Eucalyptus and Native Broadleaf Mixed Cultures Boost Soil Multifunctionality by Regulating Soil Fertility and Fungal Community Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Soil Sampling
2.3. Soil Properties and Enzymatic Activity Analysis
2.4. DNA Extracted and High-Throughput Sequencing
2.5. Soil Multifunctionality
2.6. Data and Statistical Analyses
3. Results
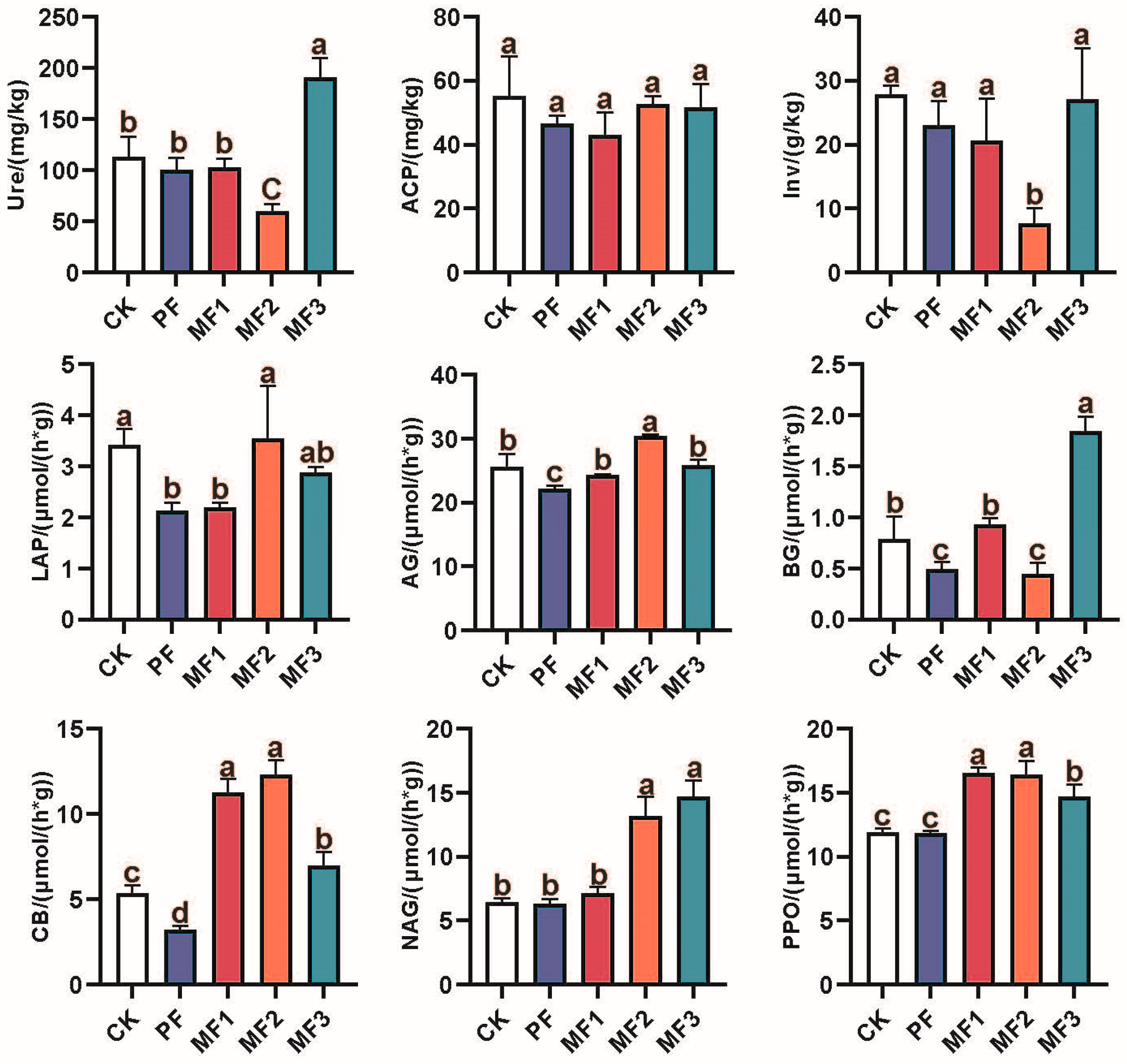
3.1. Soil Properties and Enzymatic Activities
3.2. Diversity and Species Composition of Soil Fungal Communities
3.3. Fungal Co-Occurrence Networks
3.4. Relationship between Fungal Community Structure and Soil Properties
3.5. Soil Multifunctionality and Driving Force
4. Discussion
4.1. Effects of Eucalyptus and Broadleaf Mixing on Soil Properties and Enzyme Activities
4.2. Eucalyptus Broad Mixing Enhances Fungal Network Complexity
4.3. Soil Nutrients, Fungal Diversity, and Network Complexity Jointly Drive Soil Multifunctionality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, K.; Chu, H.; Eldridge, D.J.; Gaitan, J.J.; Liu, Y.-R.; Sokoya, B.; Wang, J.-T.; Hu, H.-W.; He, J.-Z.; Sun, W.; et al. Soil Biodiversity Supports the Delivery of Multiple Ecosystem Functions in Urban Greenspaces. Nat. Ecol. Evol. 2023, 7, 113–126. [Google Scholar] [CrossRef]
- Creamer, R.E.; Barel, J.M.; Bongiorno, G.; Zwetsloot, M.J. The Life of Soils: Integrating the Who and How of Multifunctionality. Soil Biol. Biochem. 2022, 166, 108561. [Google Scholar] [CrossRef]
- Li, J.; Delgado-Baquerizo, M.; Wang, J.-T.; Hu, H.-W.; Cai, Z.-J.; Zhu, Y.-N.; Singh, B.K. Fungal Richness Contributes to Multifunctionality in Boreal Forest Soil. Soil Biol. Biochem. 2019, 136, 107526. [Google Scholar] [CrossRef]
- Yang, A.; Zhu, D.; Zhang, W.; Shao, Y.; Shi, Y.; Liu, X.; Lu, Z.; Zhu, Y.-G.; Wang, H.; Fu, S. Canopy Nitrogen Deposition Enhances Soil Ecosystem Multifunctionality in a Temperate Forest. Glob. Chang. Biol. 2024, 30, e17250. [Google Scholar] [CrossRef]
- Hu, Z.; Delgado-Baquerizo, M.; Fanin, N.; Chen, X.; Zhou, Y.; Du, G.; Hu, F.; Jiang, L.; Hu, S.; Liu, M. Nutrient-Induced Acidification Modulates Soil Biodiversity-Function Relationships. Nat. Commun. 2024, 15, 2858. [Google Scholar] [CrossRef]
- Luo, J.; Liao, G.; Banerjee, S.; Gu, S.; Liang, J.; Guo, X.; Zhao, H.; Liang, Y.; Li, T. Long-Term Organic Fertilization Promotes the Resilience of Soil Multifunctionality Driven by Bacterial Communities. Soil Biol. Biochem. 2023, 177, 108922. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Chen, Z.; Wu, Q.; Huang, K.; Ke, Q.; Zhu, L.; Lu, S.; Tang, Y.; Li, H.; et al. Ecological Niche Differences Regulate the Assembly of Bacterial Community in Endophytic and Rhizosphere of Eucalyptus. For. Ecol. Manag. 2022, 524, 120521. [Google Scholar] [CrossRef]
