Azole Combinations and Multi-Targeting Drugs That Synergistically Inhibit Candidozyma auris
Abstract
1. Introduction
2. Antifungal Agents
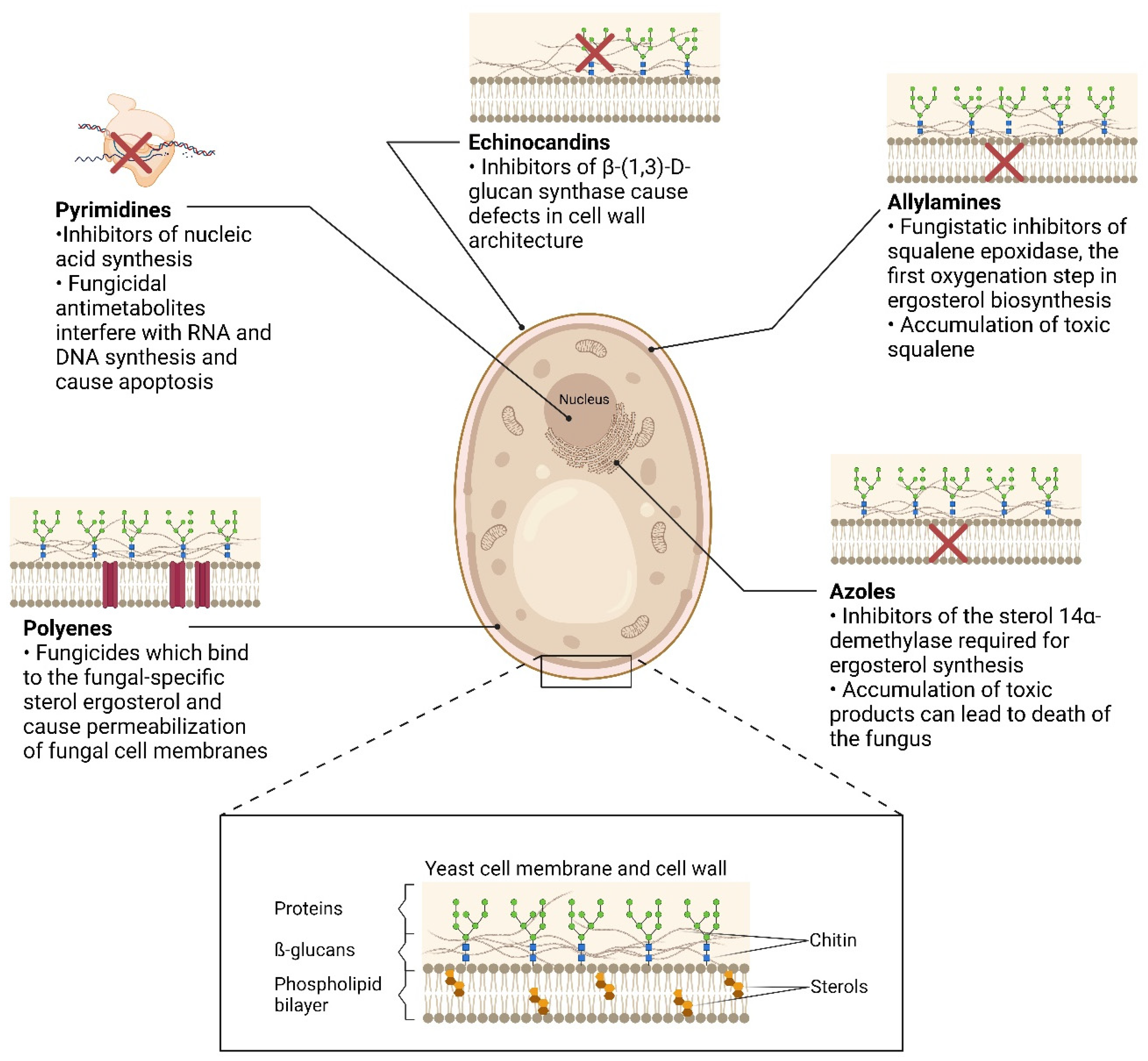
2.1. Echinocandins
2.2. Polyenes
2.3. Allylamines
2.4. Pyrimidines
2.5. Azoles
2.6. The Antifungal Pipeline
3. Antifungal Combination Therapy
3.1. Combination Therapies Used in the Clinic
3.2. Combination Therapies in Development
- Synergistic combinations affecting the efflux of azole drugs
- Inhibitors of transcriptional regulators of drug efflux
- Synergy mediated by inhibitors of Pma1p
- Synergy of azoles with inhibitors of glucan synthase
- Single molecule dual target inhibitors
- Animal trials of combination treatments
| Azole | Combination | Drug Information | Result | Setting | Source |
|---|---|---|---|---|---|
| FLC, VRC, ITC | Sulfamethoxazole | Antibiotic, CYPC9 inhibitor | Active against Erg11, ineffective against efflux pump overexpression; FLC limited effect (SYN one strain only); SUL+VRC or ITC: SYN or ADD in vivo: VRC + SUL 70% survival rate after 5 d | in vitro, C. elegans model | [197] |
| FLC, VRC, ITC | Lopinavir | HIV protease inhibitor, CYP3A substrate | LPV+FLC or VRC: SYN or ADD; LPV+ITC: SYN in vivo: LPV+ITC reduced burden | in vitro, C. elegans model | [199] |
| FLC, VRC, ITC | Lopinavir, Ritonavir | RTV interfered with efflux pump; RTV+FLC, VRC, or ITC: SYN or ADD; LPV+FLC or VRC: SYN or ADD; LPV+ITC: SYN in vivo: LPV+RTV+FLC or ITC reduced burden in kidneys | in vitro, mouse model | [200] | |
| VRC, ITC, POS | AZD8055 | ATP-competitive inhibitor of mTOR kinase activity; controller of cell growth and proliferation in eukaryotes | AZD8055+VRC, ITC, or POS: SYN or ADD in vivo: ITC+AZD8055 better than ITC alone | in vitro, G. mellonella model | [201] |
| FLC, VRC, ITC | Aprepitant | Antiemeticum, neurokinin-1 antagonist, dose-dependent inhibitor and inducer of CYP3A4 | APR+FLC, VRC, or ITC: SYN or ADD; APR+ITC active against biofilm in vivo: APR+ITC fungicidal in time killing assay C. elegans: APR+ITC reduced burden | in vitro, biofilm, C. elegans model | [203] |
| ITC | Ospemifene | Selective estrogen receptor modulator | OSP+ITC: SYN | in vitro, C. elegans model | [204] |
| FLC, ITC | RC21v3 | D-decapeptide Derivative | Combination reduced oral lesions and CFU count | Oral murine infection model | [175] |
- Combinations in clinical practice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. 2022. Available online: https://iris.who.int/bitstream/handle/10665/363682/9789240060241-eng.pdf?sequence=1 (accessed on 10 October 2022).
