Abstract
Grey mould disease, caused by various Botrytis species, poses a significant threat to important plants worldwide. This study aimed to characterize Botrytis populations on strawberry and roses, economically relevant host plants, and raspberry, used as a representative of wild plants, in Iran. A total of 389 isolates were collected and analyzed based on morphological features and haplotyping using molecular markers, transposable elements (Boty and Flipper), and fungicide response. Moreover, 60 isolates were used for phylogenetic analysis based on the rpb2 gene, and 16 selected isolates from each clade were further characterized using the g3pdh, hsp60, and nep2 genes. The results revealed the presence of three distinct species, Botrytis cinerea, Botrytis sinoviticola, and Botrytis prunorum, among the sampled isolates. Additionally, this study reports for the first time the presence of B. sinoviticola on strawberry and isolates belonging to B. cinerea group S in Iran. These findings provide insights into the diversity and composition of Botrytis populations on Iranian host plants.
1. Introduction
In recent decades, Iran has emerged as a significant global producer of various crops susceptible to Botrytis species, as reported by FAOSTAT [1]. The cultivation of high-value crops, such as strawberry (Fragaria ananassa Duchesne) and roses (several species of the genus Rosa L.), has increased, particularly in greenhouse environments, which often provide favourable conditions for Botrytis growth. Botrytis species can cause various diseases, such as blossom blight, leaf blight, and onion neck rot, but the most relevant is grey mould [2]. Grey mould disease, induced by Botrytis cinerea, results in heavy yield losses, causing severe economic losses all over the world, especially during periods of high humidity before harvest [3]. The ability of Botrytis species to cause latent infections in host tissues also poses significant challenges during transport and storage, making them formidable postharvest pathogens.
The genus Botrytis belongs to the Leotiomycetes class, Helotiales order, and Sclerotiniaceae family within the Ascomycota phylum [4], comprising approximately 38 species, either polyphagous or specialized, that collectively infect over 1400 plant species [5,6]. Notably, Botrytis, as a saprophyte, can continue to grow and survive on host plants even after their decay, posing a considerable challenge in disease management [7].
Traditionally, species identification relied on morphological and cultural characteristics [8]. However, morphological similarities hampered identification, and difficulties were overcome by the development of molecular methods based on DNA sequencing [9]. Molecular techniques provide valuable insights into pathogen detection, species identification, and genetic variability within Botrytis species and are helpful in early diagnosis, early-stage treatments, and the adoption of suitable antifungal control measures [10].
B. cinerea, the most prominent species within the genus, is in second place in the list of the top 10 fungal plant pathogens in the world based on their scientific and economic importance, preceded only by Magnaporthe oryzae [11].
B. cinerea shows considerable genetic variation, which has been well documented in the literature from the early studies of the fungus [9], and which makes it a high-risk pathogen for the development of fungicide resistance (FRAC; http://www.frac.info (accessed on 20 July 2024)). Two transposable elements (TEs), the retrotransposon Boty and the DNA transposon Flipper, have been identified in B. cinerea and used to define two sibling sympatric species or biotypes, named transposa and vacuma, based on the presence or absence of the two TEs [12]. Significant differences in the frequency and distribution of the two transposons among Botrytis isolates collected from different host plants and/or geographic regions have been reported [13,14]. Moreover, a polymorphic exon/intron structure in the mitochondrial cytb gene was reported in B. cinerea, with isolates possessing or lacking a group I intron interrupting the coding sequence of the gene [15].
Several molecular markers have been used for genetic identification and phylogenetic relationships within Botrytis species. Phylogenetic analysis has divided the genus Botrytis into two clades based on the Bc-hch gene sequence, with B. cinerea falling under the clade of species (Botrytis clade 1) that infects a wide host range of dicotyledonous plants and is clearly distinguished from clade 2, which contains Botrytis species with a restricted host range in both monocotyledonous and dicotyledonous plants [16]. Three housekeeping genes, glyceraldehyde-3-phosphate dehydrogenase (g3pdh), heat shock protein 60 (hsp60), and subunit II RNA polymerase (rpb2), and the necrosis- and ethylene-inducing protein (nep1 and nep2) genes, have been used to corroborate the identification of Botrytis species [17,18,19,20]. Additionally, the sequencing of the multidrug resistance regulator 1 (mrr1) gene and several other genes distinguished a subgroup of isolates within grey mould populations, named B. cinerea group S, that was found to be predominant on strawberry in Germany [21]. These findings further highlight the need for accurate species identification and understanding intraspecific variability.
Numerous studies have examined genetic polymorphisms in Botrytis isolates collected from diverse host plants and growing systems worldwide [4].
The commercial significance of strawberry and roses in Iran and the potential transfer of isolates between different host plants prompted us to conduct the morphological and molecular characterization of Botrytis isolates from these crops and wild Rosaceae raspberry plants, in different provinces, to shed light on the diversity and distribution of Botrytis populations in Iran.
2. Materials and Methods
2.1. Fungal Isolates
The collection of Botrytis isolates sampled from various host plants exhibiting typical grey mould symptoms in open fields or in greenhouses is detailed in Table 1. Isolates were obtained by extracting small fragments of developed mycelia and/or conidia, or from surface-sterilized petals, leaves, or fruits without visible fungal material, which were then cultured on PDA medium (infusion from 200 g peeled and sliced potatoes kept for 1 h at 60 °C, 20 g dextrose, adjusted to pH 6.5, 20 g LLG-European bacteriological agar, per litre of distilled water). The cultures were maintained at 21 ± 1 °C in the dark for 7 days. Subsequently, fungal isolates were purified using the single-spore method and identified based on their morphological macroscopic and microscopic traits (e.g., colony morphology, conidia shape and size, sclerotia shape, and size and distribution pattern on Petri plates). Regarding storage, the isolates were maintained on paper slants at −20 °C for short-term storage or in 10% glycerol at −80 °C for long-term storage.

Table 1.
Origin of the Botrytis isolates used in this study.
2.2. Genetic Analysis and Fungicide Resistance
The single-spore isolates were cultured on malt extract agar (MEA; 20 g malt extract Oxoid, 20 g agar, per litre) at 21 ± 1 °C in darkness for 2–3 days. Subsequently, five small (2–4 mm) mycelial agar plugs from each isolate were singularly transferred onto 90 mm MEA plates overlaid with sterile cellophane films. The cultures were then incubated at 21 ± 1 °C for an additional two days to obtain fresh mycelium. Genomic DNA extraction was carried out from freeze-dried mycelium using the CTAB method as described by De Miccolis Angelini et al. [22]. The quality and concentration of the extracted DNA were determined using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA), and it was stored at −20 °C until use.