- Chen, L.; Dini-Andreote, F.; Liu, H.; Wang, H.; Dumbrell, A.; Wang, Z.; Chen, X.; Chen, F.; Chen, X.; Wu, L.; et al. Integrating Variation in Bacterial-fungal Co-occurrence Network with Soil Carbon Dynamics. J. Appl. Ecol. 2024, 61, 36–50. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Wang, H.; Wu, Q.; Ke, Q.; Zhu, L.; Wu, L.; Chen, L. Harvest Residue Recycling Rather than Slash-Burning Results in the Enhancement of Soil Fertility and Bacterial Community Stability in Eucalyptus Plantations. Sci. Total Environ. 2024, 945, 173850. [Google Scholar] [CrossRef]
- Qin, F.; Yang, F.; Ming, A.; Jia, H.; Zhou, B.; Xiong, J.; Lu, J. Mixture Enhances Microbial Network Complexity of Soil Carbon, Nitrogen and Phosphorus Cycling in Eucalyptus Plantations. For. Ecol. Manag. 2024, 553, 121632. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Zhang, L.; Liu, S.; Wang, J.; Tian, Y.; Campbell, D.E.; Lin, R.; Ren, H.; Lu, H. Long-term Effects of Intercropping on Multi-trophic Structure and Bio-thermodynamic Health of Mixed Eucalyptus-native Tree Plantations. J. Appl. Ecol. 2024, 61, 103–119. [Google Scholar] [CrossRef]
- Zhang, W.; You, Y.; Su, X.; Yan, J.; Gao, G.; Ming, A.; Shen, W.; Huang, X. Introducing N2-Fixing Tree Species into Eucalyptus Plantations Promotes Soil Organic Carbon Sequestration in Aggregates by Increasing Microbial Carbon Use Efficiency. CATENA 2023, 231, 107321. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; You, Y.; Xiang, M.; Li, C.; Ming, A.; Ma, H.; Huang, X. N2-Fixing Tree Species Help to Alleviate C- and P-Limitation in Both Rhizosphere and Non-Rhizosphere Soils in the Eucalyptus Plantations of Subtropical China. Forests 2023, 14, 2070. [Google Scholar] [CrossRef]
- Bouillet, J.-P.; Laclau, J.-P.; de Moraes Gonçalves, J.L.; Voigtlaender, M.; Gava, J.L.; Leite, F.P.; Hakamada, R.; Mareschal, L.; Mabiala, A.; Tardy, F.; et al. Eucalyptus and Acacia Tree Growth over Entire Rotation in Single- and Mixed-Species Plantations across Five Sites in Brazil and Congo. For. Ecol. Manag. 2013, 301, 89–101. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; He, C.; Wang, Z.; Zhu, W.; Wang, Z.; Chen, L.; Wu, L.; Du, A. Impact of Native Tree Species Introduction on Soil Nutrient and Bacterial Community in Eucalyptus Plantations. Eur. J. For. Res. 2023, 142, 1369–1383. [Google Scholar] [CrossRef]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The Global Soil Community and Its Influence on Biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil Bacterial Networks Are Less Stable under Drought than Fungal Networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Guo, X.; Feng, J.; Shi, Z.; Zhou, X.; Yuan, M.; Tao, X.; Hale, L.; Yuan, T.; Wang, J.; Qin, Y.; et al. Climate Warming Leads to Divergent Succession of Grassland Microbial Communities. Nat. Clim. Chang. 2018, 8, 813–818. [Google Scholar] [CrossRef]
- Anthony, M.A.; Tedersoo, L.; De Vos, B.; Croisé, L.; Meesenburg, H.; Wagner, M.; Andreae, H.; Jacob, F.; Lech, P.; Kowalska, A.; et al. Fungal Community Composition Predicts Forest Carbon Storage at a Continental Scale. Nat. Commun. 2024, 15, 2385. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Lucas-Borja, M.E.; Wang, Z.; Li, X.; Huang, Z. Microbial Diversity Regulates Ecosystem Multifunctionality during Natural Secondary Succession. J. Appl. Ecol. 2021, 58, 2833–2842. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Sun, S.; Jin, C.; Su, J.; Wei, J.; Luo, X.; Wen, J.; Wei, T.; Sahu, S.K.; et al. GWAS, MWAS and mGWAS Provide Insights into Precision Agriculture Based on Genotype-Dependent Microbial Effects in Foxtail Millet. Nat. Commun. 2022, 13, 5913. [Google Scholar] [CrossRef]
- Jiao, S.; Chu, H.; Zhang, B.; Wei, X.; Chen, W.; Wei, G. Linking Soil Fungi to Bacterial Community Assembly in Arid Ecosystems. iMeta 2022, 1, e2. [Google Scholar] [CrossRef]
- Zhai, C.; Han, L.; Xiong, C.; Ge, A.; Yue, X.; Li, Y.; Zhou, Z.; Feng, J.; Ru, J.; Song, J.; et al. Soil Microbial Diversity and Network Complexity Drive the Ecosystem Multifunctionality of Temperate Grasslands under Changing Precipitation. Sci. Total Environ. 2024, 906, 167217. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Singh, B.K.; Maestre, F.T. Soil Microbial Communities Drive the Resistance of Ecosystem Multifunctionality to Global Change in Drylands across the Globe. Ecol. Lett. 2017, 20, 1295–1305. [Google Scholar] [CrossRef]
- Walkley, A. An Examination of Methods for Determining Organic Carbon and Nitrogen in Soils. (With One Text-Figure.). J. Agric. Sci. 1935, 25, 598–609. [Google Scholar] [CrossRef]
- Tsiknia, M.; Tzanakakis, V.A.; Oikonomidis, D.; Paranychianakis, N.V.; Nikolaidis, N.P. Effects of Olive Mill Wastewater on Soil Carbon and Nitrogen Cycling. Appl. Microbiol. Biotechnol. 2014, 98, 2739–2749. [Google Scholar] [CrossRef]