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Dollive, S.; Peterfreund, G.L.; Sherrill-Mix, S.; Bittinger, K.; Sinha, R.; Hoffmann, C.; Nabel, C.S.; Hill, D.A.; Artis, D.; Bachman, M.A.; et al. A tool kit for quantifying eukaryotic rRNA gene sequences from human microbiome samples. Genome Biol. 2012, 13, R60. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Köhler, J.R.; Casadevall, A.; Perfect, J. The spectrum of fungi that infects humans. Cold Spring Harb. Perspect. Med. 2014, 5, a019273. [Google Scholar] [CrossRef]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease among Adults, Adolescents and Children Living with HIV. Available online: https://www.who.int/publications/i/item/9789240052178 (accessed on 22 April 2023).
- Cleveland, A.A.; Harrison, L.H.; Farley, M.M.; Hollick, R.; Stein, B.; Chiller, T.M.; Lockhart, S.R.; Park, B.J. Declining Incidence of Candidemia and the Shifting Epidemiology of Candida Resistance in Two US Metropolitan Areas, 2008–2013: Results from Population-Based Surveillance. PLoS ONE 2015, 10, e0120452. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Pappas, P.G.; Rex, J.H.; Lee, J.; Hamill, R.J.; Larsen, R.A.; Powderly, W.; Kauffman, C.A.; Hyslop, N.; Mangino, J.E.; Chapman, S.; et al. A prospective observational study of candidemia: Epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003, 37, 634–643. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Papon, N.; Courdavault, V.; Clastre, M.; Bennett, R.J. Emerging and emerged pathogenic Candida species: Beyond the Candida albicans paradigm. PLoS Pathog. 2013, 9, e1003550. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Candida auris: A Drug-Resitant Fungus That Spreads in Healthcare Facilities. Available online: https://www.cdc.gov/candida-auris/hcp/laboratories/?CDC_AAref_Val=https://www.cdc.gov/fungal/candida-auris/fact-sheets/fact-sheet-lab-staff.html (accessed on 13 January 2023).
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Brown, C.S. Candida auris: A Review of the Literature. Clin. Microbiol. Rev. 2017, 31, e00029-17. [Google Scholar] [CrossRef] [PubMed]
- New Zealand Ministry of Health. One Case of Candida auris Detected in New Zealand. Available online: https://www.health.govt.nz/news-media/news-items/one-case-candida-auris-detected-new-zealand (accessed on 18 April 2023).
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18 (Suppl. S7), 19–37. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Djenontin, E.; Botterel, F.; Chowdhary, A.; Dannaoui, E. Colistin interacts synergistically with echinocandins against Candida auris. Int. J. Antimicrob. Agents 2020, 55, 105901. [Google Scholar] [CrossRef]
- Caballero, U.; Kim, S.; Eraso, E.; Quindós, G.; Vozmediano, V.; Schmidt, S.; Jauregizar, N. In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris. Antibiotics 2021, 10, 335. [Google Scholar] [CrossRef]
- Kaneko, Y.; Fukazawa, H.; Ohno, H.; Miyazaki, Y. Combinatory effect of fluconazole and FDA-approved drugs against Candida albicans. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2013, 19, 1141–1145. [Google Scholar] [CrossRef]
- Wu, Y.; Totten, M.; Memon, W.; Ying, C.; Zhang, S.X. In Vitro Antifungal Susceptibility of the Emerging Multidrug-Resistant Pathogen Candida auris to Miltefosine Alone and in Combination with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, e02063-19. [Google Scholar] [CrossRef]
- Schwarz, P.; Bidaud, A.L.; Dannaoui, E. In vitro synergy of isavuconazole in combination with colistin against Candida auris. Sci. Rep. 2020, 10, 21448. [Google Scholar] [CrossRef]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial strategies for combating invasive fungal infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.R.; Cardno, T.S.; Strouse, J.J.; Ivnitski-Steele, I.; Keniya, M.V.; Lackovic, K.; Monk, B.C.; Sklar, L.A.; Cannon, R.D. Targeting efflux pumps to overcome antifungal drug resistance. Future Med. Chem. 2016, 8, 1485–1501. [Google Scholar] [CrossRef] [PubMed]
- Fioriti, S.; Brescini, L.; Pallotta, F.; Canovari, B.; Morroni, G.; Barchiesi, F. Antifungal Combinations against Candida Species: From Bench to Bedside. J. Fungi 2022, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef]
- Van Dijck, P.; Sjollema, J.; Cammue, B.P.; Lagrou, K.; Berman, J.; d’Enfert, C.; Andes, D.R.; Arendrup, M.C.; Brakhage, A.A.; Calderone, R.; et al. Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms. Microb. Cell 2018, 5, 300–326. [Google Scholar] [CrossRef]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, mechanism of action and use in antifungal therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef]
- Monk, B.C.; Goffeau, A. Outwitting Multidrug Resistance to Antifungals. Science 2008, 321, 367–369. [Google Scholar] [CrossRef]
- Monk, B.C.; Keniya, M.V. Using Yeast to Discover Inhibitors of Multidrug Efflux in Candida albicans. In Candida albicans: Cellular and Molecular Biology; Prasad, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 491–543. [Google Scholar] [CrossRef]
- Su, H.; Han, L.; Huang, X. Potential targets for the development of new antifungal drugs. J. Antibiot. 2018, 71, 978–991. [Google Scholar] [CrossRef]
- Anderson, J.B. Evolution of antifungal-drug resistance: Mechanisms and pathogen fitness. Nat. Rev. Microbiol. 2005, 3, 547–556. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.C.; Cannon, R.D. Genomic pathways to antifungal discovery. Curr. Drug Targets Infect. Disord. 2002, 2, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Healy, M.D.; Dougherty, B.A.; Esposito, K.M.; Maurice, T.C.; Mazzucco, C.E.; Bruccoleri, R.E.; Davison, D.B.; Frosco, M.; Barrett, J.F.; et al. Conserved fungal genes as potential targets for broad-spectrum antifungal drug discovery. Eukaryot. Cell 2006, 5, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Grover, N.D. Echinocandins: A ray of hope in antifungal drug therapy. Indian J. Pharmacol. 2010, 42, 9–11. [Google Scholar] [CrossRef]