All isolates underwent molecular analysis: (i) TE profiles were determined using PCR primers specific to Boty and Flipper [23]; (ii) cytb gene structure was assessed according to De Miccolis Angelini et al. [15] and Habib et al. [24]; (iii) strains belonging to group I or group II of the Botrytis genus were distinguished by Bc-hch RFLP analysis [25]; (iv) B. cinerea group S strains were identified by the mrr1 PCR assay [21]. In detail, the PCR mixtures (25 μL) consisted of 1× Green GoTaq Flexi Buffer (Mg2+ free), 2 mM MgCl2, 75 μM of each dNTP nucleotide, 0.5 μM of each primer, 0.75 U of GoTaq DNA polymerase (all PCR reagents were from Promega Corp., Madison, WI, USA), and 50 ng of genomic DNA. The primer pairs used are listed in Table 2. A control with no template was always run. HhaI restriction enzyme (New England Biolabs Ltd., Hitchin, UK) was employed for Bc-hch RFLP analysis in a 10 µL mixture consisting of 1× CutSmart™ Buffer, 2 U of enzyme, and 4 µL of PCR product, and the digestion reaction was carried out at 37 °C for 30 min. The results were visualized after 110 min of electrophoresis run on a 1.5% agarose gel at 110 V using SYBR Safe DNA gel stain (Thermo Fisher Scientific, Waltham, MA, USA).

Table 2.
PCR primer pairs used in this study.
Sensitivity tests were conducted for six selected fungicides, namely, the SDHIs boscalid and isofetamid, the class III sterol biosynthesis inhibitor (SBI-III) fenhexamid, the phenylpyrrole fludioxonil, the anilinopyrimidine pyrimethanil, and the Quinone outside Inhibitor (QoI) trifloxystrobin, due to their frequent usage and/or having a common mode of action with the fungicides most frequently used against B. cinerea in recent years in Iran. Isolates were individually tested for their response to each fungicide by colony growth tests. In brief, mycelial plugs (2–4 mm) from the margins of actively growing colonies were placed upside-down on fungicide-amended or unamended media. The composition of the media and the discriminating doses of the fungicides were as previously described by De Miccolis Angelini et al. [26]. After incubation at 21 ± 1 °C in darkness for 2–5 days, isolates showing colony growth were considered to be resistant, while isolates whose growth was inhibited were considered to be sensitive to the tested fungicide. Reference B. cinerea strains already known for their sensitivity or resistance to each fungicide were used as controls in each test.
The haplotyping categorization integrated data from both genetic analysis and fungicide response. One isolate from each haplotype was then selected for further genetic analysis.
2.3. Phylogenetic Analysis
Phylogenetic analysis was conducted using the rpb2, g3pdh, hsp60, and nep2 gene sequences. The PCR was performed in a 20 μL reaction mixture consisting of 1× Phusion™ HF Buffer, 0.2 mM dNTPs, 0.5 μM of each primer, 0.4 U of Phusion™ high-fidelity DNA polymerase (all PCR reagents were from Thermo Fisher Scientific), and 25 ng of template DNA. The reaction was carried out in a MyCyclerTM thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA) programmed for initial denaturation at 98 °C for 30 s, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 51–64 °C for 10 s, and extension and final extension at 72 °C for 0.5 and 8 min, respectively. The primer sequences and annealing temperatures related to all the markers used in the analysis are shown in Table 2. It should be noticed that the NEP2forE/NEP2revE primer pair was used in addition to the more commonly used NEP2(−200)for/NEP2(+1147)rev primer pair to obtain high-quality nep2 sequences from B. prunorum isolates, according to Staats et al. [17].
PCR products were directly sequenced in both forward and reverse directions, using the same primers as for PCR, from an external service (Genewiz from Azenta Life Sciences, Leipzig, Germany). DNA sequence analysis was carried out using the Lasergene software package (v. 15.0.1; DNASTAR Inc., Madison, WI, USA). In detail, the nucleotide sequences for each gene were aligned using ClustalW and default settings in MegAlign Pro software (Lasergene v. 15.0.1; DNASTAR Inc.). After filtering out the poorly aligned regions, alignments for rpb2, g3pdh, and hsp60 were concatenated and used to reconstruct the maximum likelihood (ML) tree with 1000 bootstrap replicates with ASTRAL-II, according to Eyvazi et al. [27]. The ML tree for rpb2 and nep2 was inferred using MEGA7 [28] with similar parameters. Sclerotinia sclerotiorum strain 484 was used as the outgroup. The code of each sequence used for building phylogenetic trees is listed in Supplementary Table S1.
2.4. Pathogenicity Assay
The pathogenicity of the B. sinoviticola strain, three strains of B. prunorum, and three strains of B. cinerea (Supplementary Table S2) was evaluated by artificial inoculation on healthy organic strawberry fruits (cv ‘Candonga’) and cucumber cotyledons (cv ‘Mezzo Lungo di Polignano’), which were previously decontaminated by immersion in 2% sodium hypochlorite for 1 min, washed twice with sterilized distilled water, and dried at room temperature. Strawberry fruits were inoculated with conidial suspensions prepared using sterile distilled water containing 0.01% Tween 20 from 7-day-old cultures grown on PDA. Conidial suspensions were then filtered through a layer of Miracloth (Calbiochem, San Diego, CA, USA) to remove mycelium fragments and adjusted to 1 × 105 conidia mL−1 using a haemocytometer. Each fruit was punctured with a sterile needle after being inoculated with 20 µL of conidial suspension. Cucumber cotyledons were wounded and inoculated with a mycelium plug (2 to 4 mm in diameter) excised from the margin of actively growing cultures on MEA. Fruit inoculated with sterile distilled water and cotyledons inoculated with plugs of sterile MEA were used as a control. After inoculation, fruit and cotyledons were incubated in a moist chamber at 21 ± 1 °C in darkness. Starting from 2 days after inoculation, rotting around the inoculation point was recorded according to an empirical scale with seven classes of severity (0 = absence of infections, 1 = a lesion < 1 mm of rotted area (r.a.); 2 = 1–3 mm of r.a.; 3 = 4–5 mm r.a.; 4 = 6–7 mm r.a.; 5 = up to 50% r.a.; 6 = 51–75% r.a.; 7 = 76–100% r.a.). Data from five replicated fruits/cotyledons were used to calculate the mean disease severity. The assay was repeated twice. All data were analyzed by analysis of variance followed by Tukey’s honestly significant different test using CoStat software version 6.451 (CoHort Software, Monterey, CA, USA) at the significance level p = 0.05.
3. Results
3.1. Fungal Isolates
The typical symptoms associated with grey mould included spreading lesions on fruits or petal decay, with or without visible conidia, and/or mycelium on the surface. Some examples of symptomatic tissues are reported in Figure 1. In this study, both symptomatic and asymptomatic materials were utilized, showing the presence of latent infections of B. cinerea in the analyzed samples. Following an incubation period on PDA medium of 4–7 days at 21 °C in darkness, fungal mycelium and sporulation appeared and were utilized for single-spore purification to obtain a collection of Botrytis isolates. A total of 389 Botrytis isolates, first identified on the grounds of morphological characteristics, were obtained. The colony morphology of isolates was classified into three main categories (mycelial, conidial, and sclerotial) and eleven different morphotypes, as detailed in Supplementary Table S3. The isolates encompassed all categories, with the conidial one being dominant, but morphology could vary among subcultures from the same isolate. However, based on morphological features, all isolates appeared to be consistent with B. cinerea.