- Lu, K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Olsen, S.; Sommers, L. Methods of Soil Analysis. In Methods of Soil Analysis; Page, A.L., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 403–430. ISBN 978-0-89118-977-0. [Google Scholar]
- Daniels, M.B.; Delaune, P.; Moore, P.A.; Mauromoustakos, A.; Chapman, S.L.; Langston, J.M. Soil Phosphorus Variability in Pastures: Implications for Sampling and Environmental Management Strategies. J. Environ. Qual. 2001, 30, 2157–2165. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Allison, S.D.; Jastrow, J.D. Activities of Extracellular Enzymes in Physically Isolated Fractions of Restored Grassland Soils. Soil Biol. Biochem. 2006, 38, 3245–3256. [Google Scholar] [CrossRef]
- Bazzicalupo, A.L.; Bálint, M.; Schmitt, I. Comparison of ITS1 and ITS2 rDNA in 454 Sequencing of Hyperdiverse Fungal Communities. Fungal Ecol. 2013, 6, 102–109. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Zhang, Y.; Hobson, S.A.; Garcia Lloret, M.; Chehoud, C.; Kuczynski, J.; DeSantis, T.; Warrington, J.; et al. A Microbiota Signature Associated with Experimental Food Allergy Promotes Allergic Sensitization and Anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.; Bahram, M.; Bates, S.; Bruns, T.; Bengtsson-Palme, J.; Callaghan, T.; et al. Towards a Unified Paradigm for Sequence-Based Identification of Fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical Methods for Research Workers. In Breakthroughs in Statistics: Methodology and Distribution; Kotz, S., Johnson, N.L., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1992; pp. 66–70. ISBN 978-1-4612-4380-9. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, B.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 22-1. 2015; Volume 2, pp. 1–2. Available online: https://www.researchgate.net/publication/258996451_Vegan_Community_Ecology_Package_R_Package_Version_20-10 (accessed on 7 October 2024).
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Friedman, J.; Alm, E.J. Inferring Correlation Networks from Genomic Survey Data. PLoS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef]
- Sanchez, G.; Trinchera, R.G. plspm: Tools for Partial Least Squares Path Modeling (PLS-PM) 2014, 0.5.1. Available online: https://www.researchgate.net/publication/285763042_Tools_for_partial_least_squares_path_modeling_PLS-PM (accessed on 7 October 2024).