- Fujii, G.; Chang, J.E.; Coley, T.; Steere, B. The formation of amphotericin B ion channels in lipid bilayers. Biochemistry 1997, 36, 4959–4968. [Google Scholar] [CrossRef]
- Holz, R.; Finkelstein, A. The water and nonelectrolyte permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J. Gen. Physiol. 1970, 56, 125–145. [Google Scholar] [CrossRef]
- Petranyi, G.; Ryder, N.S.; Stütz, A. Allylamine derivatives: New class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science 1984, 224, 1239–1241. [Google Scholar] [CrossRef]
- Parker, W.B. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem. Rev. 2009, 109, 2880–2893. [Google Scholar] [CrossRef]
- Sanati, H.; Belanger, P.; Fratti, R.; Ghannoum, M. A new triazole, voriconazole (UK-109,496), blocks sterol biosynthesis in Candida albicans and Candida krusei. Antimicrob. Agents Chemother. 1997, 41, 2492–2496. [Google Scholar] [CrossRef]
- Balkovec, J.M.; Hughes, D.L.; Masurekar, P.S.; Sable, C.A.; Schwartz, R.E.; Singh, S.B. Discovery and development of first in class antifungal caspofungin (CANCIDAS®)—A case study. Nat. Prod. Rep. 2014, 31, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Sucher, A.J.; Chahine, E.B.; Balcer, H.E. Echinocandins: The Newest Class of Antifungals. Ann. Pharmacother. 2009, 43, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61 (Suppl. S6), 612–617. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Niimi, K.; Monk, B.C.; Hirai, A.; Hatakenaka, K.; Umeyama, T.; Lamping, E.; Maki, K.; Tanabe, K.; Kamimura, T.; Ikeda, F.; et al. Clinically significant micafungin resistance in Candida albicans involves modification of a glucan synthase catalytic subunit GSC1 (FKS1) allele followed by loss of heterozygosity. J. Antimicrob. Chemother. 2010, 65, 842–852. [Google Scholar] [CrossRef]
- Walker, L.A.; Munro, C.A.; de Bruijn, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef]
- Perlin, D.S. Mechanisms of echinocandin antifungal drug resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 1–11. [Google Scholar] [CrossRef]
- Niimi, K.; Harding, D.R.K.; Holmes, A.R.; Lamping, E.; Niimi, M.; Tyndall, J.D.A.; Cannon, R.D.; Monk, B.C. Specific interactions between the Candida albicans ABC transporter Cdr1p ectodomain and a d-octapeptide derivative inhibitor: A surface active CaCdr1p-specific inhibitor. Mol. Microbiol. 2012, 85, 747–767. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 18 January 2023).
- Zotchev, S.B. Polyene macrolide antibiotics and their applications in human therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [CrossRef]
- Yang, T.S.; Ou, K.L.; Peng, P.W.; Liou, B.C.; Wang, W.T.; Huang, Y.C.; Tsai, C.M.; Su, C.H. Quantifying membrane permeability of amphotericin B ion channels in single living cells. Biochim. Biophys. Acta 2013, 1828, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Li, X.; Xiao, E.; Lange, J.D.; Rienstra, C.M.; Burke, M.D.; Mitchell, D.A. Sterol Sponge Mechanism Is Conserved for Glycosylated Polyene Macrolides. ACS Cent. Sci. 2021, 7, 781–791. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Mishra, J.; Dey, A.; Singh, N.; Somvanshi, R.; Singh, S. Evaluation of toxicity & therapeutic efficacy of a new liposomal formulation of amphotericin B in a mouse model. Indian J. Med. Res. 2013, 137, 767–776. [Google Scholar]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Wang, X.; Mohammad, I.S.; Fan, L.; Zhao, Z.; Nurunnabi, M.; Sallam, M.A.; Wu, J.; Chen, Z.; Yin, L.; He, W. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharm. Sin. B 2021, 11, 2585–2604. [Google Scholar] [CrossRef]
- Safe, L.M.; Safe, S.H.; Subden, R.E.; Morris, D.C. Sterol content and polyene antibiotic resistance in isolates of Candida krusei, Candida parakrusei, and Candida tropicalis. Can. J. Microbiol. 1977, 23, 398–401. [Google Scholar] [CrossRef]
- Yoon, S.A.; Vazquez, J.A.; Steffan, P.E.; Sobel, J.D.; Akins, R.A. High-frequency, in vitro reversible switching of Candida lusitaniae clinical isolates from amphotericin B susceptibility to resistance. Antimicrob. Agents Chemother. 1999, 43, 836–845. [Google Scholar] [CrossRef]
- Keniya, M.V.; Ruma, Y.N.; Tyndall, J.D.A.; Monk, B.C. Heterologous Expression of Full-Length Lanosterol 14α-Demethylases of Prominent Fungal Pathogens Candida albicans and Candida glabrata Provides Tools for Antifungal Discovery. Antimicrob. Agents Chemother. 2018, 62, e01131-18. [Google Scholar] [CrossRef]
- Ryder, N.S. Mechanism of action and biochemical selectivity of allylamine antimycotic agents. Ann. N. Y. Acad. Sci. 1988, 544, 208–220. [Google Scholar] [CrossRef]
- Kovarik, J.M.; Mueller, E.A.; Zehender, H.; Denouël, J.; Caplain, H.; Millerioux, L. Multiple-dose pharmacokinetics and distribution in tissue of terbinafine and metabolites. Antimicrob. Agents Chemother. 1995, 39, 2738–2741. [Google Scholar] [CrossRef] [PubMed]
- Degreef, H.J.; DeDoncker, P.R. Current therapy of dermatophytosis. J. Am. Acad. Dermatol. 1994, 31, 25–30. [Google Scholar] [CrossRef]
- Roberts, D.T.; Taylor, W.D.; Boyle, J. Guidelines for treatment of onychomycosis. J. Am. Acad. Dermatol. 2003, 148, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Bebawi, E.; Jouni, S.S.; Tessier, A.A.; Frenette, A.J.; Brindamour, D.; Doré, M. A metoprolol-terbinafine combination induced bradycardia. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.R.; Westley, I.; Sallustio, B.; Horowitz, J.D.; Beltrame, J.F. Interaction of terbinafine (anti-fungal agent) with perhexiline: A case report. Heart Lung Circ. 2014, 23, e149–e151. [Google Scholar] [CrossRef] [PubMed]
- Vanden Bossche, H.; Dromer, F.; Improvisi, I.; Lozano-Chiu, M.; Rex, J.H.; Sanglard, D. Antifungal drug resistance in pathogenic fungi. Med. Mycol. 1998, 36, 119–128. [Google Scholar]