Figure 1.
Grey mould symptoms on strawberry fruits (A–F) and rose petals (G–J).
3.2. Grouping of Isolates Based on Genetic Analyses and Fungicide Resistance
DNA amplification for the detection of the TEs Boty and Flipper revealed, in the analyzed isolates, all four transposon combinations, namely, transposa (Boty+Flipper+; 65%), Boty (Boty+Flipper−; 25%), Flipper (Boty−Flipper+; 7%), and vacuma (Boty−Flipper−; 2%). Further analysis involving the restriction of Bc-hch amplicons indicated that one isolate belonged to group I and the remaining ones belonged to group II (Figure 2). Both intron-possessing (T1; 42%) and intron-lacking (T2; 58%) variants of the cytb gene were detected. Additionally, 24 isolates (6.1%) from different host plants belonged to B. cinerea group S.
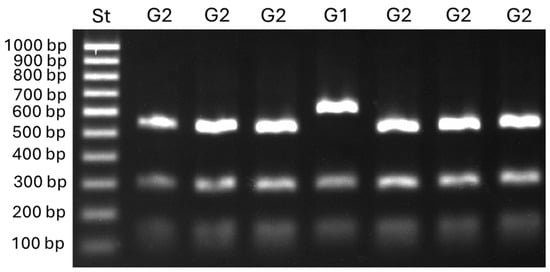
Figure 2.
Restriction profiles of Botrytis group 1 (G1) and group 2 (G2) isolates obtained with the Bc-hch PCR-RPLP assay according to Fournier et al. [26] (PCR amplification with the primer pair 262/520L followed by digestion with the restriction enzyme HhaI).
The fungicide response of 345 out of the 389 isolates was categorized as sensitive (S) or resistant (R) to each fungicide (Supplementary Table S3). In addition, isolates showing low (LR) to high (HR) levels of resistance were distinguished for fenhexamid and fludioxonil. As a result, based on their response profiles for the assayed fungicides, the isolates were classified into a total of 44 different groups, with most of the isolates resistant to at least one class of fungicide (95%).
Overall, 60 haplotypes were identified among the analyzed Botrytis isolates based on data from molecular data and fungicide responses (Table 3).

Table 3.
Description of the haplotypes detected among the collected isolates.
3.3. Phylogenetic Analysis
Preliminary, the rpb2 gene sequences of 60 selected isolates representative of different haplotypes were analyzed. The two B. cinerea group S isolates, P12-28 and P16-24, and the isolate P15-2 did not produce sequences of good quality with rpb2 primers and were the excluded from subsequent analysis. Afterward, 16 isolates were selected based on macroscopic and microscopic morphology and the rpb2 gene sequences and subjected to molecular species identification based on the concatenated sequences of the rpb2, g3pdh, hsp60, and nep2 genes. Molecular analysis, with few exceptions, confirmed the results of the morphological observations. The initial analysis based on the rpb2 gene (Supplementary Figure S1A) grouped 38 of the 57 examined isolates in the previously described Botrytis clade 1 [16,17,20], including B. cinerea and the closely related species Botrytis fabae, Botrytis pseudocinerea, B. sinoviticola, Botrytis californica, Botryotinia calthae (syn. Botrytis calthae), and Botrytis medusae used in our analysis as references. Among them, the P14-2 strain was closely related to B. pseudocinerea and B. sinoviticola, while all the other isolates grouped close to B. cinerea. The remaining 19 isolates clustered together in a separated clade with the reference sequences of B. prunorum. Sequence analysis of nep2 (Supplementary Figure S1B) corroborated these results. It should be mentioned that the second group of nep2 primers (Table 2) produced more qualified amplicons and was able to place B. prunorum isolates into separated subgroups. The phylogenetic analysis using the combined rpb2, g3pdh, and hsp60 sequences on the selected 16 isolates (Figure 3) consistently placed the strain P14-2 with B. sinoviticola in one subclade, forming a single lineage within Botrytis clade 1, and confirmed the molecular identification of 5 isolates as B. prunorum and 10 isolates as B. cinerea (Table 4). The nep2 data were not included in the construction of the combined phylogenetic tree due to the lack of information related to all the reference sequences used for this gene in the NCBI database. On the other hand, our results confirmed the insufficiency of single marker genes in species identification.
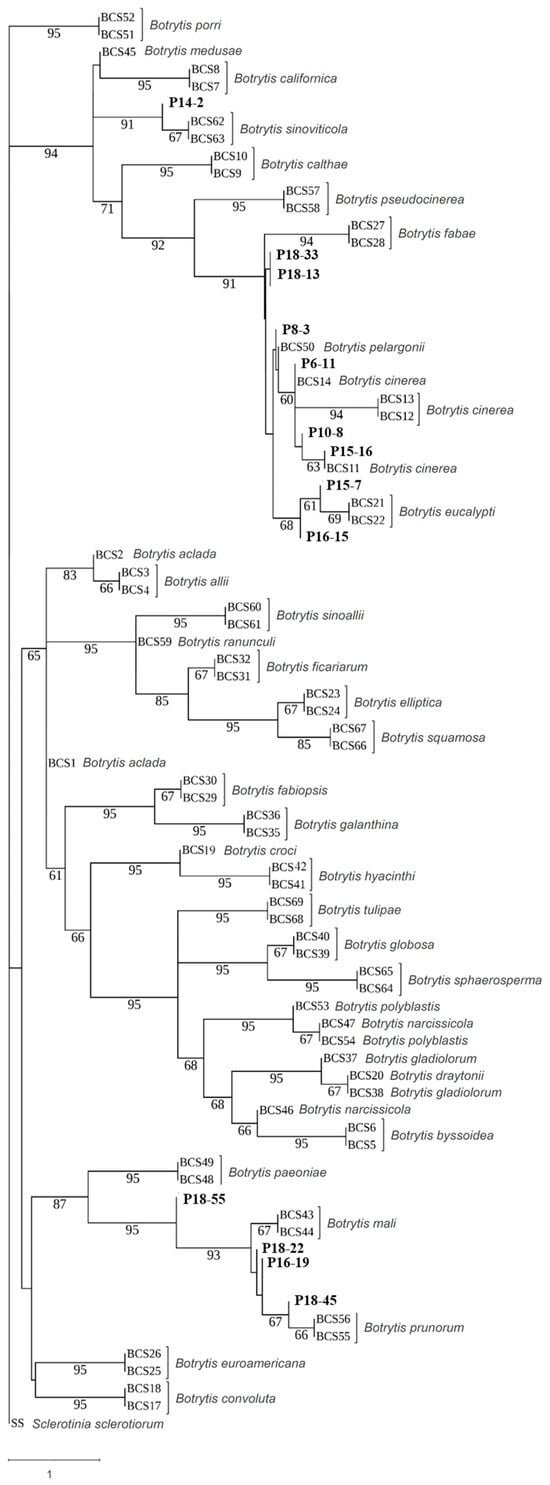
Figure 3.