- Wetzels, M.; Odekerken-Schröder, G.; van Oppen, C. Using PLS Path Modeling for Assessing Hierarchical Construct Models: Guidelines and Empirical Illustration. MIS Q. 2009, 33, 177–195. [Google Scholar] [CrossRef]
- Guo, J.; Feng, H.; McNie, P.; Liu, Q.; Xu, X.; Pan, C.; Yan, K.; Feng, L.; Adehanom Goitom, E.; Yu, Y. Species Mixing Improves Soil Properties and Enzymatic Activities in Chinese Fir Plantations: A Meta-Analysis. CATENA 2023, 220, 106723. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Zhu, W.; Wang, Z.; Wu, L.; Du, A. Effects of Enrichmemt Planting with Native Tree Species on Bacterial Community Structure and Potential Impact on Eucalyptus Plantations in Southern China. J. For. Res. 2022, 33, 1349–1363. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, J.; Ding, J.; Zou, T.; Zhang, Z.; Liu, Q.; Yin, H. Differences in Root Exudate Inputs and Rhizosphere Effects on Soil N Transformation between Deciduous and Evergreen Trees. Plant Soil 2021, 458, 277–289. [Google Scholar] [CrossRef]
- Chen, X.; Taylor, A.R.; Reich, P.B.; Hisano, M.; Chen, H.Y.H.; Chang, S.X. Tree Diversity Increases Decadal Forest Soil Carbon and Nitrogen Accrual. Nature 2023, 618, 94–101. [Google Scholar] [CrossRef]
- Duan, P.; Fu, R.; Nottingham, A.T.; Domeignoz-Horta, L.A.; Yang, X.; Du, H.; Wang, K.; Li, D. Tree Species Diversity Increases Soil Microbial Carbon Use Efficiency in a Subtropical Forest. Glob. Chang. Biol. 2023, 29, 7131–7144. [Google Scholar] [CrossRef]
- Liu, D.; Wang, T.; Peñuelas, J.; Piao, S. Drought Resistance Enhanced by Tree Species Diversity in Global Forests. Nat. Geosci. 2022, 15, 800–804. [Google Scholar] [CrossRef]
- Xie, Y.; Ouyang, Y.; Han, S.; Se, J.; Tang, S.; Yang, Y.; Ma, Q.; Wu, L. Crop Rotation Stage Has a Greater Effect than Fertilisation on Soil Microbiome Assembly and Enzymatic Stoichiometry. Sci. Total Environ. 2022, 815, 152956. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; Van Der Heijden, M.G.A. Agricultural Intensification Reduces Microbial Network Complexity and the Abundance of Keystone Taxa in Roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Hättenschwiler, S.; Milcu, A.; Wambsganss, J.; Shihan, A.; Fromin, N. Tree Species Mixing Affects Soil Microbial Functioning Indirectly via Root and Litter Traits and Soil Parameters in European Forests. Funct. Ecol. 2021, 35, 2190–2204. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Fang, J.; Chu, H.; Adams, J.M. Soil Mcrobial Network Complexity Varies with pH as a Continuum, Not a Threshold, across the North China Plain. Front. Microbiol. 2022, 13, 895687. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Wang, L.; Xu, R.; Tigabu, M.; Li, M.; Wang, D. Effects of Species Mixture on Understory Vegetation, Soil Properties and Bacterial Diversity of Acacia Cincinnata, Eucalyptus Robusta and Acacia Mangium Plantations in Southeastern China. Plant Stress 2023, 10, 100278. [Google Scholar] [CrossRef]
- Gurevitch, J. Managing Forests for Competing Goals. Science 2022, 376, 792–793. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-Species versus Monocultures in Plantation Forestry: Development, Benefits, Ecosystem Services and Perspectives for the Future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-Term Nutrient Inputs Shift Soil Microbial Functional Profiles of Phosphorus Cycling in Diverse Agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Chen, X.; Meng, Z.; Xu, R.; Duoji, D.; Zhang, J.; He, J.; Wang, Z.; Chen, J.; et al. Soil Microbial Network Complexity Predicts Ecosystem Function along Elevation Gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Wang, A.-Y.; Peng, Y.-Q.; Harder, L.D.; Huang, J.-F.; Yang, D.-R.; Zhang, D.-Y.; Liao, W.-J. The Nature of Interspecific Interactions and Co-Diversification Patterns, as Illustrated by the Fig Microcosm. New Phytol. 2019, 224, 1304–1315. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Van Der Heijden, M.G.A. Fungal-Bacterial Diversity and Microbiome Complexity Predict Ecosystem Functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]





| CK | PF | MF1 | MF2 | MF3 | |
|---|---|---|---|---|---|
| pH | 4.4 ± 0.09 a | 4.08 ± 0.07 b | 4.23 ± 0.06 ab | 4.39 ± 0.27 a | 4.15 ± 0.05 ab |
| SOC/(g/kg) | 33.18 ± 3.3 ab | 29.19 ± 1.37 bc | 31.28 ± 0.77 bc | 28.7 ± 1.11 c | 36.14 ± 2.35 a |
| TN/(g/kg) | 2.25 ± 0.11 a | 2.15 ± 0.34 a | 2.01 ± 0.1 ab | 1.76 ± 0.01 b | 2.33 ± 0.08 a |
| TP/(g/kg) | 1.11 ± 0.12 a | 0.56 ± 0.08 bc | 0.48 ± 0.03 c | 0.64 ± 0.16 bc | 0.72 ± 0.08 b |
| TK/(g/kg) | 4.6 ± 0.09 b | 18.55 ± 1.49 a | 16.27 ± 3.65 a | 13.98 ± 3.82 a | 5.16 ± 1.1 b |
| AP/(mg/kg) | 35.48 ± 8.2 a | 21.08 ± 1.34 b | 9.85 ± 4.13 c | 26.96 ± 0.55 ab | 10.78 ± 4.21 c |
| AK/(mg/kg) | 65.83 ± 6.37 c | 100.54 ± 8.41 ab | 82.27 ± 9.85 bc | 107.13 ± 16.31 a | 72.71 ± 6.43 c |
| NH4+-N/(mg/kg)) | 2.39 ± 0.23 c | 4.5 ± 1.34 b | 3.92 ± 0.2 b | 6.85 ± 0.9 a | 4.28 ± 0.4 b |
| NO3−-N/(mg/kg) | 6.11 ± 0.84 c | 22.1 ± 3.8 ab | 22.4 ± 4.8 ab | 22.94 ± 2.78 a | 16.03 ± 2.95 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Tian, D.; Cao, J.; Ren, S.; Zhu, Y.; Wang, H.; Wu, L.; Chen, L. Eucalyptus and Native Broadleaf Mixed Cultures Boost Soil Multifunctionality by Regulating Soil Fertility and Fungal Community Dynamics. J. Fungi 2024, 10, 709. https://doi.org/10.3390/jof10100709
Wang H, Tian D, Cao J, Ren S, Zhu Y, Wang H, Wu L, Chen L. Eucalyptus and Native Broadleaf Mixed Cultures Boost Soil Multifunctionality by Regulating Soil Fertility and Fungal Community Dynamics. Journal of Fungi. 2024; 10(10):709. https://doi.org/10.3390/jof10100709
Chicago/Turabian StyleWang, Huaxiang, Dian Tian, Jizhao Cao, Shiqi Ren, Yuanli Zhu, Huili Wang, Lichao Wu, and Lijun Chen. 2024. "Eucalyptus and Native Broadleaf Mixed Cultures Boost Soil Multifunctionality by Regulating Soil Fertility and Fungal Community Dynamics" Journal of Fungi 10, no. 10: 709. https://doi.org/10.3390/jof10100709
APA StyleWang, H., Tian, D., Cao, J., Ren, S., Zhu, Y., Wang, H., Wu, L., & Chen, L. (2024). Eucalyptus and Native Broadleaf Mixed Cultures Boost Soil Multifunctionality by Regulating Soil Fertility and Fungal Community Dynamics. Journal of Fungi, 10(10), 709. https://doi.org/10.3390/jof10100709