- Vermes, A.; Guchelaar, H.J.; Dankert, J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef]
- Waldorf, A.R.; Polak, A. Mechanisms of action of 5-fluorocytosine. Antimicrob. Agents Chemother. 1983, 23, 79–85. [Google Scholar] [CrossRef]
- Hospenthal, D.R.; Bennett, J.E. Flucytosine monotherapy for cryptococcosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1998, 27, 260–264. [Google Scholar] [CrossRef]
- Bicanic, T.; Wood, R.; Meintjes, G.; Rebe, K.; Brouwer, A.; Loyse, A.; Bekker, L.G.; Jaffar, S.; Harrison, T. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: A randomized trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 47, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Graybill, R.J.; Larsen, R.A.; Pappas, P.G.; Perfect, J.R.; Powderly, W.G.; Sobel, J.D.; Dismukes, W.E. Practice guidelines for the management of cryptococcal disease. Infect. Dis. Soc. Am. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 30, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Janbon, G.; Dromer, F.; Lortholary, O.; Dannaoui, E. Combination of amphotericin B with flucytosine is active in vitro against flucytosine-resistant isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 2007, 51, 383–385. [Google Scholar] [CrossRef]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef]
- Sigera, L.S.M.; Denning, D.W. Flucytosine and its clinical usage. Ther. Adv. Infect. Dis. 2023, 10, 20499361231161387. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Carvalho, D.T.; Sousa, E.; Pinto, E. New Antifungal Agents with Azole Moieties. Pharmaceuticals 2022, 15, 1427. [Google Scholar] [CrossRef]
- Vardanyan, R.; Hruby, V. Antifungal Drugs. In Synthesis of Best-Seller Drugs; Vardanyan, R., Hruby, V., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 677–686. [Google Scholar] [CrossRef]
- Hoekstra, W.J.; Garvey, E.P.; Moore, W.R.; Rafferty, S.W.; Yates, C.M.; Schotzinger, R.J. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorganic Med. Chem. Lett. 2014, 24, 3455–3458. [Google Scholar] [CrossRef]
- Lakshmi Krishnasamy, S.K.; Kumaramanickavel, G.; Saikumar, C. Molecular Mechanisms of Antifungal Drug Resistance in Candida Species. J. Clin. Diagn. Res. 2018, 12, DE01–DE06. [Google Scholar] [CrossRef]
- White, T.C.; Marr, K.A.; Bowden, R.A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [CrossRef]
- Yoshida, Y.; Aoyama, Y. Yeast cytochrome P-450 catalyzing lanosterol 14 alpha-demethylation. I. Purification and spectral properties. J. Biol. Chem. 1984, 259, 1655–1660. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim. Biophys. Acta 2007, 1770, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Garvey, E.P.; Hoekstra, W.J.; Yates, C.M.; Wawrzak, Z.; Rachakonda, G.; Villalta, F.; Lepesheva, G.I. Crystal Structure of the New Investigational Drug Candidate VT-1598 in Complex with Aspergillus fumigatus Sterol 14α-Demethylase Provides Insights into Its Broad-Spectrum Antifungal Activity. Antimicrob. Agents Chemother. 2017, 61, e00570-17. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.C.; Sagatova, A.A.; Hosseini, P.; Ruma, Y.N.; Wilson, R.K.; Keniya, M.V. Fungal Lanosterol 14α-demethylase: A target for next-generation antifungal design. Biochim. Biophys. Acta 2020, 1868, 140206. [Google Scholar] [CrossRef]
- Sagatova, A.A.; Keniya, M.V.; Wilson, R.K.; Monk, B.C.; Tyndall, J.D.A. Structural Insights into Binding of the Antifungal Drug Fluconazole to Saccharomyces cerevisiae Lanosterol 14α-Demethylase. Antimicrob. Agents Chemother. 2015, 59, 4982–4989. [Google Scholar] [CrossRef]
- Sabatelli, F.; Patel, R.; Mann, P.A.; Mendrick, C.A.; Norris, C.C.; Hare, R.; Loebenberg, D.; Black, T.A.; McNicholas, P.M. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 2006, 50, 2009–2015. [Google Scholar] [CrossRef]
- Morris, M.I. Posaconazole: A new oral antifungal agent with an expanded spectrum of activity. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2009, 66, 225–236. [Google Scholar] [CrossRef]
- Schmitt-Hoffmann, A.; Roos, B.; Heep, M.; Schleimer, M.; Weidekamm, E.; Brown, T.; Roehrle, M.; Beglinger, C. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 2006, 50, 279–285. [Google Scholar] [CrossRef]
- Jović, Z.; Janković, S.M.; Ružić Zečević, D.; Milovanović, D.; Stefanović, S.; Folić, M.; Milovanović, J.; Kostić, M. Clinical Pharmacokinetics of Second-Generation Triazoles for the Treatment of Invasive Aspergillosis and Candidiasis. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 139–157. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Mellado, E.; Garcia-Effron, G.; Rodriguez-Tudela, J.L. In vitro activities of ravuconazole and four other antifungal agents against fluconazole-resistant or -susceptible clinical yeast isolates. Antimicrob. Agents Chemother. 2004, 48, 3107–3111. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Hollis, R.J.; Jones, R.N. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: Report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 2002, 46, 1032–1037. [Google Scholar] [CrossRef]
- Shimoyama, H.; Yo, A.; Sei, Y.; Kuwano, Y. Treatment Outcome with Fosravuconazole for Onychomycosis. Mycopathologia 2021, 186, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Tsubouchi, I.; Okubo, A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: A multicenter, double-blind, randomized phase III study. J. Dermatol. 2018, 45, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- DNDi. A Randomized, Double Blind Phase II Proof-of-Concept Superiority Trial of Fosravuconazole 200 mg or 300 mg Weekly Dose Versus Itraconazole 400 mg Daily, All Three Arms in Combination with Surgery, in Patient with Eumycetoma in Sudan. Available online: http://dndi.org/research-development/portfolio/fosravuconazole (accessed on 28 June 2024).
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect. Dis. 2020, 7, ofaa016. [Google Scholar] [CrossRef]
- Hoenigl, M.; Sprute, R.; Egger, M.; Arastehfar, A.; Cornely, O.A.; Krause, R.; Lass-Flörl, C.; Prattes, J.; Spec, A.; Thompson, G.R., 3rd; et al. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs 2021, 81, 1703–1729. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Zagaliotis, P.; Walsh, T.J. Novel antifungal agents in clinical trials. F1000Research 2021, 10, 507. [Google Scholar] [CrossRef]
- Chang, Y.L.; Yu, S.J.; Heitman, J.; Wellington, M.; Chen, Y.L. New facets of antifungal therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef]
- McCarty, T.P.; Pappas, P.G. Antifungal Pipeline. Front. Cell. Infect. Microbiol. 2021, 11, 732223. [Google Scholar] [CrossRef]
- Watanabe, N.A.; Miyazaki, M.; Horii, T.; Sagane, K.; Tsukahara, K.; Hata, K. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother 2012, 56, 960–971. [Google Scholar] [CrossRef]
- Miyazaki, M.; Horii, T.; Hata, K.; Watanabe, N.A.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; Inoue, S.; Matsukura, M.; et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother 2011, 55, 4652–4658. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Hata, K.; Jones, R.N.; Messer, S.A.; Moet, G.J.; Castanheira, M. In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Candida spp. as determined by CLSI broth microdilution method. Diagn. Microbiol. Infect. Dis. 2011, 71, 167–170. [Google Scholar] [CrossRef]
- Wring, S.A.; Randolph, R.; Park, S.; Abruzzo, G.; Chen, Q.; Flattery, A.; Garrett, G.; Peel, M.; Outcalt, R.; Powell, K.; et al. Preclinical Pharmacokinetics and Pharmacodynamic Target of SCY-078, a First-in-Class Orally Active Antifungal Glucan Synthesis Inhibitor, in Murine Models of Disseminated Candidiasis. Antimicrob. Agents Chemother. 2017, 61, e02068-16. [Google Scholar] [CrossRef] [PubMed]
- Schell, W.A.; Jones, A.M.; Borroto-Esoda, K.; Alexander, B.D. Antifungal Activity of SCY-078 and Standard Antifungal Agents against 178 Clinical Isolates of Resistant and Susceptible Candida Species. Antimicrob. Agents Chemother. 2017, 61, e01102-17. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Motyl, M.R.; Jones, R.N.; Castanheira, M. Activity of MK-3118, a new oral glucan synthase inhibitor, tested against Candida spp. by two international methods (CLSI and EUCAST). J. Antimicrob. Chemother. 2013, 68, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ortigosa, C.; Paderu, P.; Motyl, M.R.; Perlin, D.S. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida Species and Aspergillus species isolates. Antimicrob. Agents Chemother. 2014, 58, 1248–1251. [Google Scholar] [CrossRef]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef]
- Berkow, E.L.; Angulo, D.; Lockhart, S.R. In Vitro Activity of a Novel Glucan Synthase Inhibitor, SCY-078, against Clinical Isolates of Candida auris. Antimicrob. Agents Chemother. 2017, 61, e00435-17. [Google Scholar] [CrossRef]
- Jiménez-Ortigosa, C.; Perez, W.B.; Angulo, D.; Borroto-Esoda, K.; Perlin, D.S. De Novo Acquisition of Resistance to SCY-078 in Candida glabrata Involves FKS Mutations That both Overlap and Are Distinct from Those Conferring Echinocandin Resistance. Antimicrob. Agents Chemother. 2017, 61, e00833-17. [Google Scholar] [CrossRef]
- Krishnan, B.R.; James, K.D.; Polowy, K.; Bryant, B.J.; Vaidya, A.; Smith, S.; Laudeman, C.P. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J. Antibiot. 2017, 70, 130–135. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Jones, R.N.; Castanheira, M. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J. Antimicrob. Chemother. 2016, 71, 2868–2873. [Google Scholar] [CrossRef]
- Ong, V.; Hough, G.; Schlosser, M.; Bartizal, K.; Balkovec, J.M.; James, K.D.; Krishnan, B.R. Preclinical Evaluation of the Stability, Safety, and Efficacy of CD101, a Novel Echinocandin. Antimicrob. Agents Chemother. 2016, 60, 6872–6879. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Activity of CD101, a long-acting echinocandin, against clinical isolates of Candida auris. Diagn. Microbiol. Infect. Dis. 2018, 90, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.G.; Hull, C.M.; Parker, J.E.; Garvey, E.P.; Hoekstra, W.J.; Moore, W.R.; Schotzinger, R.J.; Kelly, D.E.; Kelly, S.L. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob. Agents Chemother. 2014, 58, 7121–7127. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Lockhart, S.R.; Najvar, L.K.; Berkow, E.L.; Jaramillo, R.; Olivo, M.; Garvey, E.P.; Yates, C.M.; Schotzinger, R.J.; Catano, G.; et al. The Fungal Cyp51-Specific Inhibitor VT-1598 Demonstrates In Vitro and In Vivo Activity against Candida auris. Antimicrob. Agents Chemother. 2019, 63, e02233-18. [Google Scholar] [CrossRef]
- Schell, W.A.; Jones, A.M.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Alexander, B.D. Fungal CYP51 Inhibitors VT-1161 and VT-1129 Exhibit Strong In Vitro Activity against Candida glabrata and C. krusei Isolates Clinically Resistant to Azole and Echinocandin Antifungal Compounds. Antimicrob. Agents Chemother. 2017, 61, e01817-16. [Google Scholar] [CrossRef]
- Gu, K.; Spitz, R.; Hammett, E.; Jaunarajs, A.; Ghazaryan, V.; Garvey, E.P.; Degenhardt, T. Safety and pharmacokinetics of antifungal agent VT-1598 and its primary metabolite, VT-11134, in healthy adult subjects: Phase 1, first-in-human, randomized, double-blind, placebo-controlled study of single-ascending oral doses of VT-1598. Med. Mycol. 2024, 62, myae032. [Google Scholar] [CrossRef]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58 (Suppl. S2), 2–13. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Perfect, J.R. Resistance to Antifungal Agents: Mechanisms and Clinical Impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antimicrobial Resistance in Candida. Available online: https://www.cdc.gov/candidiasis/antimicrobial-resistance/?CDC_AAref_Val=https://www.cdc.gov/fungal/diseases/candidiasis/antifungal-resistant.html (accessed on 13 February 2023).
- Vallabhaneni, S.; Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Schaffner, W.; Beldavs, Z.G.; Derado, G.; Pham, C.D.; Lockhart, S.R.; Smith, R.M. Epidemiology and Risk Factors for Echinocandin Nonsusceptible Candida glabrata Bloodstream Infections: Data from a Large Multisite Population-Based Candidemia Surveillance Program, 2008–2014. Open Forum Infect. Dis. 2015, 2, ofv163. [Google Scholar] [CrossRef]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 1724–1732. [Google Scholar] [CrossRef]
- Coste, A.T.; Kritikos, A.; Li, J.; Khanna, N.; Goldenberger, D.; Garzoni, C.; Zehnder, C.; Boggian, K.; Neofytos, D.; Riat, A.; et al. Emerging echinocandin-resistant Candida albicans and glabrata in Switzerland. Infection 2020, 48, 761–766. [Google Scholar] [CrossRef]
- Papp, C.; Kocsis, K.; Tóth, R.; Bodai, L.; Willis, J.R.; Ksiezopolska, E.; Lozoya-Pérez, N.E.; Vágvölgyi, C.; Montes, H.M.; Gabaldón, T.; et al. Echinocandin-Induced Microevolution of Candida parapsilosis Influences Virulence and Abiotic Stress Tolerance. mSphere 2018, 3, e00547-18. [Google Scholar] [CrossRef] [PubMed]
- Morio, F.; Jensen, R.H.; Le Pape, P.; Arendrup, M.C. Molecular basis of antifungal drug resistance in yeasts. Int. J. Antimicrob. Agents 2017, 50, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob. Agents Chemother. 2018, 62, e00238-18. [Google Scholar] [CrossRef] [PubMed]
- Ostrowsky, B.; Greenko, J.; Adams, E.; Quinn, M.; O’Brien, B.; Chaturvedi, V.; Berkow, E.; Vallabhaneni, S.; Forsberg, K.; Chaturvedi, S.; et al. Candida auris Isolates Resistant to Three Classes of Antifungal Medications. MMWR Morb. Mortal. Wkly. Rep. 2019, 69, 6–9. [Google Scholar] [CrossRef]
- Denardi, L.B.; Oliveira, V.; de Jesus, F.P.K.; Dalla-Lana, B.H.; Santurio, J.M.; Zanette, R.A.; Alves, S.H. In vitro interactions of azoles and echinocandins against clinical strains of Aspergillus flavus. Med. Mycol. 2018, 56, 1006–1011. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Alves, D.F.; Henriques, M. Combination of Posaconazole and Amphotericin B in the Treatment of Candida glabrata Biofilms. Microorganisms 2018, 6, 123. [Google Scholar] [CrossRef]
- Keniya, M.V.; Fleischer, E.; Klinger, A.; Cannon, R.D.; Monk, B.C. Inhibitors of the Candida albicans Major Facilitator Superfamily Transporter Mdr1p Responsible for Fluconazole Resistance. PLoS ONE 2015, 10, e0126350. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar]
- Tang, J.; Wennerberg, K.; Aittokallio, T. What is synergy? The Saariselkä agreement revisited. Front. Pharmacol. 2015, 6, 181. [Google Scholar] [CrossRef]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; MacDougall, C.; Ostrosky-Zeichner, L.; Perfect, J.R.; Rex, J.H. Combination antifungal therapy. Antimicrob. Agents Chemother. 2004, 48, 693–715. [Google Scholar] [CrossRef] [PubMed]
- Lederer, S.; Dijkstra, T.M.H.; Heskes, T. Additive Dose Response Models: Defining Synergy. Front. Pharmacol. 2019, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Niimi, M.; Niimi, K.; Holmes, A.R.; Yates, J.E.; Decottignies, A.; Monk, B.C.; Goffeau, A.; Cannon, R.D. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 2001, 45, 3366–3374. [Google Scholar] [CrossRef]
- Niimi, M.; Niimi, K.; Takano, Y.; Holmes, A.R.; Fischer, F.J.; Uehara, Y.; Cannon, R.D. Regulated overexpression of CDR1 in Candida albicans confers multidrug resistance. J. Antimicrob. Chemother. 2004, 54, 999–1006. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- National Institutes of Health Office of AIDS Research. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/what-start-initial-combination-regimens (accessed on 8 August 2023).
- Mikulska, M.; Sepulcri, C.; Dentone, C.; Magne, F.; Balletto, E.; Baldi, F.; Labate, L.; Russo, C.; Mirabella, M.; Magnasco, L.; et al. Triple Combination Therapy with 2 Antivirals and Monoclonal Antibodies for Persistent or Relapsed Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Immunocompromised Patients. Clin. Infect. Dis. 2023, 77, 280–286. [Google Scholar] [CrossRef]
- Day, J.N.; Chau, T.T.; Lalloo, D.G. Combination antifungal therapy for cryptococcal meningitis. N. Engl. J. Med. 2013, 368, 2522–2523. [Google Scholar] [CrossRef]
- Chang, C.C.; Harrison, T.S.; Bicanic, T.A.; Chayakulkeeree, M.; Sorrell, T.C.; Warris, A.; Hagen, F.; Spec, A.; Oladele, R.; Govender, N.P.; et al. Global guideline for the diagnosis and management of cryptococcosis: An initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect. Dis. 2024, 24, e495–e512. [Google Scholar] [CrossRef]
- Tugume, L.; Ssebambulidde, K.; Kasibante, J.; Ellis, J.; Wake, R.M.; Gakuru, J.; Lawrence, D.S.; Abassi, M.; Rajasingham, R.; Meya, D.B.; et al. Cryptococcal meningitis. Nat. Rev. Dis. Primers 2023, 9, 62. [Google Scholar] [CrossRef]
- Marr, K.A.; Boeckh, M.; Carter, R.A.; Kim, H.W.; Corey, L. Combination Antifungal Therapy for Invasive Aspergillosis. Clin. Infect. Dis. 2004, 39, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Schlamm, H.T.; Herbrecht, R.; Rottinghaus, S.T.; Bow, E.J.; Cornely, O.A.; Heinz, W.J.; Jagannatha, S.; Koh, L.P.; Kontoyiannis, D.P.; et al. Combination antifungal therapy for invasive aspergillosis: A randomized trial. Ann. Intern. Med. 2015, 162, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Cornely, O.A.; Busca, A.; Caira, M.; Cesaro, S.; Gasbarrino, C.; Girmenia, C.; Heinz, W.J.; Herbrecht, R.; Lass-Flörl, C.; et al. Combined antifungal approach for the treatment of invasive mucormycosis in patients with hematologic diseases: A report from the SEIFEM and FUNGISCOPE registries. Haematologica 2013, 98, e127–e130. [Google Scholar] [CrossRef] [PubMed]
- Stenkiewicz-Witeska, J.S.; Ene, I.V. Azole potentiation in Candida species. PLoS Pathog. 2023, 19, e1011583. [Google Scholar] [CrossRef]
- Bandara, N.; Samaranayake, L. Emerging and future strategies in the management of recalcitrant Candida auris. Med Mycol. 2022, 60, myac008. [Google Scholar] [CrossRef]
- Jangir, P.; Kalra, S.; Tanwar, S.; Bari, V.K. Azole resistance in Candida auris: Mechanisms and combinatorial therapy. Apmis 2023, 131, 442–462. [Google Scholar] [CrossRef]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef]
- Schneider, E.; Hunke, S. ATP-binding-cassette (ABC) transport systems: Functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol. Rev. 1998, 22, 1–20. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Gaur, M.; Choudhury, D.; Prasad, R. Complete inventory of ABC proteins in human pathogenic yeast, Candida albicans. J. Mol. Microbiol. Biotechnol. 2005, 9, 3–15. [Google Scholar] [CrossRef]
- Higgins, C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Taglicht, D.; Michaelis, S. Saccharomyces cerevisiae ABC proteins and their relevance to human health and disease. Methods Enzymol. 1998, 292, 130–162. [Google Scholar] [CrossRef]
- Damas, J.M.; Oliveira, A.S.; Baptista, A.M.; Soares, C.M. Structural consequences of ATP hydrolysis on the ABC transporter NBD dimer: Molecular dynamics studies of HlyB. Protein Sci. A Publ. Protein Soc. 2011, 20, 1220–1230. [Google Scholar] [CrossRef]
- Walker, J.E.; Saraste, M.; Runswick, M.J.; Gay, N.J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. Embo J. 1982, 1, 945–951. [Google Scholar] [CrossRef]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. The “LSGGQ” motif in each nucleotide-binding domain of human P-glycoprotein is adjacent to the opposing walker A sequence. J. Biol. Chem. 2002, 277, 41303–41306. [Google Scholar] [CrossRef]
- Law, C.J.; Maloney, P.C.; Wang, D.N. Ins and outs of major facilitator superfamily antiporters. Annu. Rev. Microbiol. 2008, 62, 289–305. [Google Scholar] [CrossRef]
- Redhu, A.K.; Shah, A.H.; Prasad, R. MFS transporters of Candida species and their role in clinical drug resistance. FEMS Yeast Res. 2016, 16, fow043. [Google Scholar] [CrossRef]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H. Major Facilitator Superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar] [CrossRef]
- Nelissen, B.; De Wachter, R.; Goffeau, A. Classification of all putative permeases and other membrane plurispanners of the major facilitator superfamily encoded by the complete genome of Saccharomyces cerevisiae. FEMS Microbiol. Rev. 1997, 21, 113–134. [Google Scholar] [CrossRef]
- Saier, M.H., Jr.; Paulsen, I.T. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 2001, 12, 205–213. [Google Scholar] [CrossRef]
- Gaur, M.; Puri, N.; Manoharlal, R.; Rai, V.; Mukhopadhayay, G.; Choudhury, D.; Prasad, R. MFS transportome of the human pathogenic yeast Candida albicans. BMC Genom. 2008, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, S.; Michel, S.; Morschhäuser, J. Targeted gene disruption in Candida albicans wild-type strains: The role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol. Microbiol. 2000, 36, 856–865. [Google Scholar] [CrossRef]
- Toepfer, S.; Lackner, M.; Keniya, M.V.; Monk, B.C. Functional Expression of Recombinant Candida auris Proteins in Saccharomyces cerevisiae Enables Azole Susceptibility Evaluation and Drug Discovery. J. Fungi 2023, 9, 168. [Google Scholar] [CrossRef]
- Niimi, K.; Harding, D.R.; Parshot, R.; King, A.; Lun, D.J.; Decottignies, A.; Niimi, M.; Lin, S.; Cannon, R.D.; Goffeau, A.; et al. Chemosensitization of fluconazole resistance in Saccharomyces cerevisiae and pathogenic fungi by a D-octapeptide derivative. Antimicrob. Agents Chemother. 2004, 48, 1256–1271. [Google Scholar] [CrossRef]
- Hayama, K.; Ishibashi, H.; Ishijima, S.A.; Niimi, K.; Tansho, S.; Ono, Y.; Monk, B.C.; Holmes, A.R.; Harding, D.R.; Cannon, R.D.; et al. A D-octapeptide drug efflux pump inhibitor acts synergistically with azoles in a murine oral candidiasis infection model. FEMS Microbiol. Lett. 2012, 328, 130–137. [Google Scholar] [CrossRef]
- Yong, J.; Zu, R.; Huang, X.; Ge, Y.; Li, Y. Synergistic Effect of Berberine Hydrochloride and Fluconazole Against Candida albicans Resistant Isolates. Front. Microbiol. 2020, 11, 1498. [Google Scholar] [CrossRef]
- Holmes, A.R.; Keniya, M.V.; Ivnitski-Steele, I.; Monk, B.C.; Lamping, E.; Sklar, L.A.; Cannon, R.D. The Monoamine Oxidase A Inhibitor Clorgyline Is a Broad-Spectrum Inhibitor of Fungal ABC and MFS Transporter Efflux Pump Activities Which Reverses the Azole Resistance of Candida albicans and Candida glabrata Clinical Isolates. Antimicrob. Agents Chemother. 2012, 56, 1508–1515. [Google Scholar] [CrossRef]
- Toepfer, S.; Lackner, M.; Keniya, M.V.; Zenz, L.M.; Friemert, M.; Bracher, F.; Monk, B.C. Clorgyline Analogs Synergize with Azoles against Drug Efflux in Candida auris. J. Fungi 2023, 9, 663. [Google Scholar] [CrossRef]
- Lu, M.; Yu, C.; Cui, X.; Shi, J.; Yuan, L.; Sun, S. Gentamicin synergises with azoles against drug-resistant Candida albicans. Int. J. Antimicrob. Agents 2018, 51, 107–114. [Google Scholar] [CrossRef]
- Thakur, J.K.; Arthanari, H.; Yang, F.; Pan, S.J.; Fan, X.; Breger, J.; Frueh, D.P.; Gulshan, K.; Li, D.K.; Mylonakis, E.; et al. A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature 2008, 452, 604–609. [Google Scholar] [CrossRef]
- Blumberg, B.; Sabbagh, W., Jr.; Juguilon, H.; Bolado, J., Jr.; van Meter, C.M.; Ong, E.S.; Evans, R.M. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998, 12, 3195–3205. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, G.; Heidrich, J.; Svensson, K.; Asman, M.; Jendeberg, L.; Sydow-Bäckman, M.; Ohlsson, R.; Postlind, H.; Blomquist, P.; Berkenstam, A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc. Natl. Acad. Sci. USA 1998, 95, 12208–12213. [Google Scholar] [CrossRef] [PubMed]
- Bourbon, H.M. Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nucleic Acids Res. 2008, 36, 3993–4008. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Suetaka, S.; Hayashi, Y.; Arai, M. Rational peptide design for inhibition of the KIX–MLL interaction. Sci. Rep. 2023, 13, 6330. [Google Scholar] [CrossRef]
- Monk, B.C.; Perlin, D.S. Fungal Plasma Membrane Proton Pumps as Promising New Antifungal Targets. Crit. Rev. Microbiol. 1994, 20, 209–223. [Google Scholar] [CrossRef]
- Monk, B.C.; Mason, A.B.; Abramochkin, G.; Haber, J.E.; Seto-Young, D.; Perlin, D.S. The yeast plasma membrane proton pumping ATPase is a viable antifungal target. I. Effects of the cysteine-modifying reagent omeprazole. Biochim. Biophys. Acta 1995, 1239, 81–90. [Google Scholar] [CrossRef]
- Seto-Young, D.; Monk, B.; Mason, A.B.; Perlin, D.S. Exploring an antifungal target in the plasma membrane H+-ATPase of fungi. Biochim. Biophys. Acta (BBA)—Biomembr. 1997, 1326, 249–256. [Google Scholar] [CrossRef]
- Monk, B.C.; Niimi, K.; Lin, S.; Knight, A.; Kardos, T.B.; Cannon, R.D.; Parshot, R.; King, A.; Lun, D.; Harding, D.R.K. Surface-active fungicidal D-peptide inhibitors of the plasma membrane proton pump that block azole resistance. Antimicrob. Agents Chemother. 2005, 49, 57–70. [Google Scholar] [CrossRef]
- Fakhim, H.; Chowdhary, A.; Prakash, A.; Vaezi, A.; Dannaoui, E.; Meis, J.F.; Badali, H. In Vitro Interactions of Echinocandins with Triazoles against Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2017, 61, e01056-17. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Deshpande, L.M.; Rhomberg, P.R.; Utt, E.A.; Castanheira, M. Evaluation of Synergistic Activity of Isavuconazole or Voriconazole plus Anidulafungin and the Occurrence and Genetic Characterization of Candida auris Detected in a Surveillance Program. Antimicrob. Agents Chemother. 2021, 65, e02031-20. [Google Scholar] [CrossRef]
- Sun, B.; Liu, W.; Wang, Q.; Liu, Y.; Yu, S.; Liu, M.; Han, J. Design, Synthesis, and Activity Evaluation of Novel Dual-Target Inhibitors with Antifungal and Immunoregulatory Properties. J. Med. Chem. 2023, 66, 13007–13027. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Q.; Yu, S.; Liu, M.; Han, J.; Sun, B. Construction and Evaluation of Novel Dual-function Antifungal Inhibitors and Covalent Organic Framework Carriers Based on the Infection Microenvironment. J. Med. Chem. 2023, 66, 13838–13857. [Google Scholar] [CrossRef]
- Yu, S.; He, Y.-Q.; Liu, