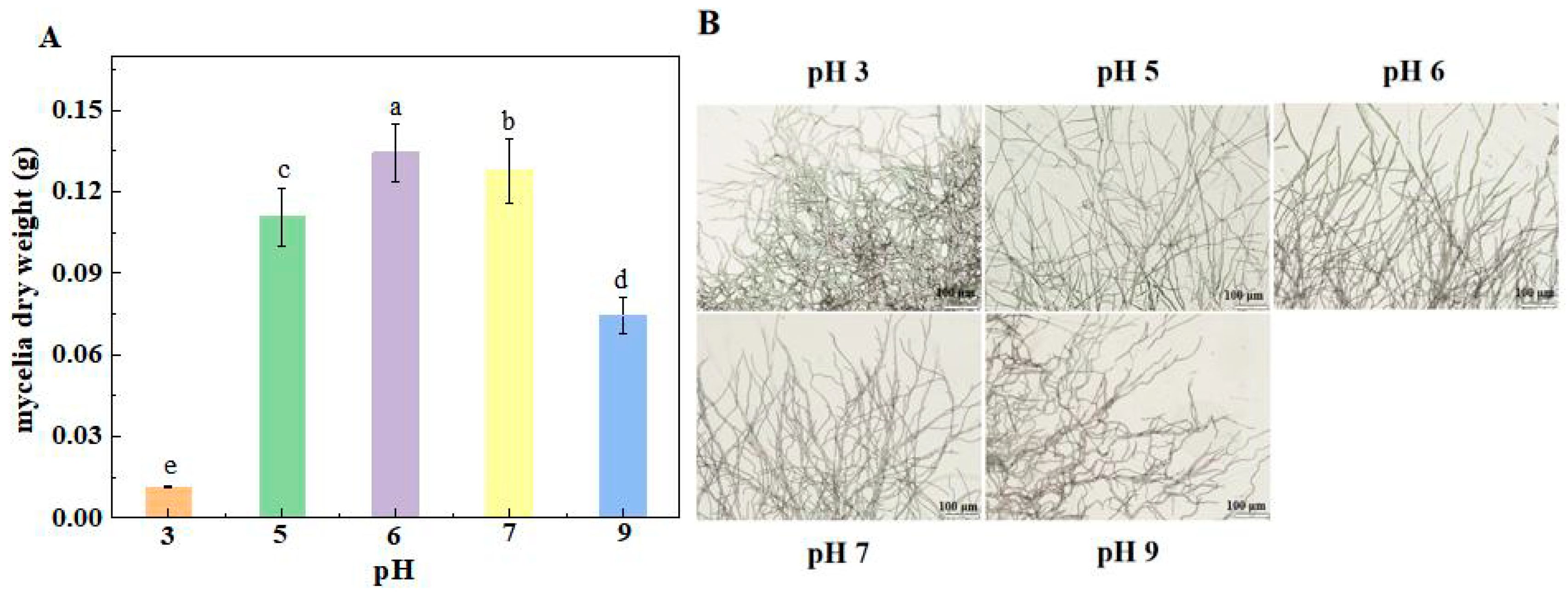
2.5. Effect of Ambient pH on Mycelial Biomass and Mycelial Morphology of F. sulphureum
F. sulphureum spore suspension (2 μL, 1 × 106 spores/mL) was inoculated in PDB medium with different pH values and cultured at 25 °C at a speed of 220 rpm for 4 days. Mycelia were collected and their dry weight measured.
Mycelium morphology was observed according to the method of Han et al. [
19]. After 3 days of
F. sulphureum culture, mycelium growth was observed under a microscope.
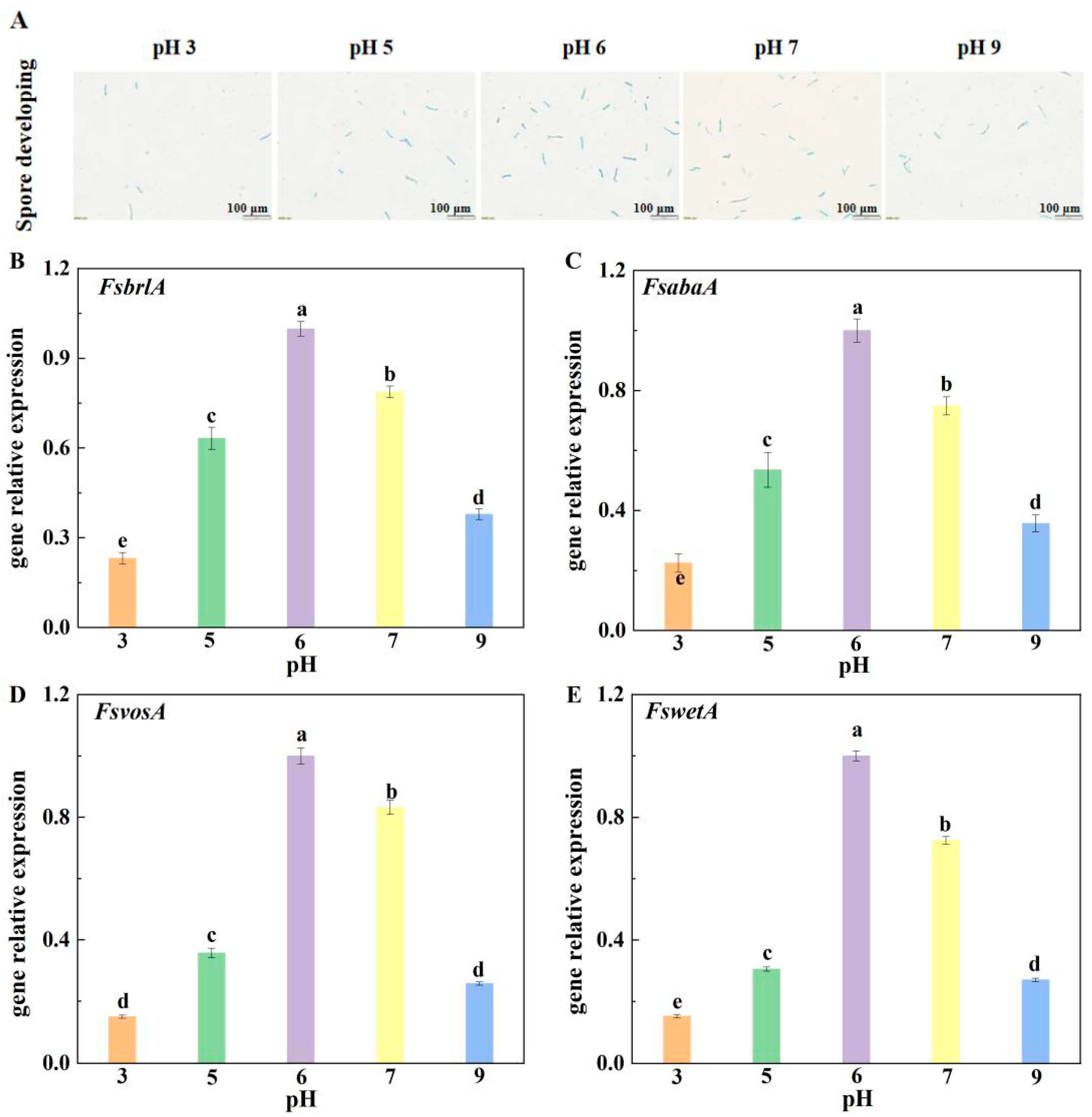
2.6. Effect of Ambient pH on Relative Gene Expression Related to Spore Germination, Sporulation, and DAS Biosynthesis
To assess relative gene expression related to spore germination and sporulation, after
F. sulphureum had been cultured for 3 days, mycelia were collected and the relative expression of
FsbrlA,
FsabaA,
FsvosA, and
FswetA genes associated with spore germination and sporulation was determined. For relative gene expression related to DAS biosynthesis, after the muskmelon had been inoculated for 7 days, fruit tissue containing mycelia at the junction of disease–health was taken for DAS biosynthesis-related gene expression determination. Primer sequences are shown in
Supporting Information Tables S1 and S2.
The determination of relative gene expression related to spore germination, sporulation, and DAS biosynthesis was based on our previous publication [
20]. After
F. sulphureum had been cultured on a PDA plate for 5 days, mycelia were collected and the expression of
FsbrlA,
FsabaA,
FsvosA and
FswetA genes associated with spore germination and sporulation analyzed according to the manufacturer’s instructions. Total RNA from
F. sulphureum mycelium was extracted using the TRNzol Universal Total RNA Extraction Kit (Tiangen Biotech, Beijing, China). RNA structural integrity was detected by 1% agarose gel electrophoresis, and the concentration and purity were checked using an Ultramicro UV-vis spectrophotometer (Kojima Tsuki Manufacturing Institute, Kyoto, Japan). First-strand cDNA was synthesized according to the instructions provided with a gDNA Eraser reverse-transcription kit (Takara, Kusatsu-shi, Japan, RR047A), which was used for the subsequent real-time quantitative PCR (RT-qPCR). The primer sequences for the RT-qPCR assay are listed in
Table S2. RT-qPCR was performed using SYBR
® Green Premix Pro Taq HS Premix (Rox plus, AG11718) according to the manufacturer’s instructions. The relative expression levels of genes were calculated by the 2
−ΔΔCt method with actin and β-tubulin genes as the reference [
21].
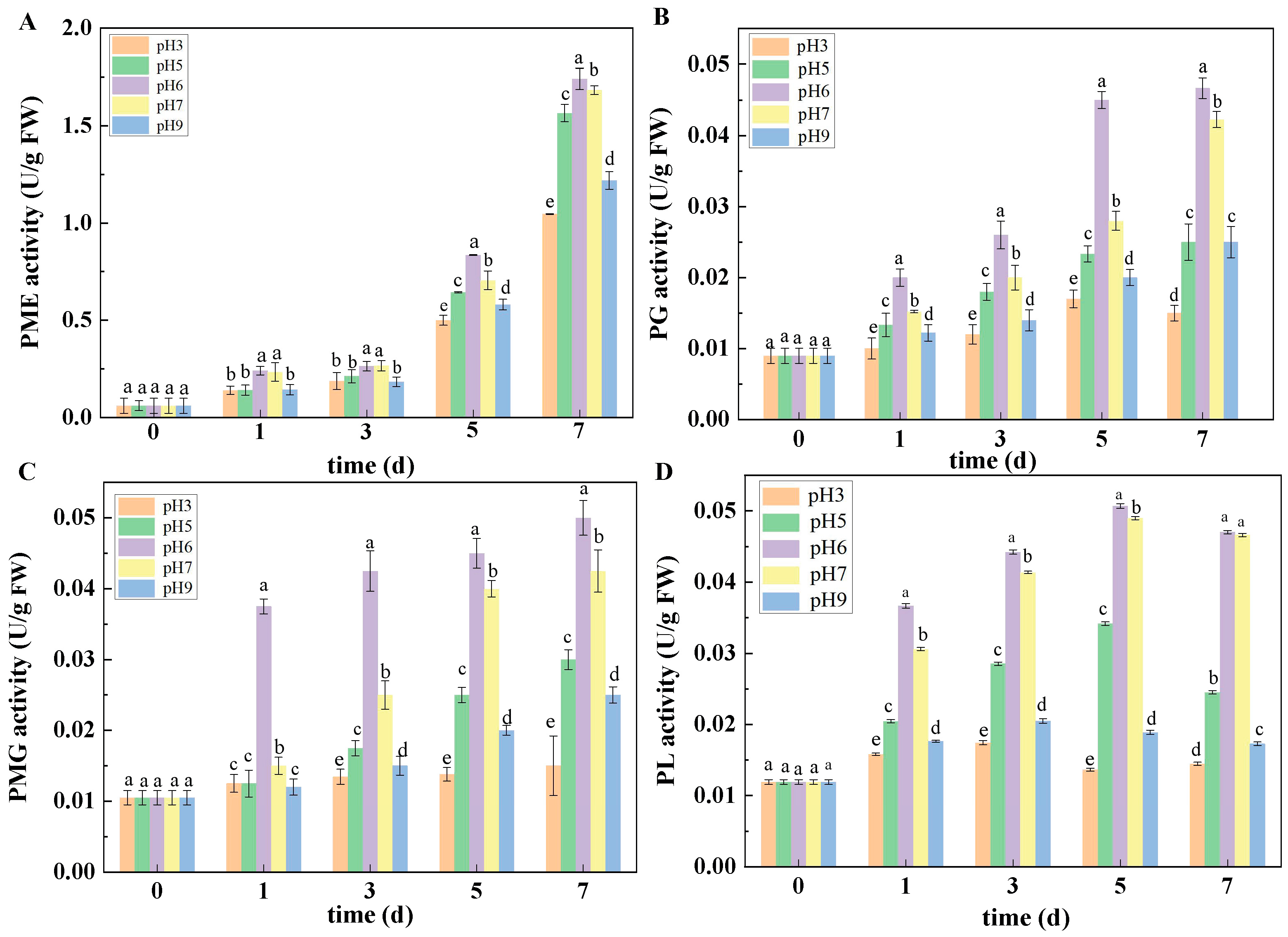
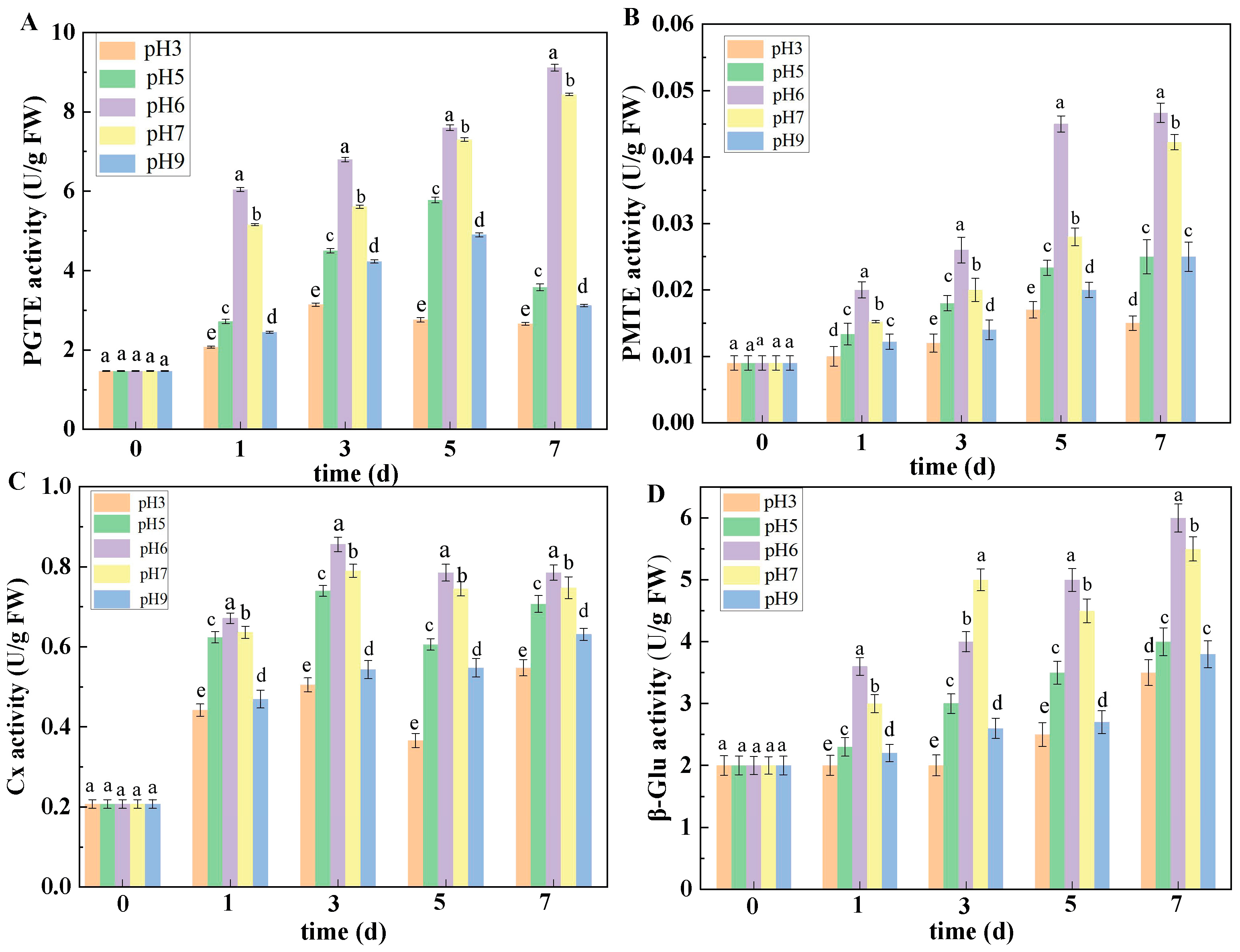
2.7. Effect of Ambient pH on the Activity of CWDEs in Inoculated Fruit
2.7.1. Extraction of Crude Enzyme Solution
Crude enzyme solutions of PMG, PG, Cx, β-Glu, PME, and PL were extracted according to the method of Wang et al. [
22]. The crude enzyme solutions of PGTE and PMTE were extracted according to the method of Reinehr et al. [
23].
The extraction of PMG, PG, Cx, and β-Glu was performed based on reference [
16]: 1.0 g frozen tissue was taken, ground under liquid nitrogen, then 2 mL 5% ethanol was added to the tissue, transferred to a 3 mL centrifuge tube, centrifuged at 4 °C, 10,000×
g for 10 min, the supernatant removed, 1 mL 80% ethanol added to the precipitation, centrifuged, 1.5 mL of extraction solution added after 20 min, and then the supernatant was centrifuged to collect the crude enzyme solution.
The extraction of PME, PL, PGTE, and PMTE was performed based on reference [
17] PME crude enzyme solution extraction: 1.0 g frozen muskmelon fruit tissue was quickly ground under liquid nitrogen, 5 mL 8.8% NaCl solution was added to the tissue to form a homogenate, then centrifuged at 4 °C, 10,000×
g for 10 min to obtain the supernatant. Then, the pH of the supernatant was adjusted to 7.5 with sodium hydroxide to obtain the crude PME crude enzyme solution.
PL crude enzyme solution extraction: 1.0 g frozen muskmelon fruit tissue was taken and rapidly ground under liquid nitrogen, then 3 mL of Tris-HCl buffer was added to the tissue, centrifuged at 4 °C, 10,000× g for 10 min, and the supernatant collected.
PGTE and PMTE crude enzyme solution extraction: 1.0 g of frozen muskmelon fruit tissue was taken, then 4 mL of extraction buffer (Tris-HCl) added to the tissue and centrifuged at 4 °C, 10,000× g, for 10 min. The supernatant was collected to obtain the crude enzyme solutions of PGTE and PMTE.
2.7.2. Enzyme Activity Assay
Determination of PME, PG, PMG, PL, Cx, and β-Glu activities were based on the method of Wang et al. [
22]. Determination of PME activity: 0.5 mL of the crude enzyme solution extracted was taken, 2 mL of pectin solution and 0.3 mL of bromothymol orchid added, the reaction of the system maintained for 2 min, then the absorption value of the mixture solution was determined.
Determination of PG activity: 0.5 mL of the crude enzyme solution extracted was taken, then 0.5 mL of 10 g/L polygalacturonic acid solution and 1.0 mL of acetate–sodium acetate buffer (pH 5.5) were added to the crude enzyme solution and the reaction maintained in a water bath at 37 °C for 1 h. After 1 h of reaction, 1.5 mL of 3,5-dinitrosalicylic acid solution was added to the reaction solution, the reaction maintained in a boiling water bath for 5 min, then the reaction solution was cooled immediately. Finally, 2 mL of distilled water was added to determine the absorption value of the mixture at 540 nm.
Determination of PMG activity: 0.5 mL of the extracted crude enzyme solution was taken, 0.5 mL of 10 g/L pectin solution and 1.0 mL of acetoacetate sodium buffer (pH 5.5) added, then the whole reaction system was kept in a water bath of 37 °C for 1 h. Then, 1.5 mL of 3,5-dinitrosalicylic acid solution was added to the reaction solution and immediately placed into a boiling water bath. The water bath was cooled after 5 min. Finally, 2 mL of distilled water was added to determine the absorbance value of the mixture at 540 nm.
Determination of PL activity: 2.0 mL 0.5% pectin solution was taken, heated in a water bath at 40 °C for 5 min, 0.5 mL crude pectin lyase added to the pectin solution, water bathed for 10 min again, then 0.5 mL of the mixture was taken and added to 4.5 mL 0.01 mol/L HCl solution. After the reaction system was completely mixed, the absorbance was measured at 235 nm.
Determination of Cx activity: 1.0 mL of the extracted crude enzyme solution was taken, 1.5 mL of 10 g/L sodium carboxymethyl cellulose (CMC) solution added, heated in a water bath at 37 °C for 1 h, 1.5 mL of 3,5-dinitrosalicylic acid quickly added to the crude enzyme solution, boiling water bath for 5 min, and 2 mL of distilled water was added after cooling. Finally, the absorbance was measured at 540 nm.
Determination of β-Glu activity: 0.5 mL of the extracted crude enzyme solution was taken, then 1.5 mL of 10 g/L salicylate solution was added and maintained for reaction in a water bath at 37 °C for 1 h. Subsequently 1.5 mL of 3,5-dinitrosalicylic acid solution was quickly added to the crude enzyme solution, the reaction maintained in a boiling water bath for 5 min, and 2 mL of distilled water was added after cooling. Finally, the absorbance was measured at 540 nm. The activity of PME, PG, PMG, PL, Cx, and β-Glu are expressed as U/g FW.
The determination of PGTE and PMTE activities was based on the method of Reinehr et al. [
23]: 1.0 mL of the extracted crude enzyme solution was taken, then 1.0 mL of 50 mmol/L Gly NaOH buffer (pH 9.0), 1.0 mL of substrate, and 1.0 mL of 3 mmol/L CaCl
2 solution were added to the crude enzyme solution. Once the reaction system was completely mixed, it was kept in a water bath at 30 °C for 5 min, and the absorbance before and after the reaction was measured at 232 nm. PGTE and PMTE activities are expressed as U/g FW.
2.8. Statistical Analysis
Experiments were carried out at least 3 times. The mean value and standard deviation of all data were calculated by Excel 2010, and the significance of differences was analyzed by SPSS 21.0 (ANOVA) (p < 0.05). Origin 9.0 was employed to make figures.
4. Discussion
In this study, it was found that the inoculation of spore suspensions with different pH values had a significant effect on disease expansion and DAS accumulation in muskmelon fruit. Among these, the lesion area in the muskmelon infected with spore suspension with pH 6 was the greatest, and the smallest lesion area of the inoculated fruit was observed at pH 3, indicating that the ambient pH 6 was more conducive to pathogenicity for
F. sulphureum. This result is similar to the report by Wang et al. [
24], who suggested that ambient pH 7 was beneficial to pathogenicity for
Trichothecium roseum to fruit. In addition, Shi et al. [
25] also observed that when pH was 6,
F. sporotrichioides grew faster in PDB medium, and too acidic or too alkaline an ambient pH was not conducive to growth or pathogenicity of
F. sporotrichioides. Furthermore, the highest DAS accumulation was also observed in the muskmelon infected with spore suspension with pH 6, and the lowest DAS content in the inoculated fruit was observed at pH 3. This result was consistent with the report by Jimdjio et al. [
9], who suggested that inoculation with spore suspension with pH 5 not only resulted in the most serious disease but also an accumulation of the highest level of patulin in fruit. The possible mechanism of action was attributed to two factors from in vitro and in vivo experiments.
An important environmental factor, ambient pH significantly affects the growth and development of pathogenic fungi in vitro [
26]. In this study, mycelial dry weight, spore germination rate, and germ tube length of
F. sulphureum at pH 7, 5, 9, and 3 were significantly inhibited compared with those at pH 6, and the growth rate of
F. sulphureum was the slowest at pH 3 and 9, which was similar to the results of Wang et al. [
24], who found that the colony diameter and spore germination rate of
T. roseum were significantly inhibited under a strong acid or alkali environment. The mycelial growth and conidial formation of pathogenic fungi under different pH were closely related to the morphology of pathogenic fungi. Our study showed that the mycelium morphology of the colony edge was significantly affected by ambient pH. The mycelium inoculated with a spore suspension at pH 6 had clear structure and smooth and full surfaces. The mycelium inoculated with pH 3 was thickened compared with that inoculated with pH 6. The mycelium inoculated with pH 5 became obviously thinner. pH 9 inoculation significantly disrupted the marginal structure of the mycelium, and the mycelium became dense and even intertwined with other branches. This phenomenon was consistent with the observation by Jimdjio et al. [
9], who also found that mycelium morphology of colony edges was more obvious and branches were longer and sparser at pH 5.0 and 7.0, while the edges were shorter and less dense at pH 2.5 and 8.5. Similarly, Li et al. [
26] found that ambient pH affected the intracellular pH and ATP levels of
P. expansum spores, and the germination of
P. expansum spores was significantly inhibited at pH 2.0 and 8.0. The reason could be that strong acids or bases destroy chromosomes and proteins’ DNA, thus inhibiting conidium growth and changing colony morphology.
In this study, compared with other treatment groups, the relative expression levels of FsbrlA, FsabaA, FswetA, and FsvosA in the mycelium inoculated with pH 6 spore suspension were up-regulated and displayed the highest expression, which made F. sulphureum produce the most spores and have the strongest growth and development ability at pH 6, followed by those infected with spore suspensions at pH 7, 5, and 9. Expression levels at pH 3 were the lowest, and accordingly, F. sulphureum had the lowest sporulation and the weakest growth and development ability at pH 3. Therefore, we speculate that ambient pH regulates the sporulation of pathogens by affecting the expression of sporulation-related genes, thereby reducing the susceptibility to pathogenicity of muskmelon fruit.
The secretion and expression of pathogenic factors was also influenced by ambient pH to a certain extent. As we know, when pathogens infect host plants, fungi will secrete plenty of CWDEs to degrade cell walls. CWDEs can degrade pectin substances of host plant cell walls, thus breaking plant defenses and promoting infections with pathogenic fungi [
27]. In the present study, the results showed that the activities of PMG, PL, PME, PG, Cx, β-Glu, PGTE, and PMTE in muskmelon inoculated with the pH 6 spore suspension were higher than those in muskmelon infected with the other pH-value spore suspensions. The above results indicated that ambient pH can regulate the secretion of CWDEs of pathogenic fungi, which is similar to the results of Juntachai et al. [
28], who indicated that ambient pH plays a promoting role in the secretion of
Malassezia furfur lipase. It has been reported that the CWDEs produced by
Colletotrichum gloesporioides and
C. coccodes during fruit infection were related to their pathogenicity [
17,
29,
30,
31]. The CWDEs secreted by
P. expansum,
P. digitatum, and
P. italicum were more conducive to the infection of apple and citrus fruit with these pathogens [
32]. Other studies have also found that
PG genes play an important role in the infection of different fruit by
Botrytis cinerea and
Alternaria citri [
33,
34]. In brief, ambient pH can regulate the secretion of CWDEs of pathogenic fungi during the infection process, which play an important role in the pathogenesis of fungi.
The occurrence of fusarium rot caused by
F. sulphureum is accompanied by DAS accumulation. DAS is one kind of trichothecene, the biosynthesis of which begins with farnesyl pyrophosphate (FPP). Under the action of the key genes
tri4 and
tri5 and main regulatory genes
tri6,
tri10, and
tri101, FPP is synthesized through a series of complex oxygenation, isomerization, cyclization, and esterification reactions under a series of enzymatic reactions. The results of this study showed that compared with the ambient pH 6 treatment, expression of
tri genes in the DAS biosynthesis pathway was down-regulated under other pH conditions, thereby reducing the accumulation of DAS in fruit inoculated with
F. sulphureum. This result was similar to Jimdjio’s report [
9], whose results showed that the expression of patulin biosynthesis-related genes could be down-regulated by ambient pH. Accordingly, the biosynthesis pathway of patulin was inhibited, thereby reducing the patulin accumulation in fruit infected with
P. expansum. In brief, the above results show that ambient pH affected the biosynthesis of DAS by down-regulating the expression of genes related to the biosynthesis pathway of DAS, thereby reducing DAS accumulation in inoculated fruit.