Multi-gene phylogenetic tree based on concatenated rpb2, g3pdh, and hsp60 gene sequences prepared by using Asteral software. Iranian strains from the current study are in bold. The tree is drawn to scale, with branch lengths measured by the number of substitutions per site. Bootstrap values > 60 based on 1000 replicates are shown.

Table 4.
Accession numbers of gene sequences of Botrytis strains obtained in the present study.
3.4. Morphological Analysis and Fungicide Resistance and Pathogenicity
For all identified species within the analyzed Botrytis populations, conidia were ovate, ellipsoidal, pyriform, globose, flat in one part, usually unicellular but occasionally septate, and with or without hilum. Their sizes exhibited broad variation, in the range of 3–19 × 3–9 μm (n = 50). Macroscopic and microscopic morphological features were not useful in distinguishing the Botrytis species investigated (Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8). The B. sinoviticola strain from this study yielded colonies without sclerotia with a creamy to grey colour on MEA and whitish to grey on PDA (Figure 4). The colony morphology of B. cinerea and B. prunorum strains was somehow affected by the medium (PDA and MEA). For example, B. cinerea strain 6 (P3-3) and B. prunorum strain 2 (P10-3) produced sclerotia on MEA but not on PDA, while B. cinerea strain 5 (P2-2) and B. prunorum strain 4 (P16-17) produced sclerotia just on PDA. Strain 3 from both species (P6-9 and P11-10) produced more sclerotia on PDA, while strain 1 of B. cinerea (P13-5) and strain 8 of B. prunorum (P18-22) had more conidia on PDA.
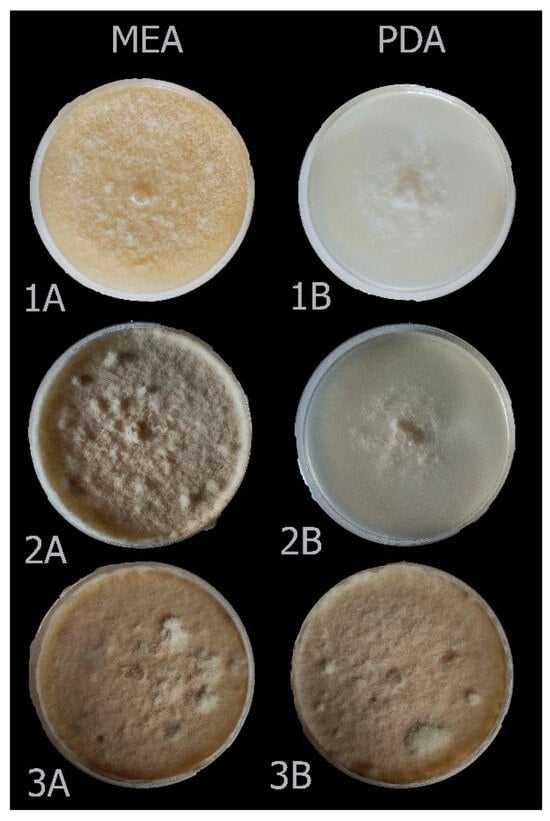
Figure 4.
Colony morphology of Botrytis sinoviticola strain P14-2 on MEA (A) and PDA (B) after 7 (1), 14 (2), and 21 (3) days.
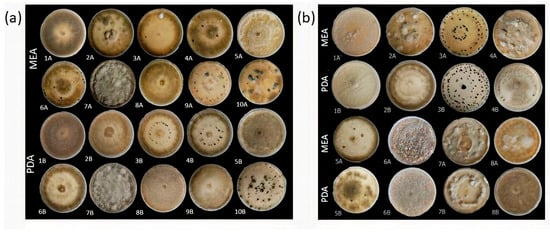
Figure 5.
Various colony morphologies of Botrytis cinerea (a) and B. prunorum (b) on MEA and PDA after 21 days of incubation. Among B. cinerea strains, 1—P13-5; 2—P11-4; 3—P6-9; 4—P8-11; 5—P2-2; 6—P3-3; 7—P7-4; 8—P18-29; 9—P18-6; and 10—P7-3; among B. prunorum strains, 1—P11-14; 2—P10-3; 3—P11-10; 4—P16-17; 5—P16-19; 6—P8-9; 7—P18-38; and 8—P18-22.
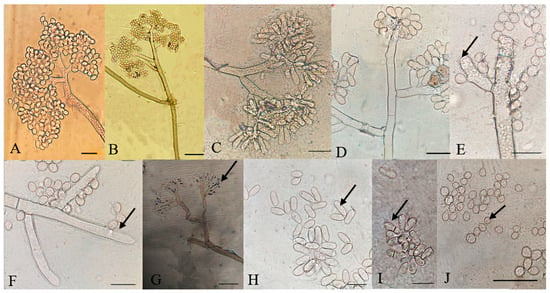
Figure 6.
Microscopic characteristics of Botrytis cinerea isolates. (A–D) Conidia attached to conidiophore, (E–G) conidiophore terminal, (H) elongated conidia, (I) septate conidia, (J) microconidia. Bar = 20 μm. The strains shown are (A) P13-5; (B) P8-11; (C) P7-4; (D) P18-6; (E) and (F) P7-3; (G) P3-3; (H) P7-4; (I) P2-2; and (J) P7-3.
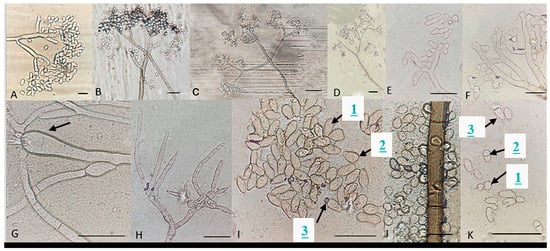
Figure 7.
Microscopic characteristics of Botrytis prunorum. (A–D) Conidia attached to conidiophore and a normal (E), swollen (F), and thin (H) conidiophore terminal. (G) Conidiophore swelling, elongated (1I), and pyriform conidia (2I), conidium with hilum (3I), (J) common conidia, and round (1K), small (2K), and large (3K) septate conidia. Bar = 20 μm. The strains shown are (A) P11-14; (B) P16-17; (C) P18-38; (D) P8-9; (E) P10-3; (F) P11-10; (G) P18-22; (H) P16-19; (I) P10-3; (J) P8-9; and (K) P10-3.
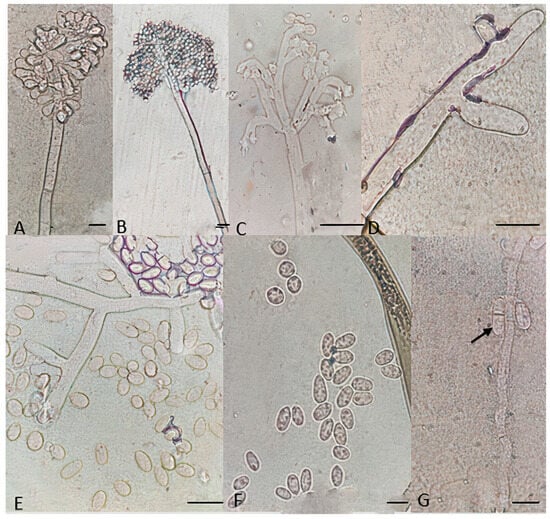
Figure 8.
Microscopic characteristics of Botrytis sinoviticola strain P14-2. (A,B) Conidia attached to conidiophore, (C,D) conidiophore terminal, (E,F) unicellular conidia, and (G) septate conidia. Bar = 20 μm.
Particular microscopic features, like swelling at the junction of conidiophore in some B. prunorum strains (Figure 7G), and a little curviness at the end of conidiophore in B. sinoviticola strains (Figure 8C), were observed. It should be noticed that the features mentioned were not specific to a single species, since they could sometimes also be observed in strains representative of other species.
With regard to fungicide response, the B. sinoviticola strain showed double resistance to fludioxonil and SDHIs and sensitivity to fenhexamid, anilinopyrimidines, and QoI fungicides (haplotype H17). The occurrence of sensitivity (S) or single (R1), double (R2), or multiple (R3–R5) resistance to the fungicides tested among B. cinerea and B. prunorum strains is summarized in Figure 9, showing similar proportions in the two species.
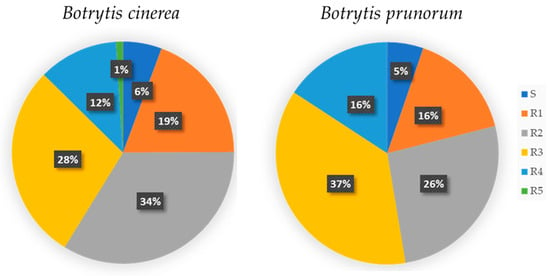
Figure 9.
Distribution pattern of fungicide resistance profiles among Botrytis cinerea and Botrytis prunorum strains, grouped in the following categories: sensitive to all used fungicides (S), and resistant to one (R1), two (R2), three (R3), four (R4), and five (R5) classes of fungicides.
A pathogenicity test was carried out for Iranian B. sinoviticola, firstly identified in this study, compared with strains of B. cinerea and B. prunorum on strawberry fruits and cucumber cotyledons. In two replicated experiments, B. sinoviticola caused lesions starting from 3 DAI on strawberry and 4 DAI on cucumber and reaching the maximum disease severity after 6 DAI and 7 DAI on the two hosts, respectively. No significant difference was observed among strains of the three Botrytis species (Supplementary Table S2).
4. Discussion
Botrytis species include plant pathogens that affect a large number of crops [4]. This study focused on the Botrytis species associated with two important economic crops, strawberry and rose, and a wild plant, raspberry, in Iran. Since different Botrytis species can convive on a single host plant showing grey mould symptoms [2,29], we aimed to investigate the composition of Botrytis populations to improve integrated disease management. The diversity observed in colony growth and colour, the number and pattern of sclerotia formations, the sequences of the genes Bc-hch and mrr1, the mitochondrial cytb gene, and the presence of the TEs Boty and Flipper [30], along with the response to different fungicides, was initially used for grouping the sampled Botrytis isolates. This led to the identification of 60 different haplotypes in fungal populations and to the selection of one isolate per haplotype for submission for phylogenetic analysis.
Species identification and phylogenetic analysis was achieved with multilocus analysis by using the gene sequences of g3pdh, hsp60, rpb2, and nep2 [16,17]. Both morphological and molecular studies showed the prevalent presence of B. cinerea, including isolates of group S. Isolates of B. prunorum and B. sinoviticola were also detected. It should be mentioned that no species-specific or haplotype-specific macroscopic or microscopic features could be recorded, and some features, like occasional septate conidia, were detected in different Botrytis species, in agreement with Mirzaei et al. [31]. These results confirm the broad intraspecific variation that is well known in B. cinerea and corroborate previous observations showing broad morphological variation in other Botrytis species [9]. Differences in colony morphologies in B. cinerea and B. prunorum grown on various culture media were like those reported in other studies [32]. We also observed that the morphology of monoconidial isolates was not always stable following repeated subculture and seriously questioned the reliability of morphological descriptions of cultured isolates in species identification [9]. It should be noticed that our morphological observations were always conducted on fungal colonies grown under dark conditions and that light exposure could affect mycelial growth, conidiation, sclerotial development, and trophic responses in Botrytis [33].
Consistent with previous findings on different hosts, B. cinerea sensu stricto was identified as the main pathogen and the one most frequently associated with grey mould on various plants [34,35], although, in some instances it may be partially replaced by B. pseudocinerea during the growing season [2]. However, B. pseudocinerea was not found among the sampled isolates in this study.
As expected, B. cinerea isolates were all recognized as belonging to Botrytis group II based on the Bc-hch RFLP test, showed considerable morphological and genetic variation, with various profiles of response to fungicides and contents in TEs, including transposa (Boty+Flipper+), Boty+Flipper−, Boty−Flipper+, and vacuma (Boty−Flipper−) isolates, as well as both structural variants of the cytb gene: possessing (T1) or not possessing (T2) the intron. Isolates belonging to Botrytis group S were identified based on the mrr1 PCR test, and this is the first report of this group in Iran. Botrytis group S was initially identified on strawberry in Germany [21], exhibiting host specificity at that time. However, subsequent findings considerably expanded its host range to various other plants across different countries [30,34,36,37], highlighting a lack of host specificity.
Several studies define B. cinerea as a species complex in which B. cinerea sensu strictu exists within a species complex, including B. pseudocinerea and new cryptic species that can live in sympatry in the same host. Although the Botrytis species in the complex show morphological similarity, they may differ with respect to ecology, host preferences, aggressiveness, and sensitivity to fungicides [12,13,19,20,25,38].
B. prunorum, one of the cryptic species in the B. cinerea species complex, shares phylogenetic similarities with B. cinerea [8]. The species has been reported in various countries and on various taxonomically unrelated plant species. It was first detected and characterized from Japanese plum [32], table grape [39], and kiwifruit in Chile [40], then it was also detected from dry pea, lentil, and chickpea in Montana, USA [41], strawberry in Norway [36], greenhouse-grown tomato in Turkey [42], and vineyards in Spain [41]. In this study, it was found in multiple provinces in Iran on strawberry (in all provinces, except Gilan), raspberry (in Mazandaran province), and roses (in Alborz and Gilan provinces). At first, it was distinguished from B. cinerea because it yielded a white-to-yellow colony on PDA, shorter and more compressed conidiophores at the base, smaller conidia and sclerotia, and less sporulation [32,39]. However, our study, in agreement with Nabizadeh et al. [2] (2022), revealed many overlapping morphological features among B. cinerea and B. prunorum. Molecular analysis using rpb2, nep2, and the combined rpb2, g3pdh, and hsp60 gene sequences clustered Iranian isolates with the reference sequences of B. prunorum. The B. prunorum isolates characterized in this study all belonged to Botrytis group II and included different haplotypes showing various TEs and fungicide resistance profiles and both intron variants of the cytb gene.
Isolates belonging to Botrytis group I based on the Bc-hch gene were identified as B. sinoviticola based on molecular and morphological analyses and according to the holotype firstly described by Zhou et al. [19]. Isolates were characterized by the presence of the only Flipper transposon (Boty−Flipper+) in their genomic DNA and the presence of the intron in the mitochondrial cytb gene and showed resistance to fludioxonil and SDHIs and normal sensitivity to fenhexamid and the other classes of fungicides tested. Pathogenicity on strawberry fruits and cucumber cotyledons was demonstrated in in vitro assays. Botrytis group I isolates were initially designated as a single new species, B. pseudocinerea [43], but additional species have been subsequently characterized, including B. calthae [44], B. californica [38], and B. sinoviticola [19]. B. sinoviticola was firstly described as another cryptic species living in sympatry with B. cinerea on table grapes in China [19], and it was found on strawberry leaves collected in an open field during spring in Mazandaran province in the current study. The species was distinguished from others based on the formation of numerous round and small sclerotia on PDA and villiform appendages on the conidial surface observed under an electron microscope [19]. Previous reports from Iran documented its presence on pomegranate from Fars province [45] and grapes and apple from Kurdistan province [2]. Our isolates showed a white-to-creamy colony and other morphological features like those mentioned in the first description of the species [19]. Mycelial colonies with no sclerotia and oval-to-ovoid conidia were also like those previously reported by Nabizadeh et al. [2]. Multilocus analysis using rpb2, g3pdh, and hsp60 sequences clearly distinguished B. sinoviticola Iranian isolates from others, originating a single lineage within Botrytis clade 1 and forming a well-supported clade together with the reference B. sinoviticola strains.
This study represents the first report of B cinerea group S and B. sinoviticola from strawberry in Iran. It should be noted that the highest proportion of B. cinerea group S isolates were related to P12 and P18 populations (both from Kurdistan province), respectively. Previously, reports of B. sinoviticola had been limited to China and Iran, from pomegranate in Fars province and apple and vine in West Kurdistan province; this study extended the host range and distribution of this species to the Mazandaran province in Iran.
In conclusion, our results highlighted several key points: (1) B. cinerea group S isolates are not host-specific, as we could identify them on both strawberry and roses in different provinces; (2) while some species-specific morphological features, such as occasionally septate conidia or the swelling of the conidiophore may be observed, especially when studying numerous isolates, reliable differentiation without molecular studies is challenging due to the broad variability and instability of morphological traits [2]; (3) despite rigorous efforts towards species distinction through a combined morphological and molecular approach, certain species might be not separated, likely due to the coevolution of genes used in multilocus analysis; (4) contrasting with previous reports by Johnston et al. [30], we could not find any relationship between the content of TEs and Botrytis species, since we detected isolates of the B−F− type among Botrytis group II, and the only Botrytis group I isolate tested was of the B+F− type.
These findings contribute to the understanding of Botrytis species diversity, emphasizing the importance of molecular techniques in accurate species identification and providing valuable insights into the genetic and ecological dynamics of Botrytis populations on cultivated and wild host plants in Iran. This information is particularly useful in improving the management of diseases on economically important hosts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof10110764/s1, Table S1: Codes of sequences of rpb2, hsp60, g3pdh, and nep2 genes from Botrytis species used in phylogenetic tree preparation; Table S2: Mean disease severity ± standard error on strawberry fruits and cucumber cotyledons at different days after inoculation (DAI) with Botrytis sinoviticola strain P14-2, three strains of Botrytis prunorum (P18-45, P16-19, and P8-9), and three strains of Botrytis cinerea (P18-13, P15-7, and P6-11); Table S3: Genetic and phenotypic data for 345 Botrytis isolates in this study; Figure S1: Single-gene rpb2 (A) and nep2 (B) phylogenetic trees prepared by using Mega7 software [46,47,48,49,50,51,52,53,54,55,56,57].
Author Contributions
S.F.: Investigation, Data curation, Formal Analysis; Writing—original draft. B.S.: Funding acquisition, Supervision, Writing—review and editing. M.J.-N.: Supervision, Writing—review and editing. S.P.: Funding acquisition, Methodology, Resources, Writing—review and editing. F.F.: Conceptualization, Supervision, Project administration, Writing—review and editing. R.M.D.M.A.: Conceptualization, Data curation, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)–MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022)—The manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All the sequence data generated in this study are publicly available from the NCBI/GenBank database (http://www.ncbi.nlm.nih.gov (accessed on 1 October 2024)) with the accession numbers listed in Table 4.
Acknowledgments
The financial and scientific support extended by Isfahan University of Technology (IUT) and the University of Bari (Italy) is greatly appreciated. Their contributions have been instrumental in facilitating and advancing this research.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- FAOSTAT. Food and Agriculture Organization. The Database of Annual Production. FAOSTAT. Statistical Database. 2019. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 30 July 2024).
- Nabizadeh, H.; Ahmadpour, A.; Gosta, Y. Study on the diversity of Botrytis spp. from different plants in West Azarbaijan province and Sanadaj city (Kurdistan province). Iran. J. Plant Pathol. 2022, 57, 237–262. [Google Scholar]
- Kulkarni, S.J. An insight into research and investigations of gray mold focused on Botrytis cinerea. In Driving Factors for Venture Creation and Success in Agricultural Entrepreneurship; Arafat, M.Y., Saleem, I., Ali, J., Khan, A., Balhareth, H.H., Eds.; IGI Global: Hershey, PA, USA, 2022; pp. 273–289. [Google Scholar]
- Gül, E.; Karakaya, A.; Ergül, A. Determination of the frequency and virulence of some Botrytis cinerea isolates and a new Botrytis prunorum cryptic species causing grey mould disease on greenhouse tomatoes. Plant Pathol. 2023, 72, 1226–1235. [Google Scholar] [CrossRef]
- Maia, J.N.; Beger, G.; Pereira, W.V.; De Mio, L.L.M.; Duarte, H.D.S.S. Gray mold in strawberries in the Paraná state of Brazil is caused by Botrytis cinerea and its isolates exhibit multiple-fungicide resistance. Crop Prot. 2021, 140, 105415. [Google Scholar] [CrossRef]
- Fekrikohan, S.; Atashi Khalilabad, A.; Fotouhifar, K.; Sharifnabi, B. First report of Botrytis cinerea and Alternaria alternata on Pelargonium grandiflorum in Iran. Mycol. Iran. 2022, 9, 105–115. [Google Scholar]
- Naeimi, S.; Zare, R. Evaluation of indigenous Trichoderma spp. isolates in biological control of Botrytis cinerea, the causal agent of strawberry gray mold disease. Biocontrol Plant Prot. 2013, 1, 55–74. [Google Scholar]
- Garfinkel, A.R.; Coats, K.P.; Sherry, D.L.; Chastagner, G.A. Genetic analysis reveals unprecedented diversity of a globally-important plant pathogenic genus. Sci. Rep. 2019, 9, 6671. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, A.R. The history of Botrytis taxonomy, the rise of phylogenetics, and implications for species recognition. Phytopathology 2021, 111, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Ziedan, E.H.; Attallah, A.G.; Abd-El-Aal, S.K.; Sahab, A.F. Molecular identification and pathogenic potential of Botrytis cinerea isolates causing fruit blight of cucumber under protective greenhouse in Egypt. Plant Arch. 2018, 18, 1563–1569. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Khahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Giraud, T.; Fortini, D.; Levis, C.; Lamarque, C.; Leroux, P.; Lobuglio, K.; Brygoo, Y. Two sibling species of the Botrytis cinerea complex, transposa and vacuma, are found in sympatry on numerous host plants. Phytopathology 1999, 89, 967–973. [Google Scholar] [CrossRef]
- Giraud, T.; Fortini, D.; Levis, C.; Leroux, P.; Brygoo, Y. RFLP markers show genetic recombination in Botryotinia fuckeliana (Botrytis cinerea) and transposable elements reveal two sympatric species. Mol. Biol. Evol. 1997, 14, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Wessels, B.; Linde, C.; Fourie, P.; Mostert, L. Genetic population structure and fungicide resistance of Botrytis cinerea in pear orchards in the Western Cape of South Africa. Plant Pathol. 2016, 65, 1473–1483. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Rotolo, C.; Masiello, M.; Pollastro, S.; Ishii, H.; Faretra, F. Genetic analysis and molecular characterisation of laboratory and field mutants of Botryotinia fuckeliana (Botrytis cinerea) resistant to QoI fungicides. Pest Manag. Sci. 2012, 68, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Staats, M.; van Baarlen, P.; Van Kan, J.A. Molecular phylogeny of the plant pathogenic genus Botrytis and the evolution of host specificity. Mol. Biol. Evol. 2005, 22, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Staats, M.; Van Baarlen, P.; Schouten, A.; Van Kan, J.A. Functional analysis of NLP genes from Botrytis elliptica. Mol. Plant Pathol. 2007, 8, 209–214. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Li, G.Q.; Yang, L.; Jiang, D.H.; Zhuang, W.Y.; Huang, H.C. Botrytis sinoallii: A new species of the grey mould pathogen on Allium crops in China. Mycoscience 2010, 51, 421–431. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, J.; Wang, X.D.; Yng, L.; Jiang, D.H.; Li, G.Q.; Hsiang, T.; Zhuang, W.Y. Morphological and phylogenetic identification of Botrytis sinoviticola, a novel cryptic species causing gray mold disease of table grapes (Vitis vinifera) in China. Mycologia 2014, 106, 43–56. [Google Scholar] [CrossRef]
- Liu, Q.; Li, G.; Li, J.; Chen, S. Botrytis eucalypti, a novel species isolated from diseased Eucalyptus seedlings in South China. Mycol. Prog. 2016, 15, 1057–1079. [Google Scholar] [CrossRef]
- Leroch, M.; Plesken, C.; Weber, R.W.; Kauff, F.; Scalliet, G.; Hahn, M. Gray mold populations in German strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl. Environ. Microbiol. 2013, 79, 159–167. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Habib, W.; Rotolo, C.; Pollastro, S.; Faretra, F. Selection, characterization and genetic analysis of laboratory mutants of Botryotinia fuckeliana (Botrytis cinerea) resistant to the fungicide boscalid. Eur. J. Plant Pathol. 2010, 128, 185–199. [Google Scholar] [CrossRef]
- Ma, Z.; Michailides, T.J. Genetic structure of Botrytis cinerea populations from different host plants in California. Plant Dis. 2005, 89, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Habib, W.; De Miccolis Angelini, R.M.; Rotolo, C.; Pollastro, S.; Faretra, F. Genetic variation in Botryotinia fuckeliana (Botrytis cinerea) populations on greenhouse vegetable crops in Lebanon. In Proceedings of the Abstract Book of XVIth International Botrytis Symposium, Bari, Italy, 23–28 June 2013; p. 37. [Google Scholar]
- Fournier, E.; Levis, C.; Fortini, D.; Leroux, P.; Giraud, T.; Brygoo, Y. Characterization of Bc-hch, the Botrytis cinerea homolog of the Neurospora crassa het-c vegetative incompatibility locus, and its use as a population marker. Mycologia 2003, 95, 251–261. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Rotolo, C.; Masiello, M.; Gerin, D.; Pollastro, S.; Faretra, F. Occurrence of fungicide resistance in populations of Botryotinia fuckeliana (Botrytis cinerea) on table grape and strawberry in southern Italy. Pest Manag. Sci. 2014, 70, 1785–1796. [Google Scholar] [CrossRef]
- Eyvazi, A.; Massah, A.; Soorni, A.; Babaie, G. Molecular phylogenetic analysis shows that causal agent of maize rough dwarf disease in Iran is closer to rice black-streaked dwarf virus. Eur. J. Plant Pathol. 2021, 160, 411–425. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol. Biol Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Rasiukevičiūtė, N.; Rugienius, R.; Šikšnianienė, J.B. Genetic diversity of Botrytis cinerea from strawberry in Lithuania. Zemdirbyste 2018, 105, 265–270. [Google Scholar] [CrossRef]
- Johnston, P.R.; Hoksbergen, K.; Park, D.; Beever, R.E. Genetic diversity of Botrytis in New Zealand vineyards and the signi–ficance of its seasonal and regional variation. Plant Pathol. 2014, 63, 888–898. [Google Scholar] [CrossRef]
- Mirzaei, S.; Goltapeh, E.M.; Shams-bakhsh, M. Taxonomical studies on the genus Botrytis in Iran. J. Agric. Technol. 2007, 3, 65–76. [Google Scholar]
- Ferrada, E.E.; Latorre, B.A.; Zoffoli, J.P.; Castillo, A. Identification and characterization of Botrytis blossom blight of Japanese plums caused by Botrytis cinerea and B. prunorum sp. nov. in Chile. Phytopathology 2016, 106, 155–165. [Google Scholar] [CrossRef]
- Schumacher, J. How light affects the life of Botrytis. Fungal Genet. Biol. 2017, 106, 26–41. [Google Scholar] [CrossRef]
- Amiri, A.; Zuniga, A.I.; Peres, N.A. Prevalence of Botrytis cryptic species in strawberry nursery transplants and strawberry and blueberry commercial fields in the eastern United States. Plant Dis. 2018, 102, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Isaza, L.; Zuluaga, Y.P.; Marulanda, M.L. Morphological, pathogenic and genetic diversity of Botrytis cinerea Pers. in blackberry cultivations in Colombia. Rev. Bras. Frutic. 2019, 41, 115. [Google Scholar] [CrossRef]
- Nielsen, K.A.; Skårn, M.N.; Strømeng, G.M.; Brurberg, M.B.; Stensvand, A. Pervasive fungicide resistance in Botrytis from strawberry in Norway: Identification of the grey mould pathogen and mutations. Plant Pathol. 2022, 71, 1392–1403. [Google Scholar] [CrossRef]
- Testempasis, S.; Puckett, R.D.; Michailides, T.J.; Karaoglanidis, G.S. Genetic structure and fungicide resistance profile of Botrytis spp. populations causing postharvest gray mold of pomegranate fruit in Greece and California. Postharvest Biol. Technol. 2020, 170, 111319. [Google Scholar] [CrossRef]
- Saito, S.; Margosan, D.; Michailides, T.J.; Xiao, C.L. Botrytis californica, a new cryptic species in the B. cinerea species complex causing gray mold in blueberries and table grapes. Mycologia 2016, 108, 330–343. [Google Scholar] [CrossRef]
- Esterio, M.; Osorio-Navarro, C.; Carreras, C.; Azócar, M.; Copier, C.; Estrada, V.; Auger, J. Botrytis prunorum associated to Vitis vinifera blossom blight in Chile. Plant Dis. 2020, 104, 2324–2329. [Google Scholar] [CrossRef]
- Riquelme, D.; Aravena, Z.; Valdés-Gómez, H.; Latorre, B.A.; Díaz, G.A.; Zoffoli, J.P. Characterization of Botrytis cinerea and B. prunorum from healthy floral structures and decayed ‘Hayward’ kiwifruit during post-harvest storage. Plant Dis. 2021, 105, 2129–2140. [Google Scholar] [CrossRef]
- Acosta Morel, W.; Marques-Costa, T.M.; Santander-Gordón, D.; Anta Fernández, F.; Zabalgogeazcoa, I.; Vázquez de Aldana, B.R.; Sukno, S.A.; Díaz-Mínguez, J.M.; Benito, E.P. Physiological and population genetic analysis of Botrytis field isolates from vineyards in Castilla y León, Spain. Plant Pathol. 2019, 68, 523–536. [Google Scholar] [CrossRef]
- Moparthi, S.; Parikh, L.P.; Gunnink Troth, E.E.; Burrows, M.E. Identification and prevalence of seedborne Botrytis spp. in dry pea, lentil, and chickpea in Montana. Plant Dis. 2023, 107, 382392. [Google Scholar] [CrossRef]
- Walker, A.S.; Gautier, A.; Confais, J.; Martinho, D.; Viaud, M.; Le P Cheur, P.; Dupont, J.; Fournier, E. Botrytis pseudocinerea, a new cryptic species causing gray mold in French vineyards in sympatry with Botrytis cinerea. Phytopathology 2011, 101, 1433–1445. [Google Scholar] [CrossRef]
- Plesken, C.; Westrich, L.D.; Hahn, M. Genetic and phenotypic characterization of Botrytis calthae. Plant Pathol. 2015, 64, 128–136. [Google Scholar] [CrossRef]
- Sadeghi, A.; Atghia, O.; Javan-Nikkhah, M. Occurrence of Botrytis sinoviticola on pomegranate fruit. In Proceedings of the 23rd Iranian Plant Protection Congress, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran, 27–30 August 2018; pp. 440–441. [Google Scholar]
- Garfinkel, A.R.; Lorenzini, M.; Zapparoli, G.; Chastagner, G.A. Botrytis euroamericana, a new species from peony and grape in North America and Europe. Mycologia 2017, 109, 495–507. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, H.Y.; Wang, X.J.; Sun, B. First report of Botrytis cinerea causing fruit rot of Pyrus sinkiangensis in China. Plant Dis. 2014, 98, 281. [Google Scholar] [CrossRef]
- Garfinkel, A.R.; Coats, K.P.; Chastagner, G.A. Identification of Botrytis paeoniae microsatellites using Ion Proton technology. In Proceedings of the XII International Symposium on Flower Bulbs and Herbaceous Perennials, Kunming, China, 28 June–2 July 2016; pp. 341–348. [Google Scholar]
- Chen, X.R.; Huang, S.X.; Wang, H.; Zhang, Y.; Ji, Z.L. First report of Botrytis cinerea causing leaf spot of Chinese quince in China. Plant Dis. 2019, 103, 1027. [Google Scholar] [CrossRef]
- Harper, L.A.; Derbyshire, M.C.; Lopez-Ruiz, F.J. Identification and characterization of Botrytis medusae, a novel cryptic species causing grey mould on wine grapes in Australia. Plant Pathol. 2019, 68, 939–953. [Google Scholar] [CrossRef]
- Brauna-Morževska, E.; Stoddard, F.L.; Bankina, B.; Kaņeps, J.; Bimšteine, G.; Petrova, I.; Fridmanis, D. Evaluation of pathogenicity of Botrytis species isolated from different legumes. Front. Plant Sci. 2023, 14, 1069126. [Google Scholar] [CrossRef]
- Rupp, S.; Weber, R.W.; Rieger, D.; Detzel, P.; Hahn, M. Spread of Botrytis cinerea strains with multiple fungicide resistance in German horticulture. Front. Microbiol. 2017, 7, 2075. [Google Scholar] [CrossRef]
- Lorenzini, M.; Zapparoli, G. An isolate morphologically and phylogenetically distinct from Botrytis cinerea obtained from withered grapes possibly represents a new species of Botrytis. Plant pathol. 2014, 63, 1326–1335. [Google Scholar] [CrossRef]
- Van Kan, J.A.; Stassen, J.H.; Mosbach, A.; Van Der Lee, T.A.; Faino, L.; Farmer, A.D.; Papasotiriou, D.G.; Zhou, S.; Seidl, M.F.; Cottam, E.; et al. A gapless genome sequence of the fungus Botrytis cinerea. Mol. Plant Pathol. 2017, 18, 75–89. [Google Scholar] [CrossRef]
- Prasannath, K.; Galea, V.J.; Akinsanmi, O.A. Diversity and pathogenicity of species of Botrytis, Cladosporium, Neopestalotiopsis and Pestalotiopsis causing flower diseases of macadamia in Australia. Plant Pathol. 2023, 72, 881–899. [Google Scholar] [CrossRef]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.; Viaud, M.; Benito, E.P.; Couloux, A.; Dickman, M. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.V.; Peres, N.A. First Report of Botrytis cinerea causing leaf spot on strawberry in Florida. Plant Dis. 2022, 106, 1298. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).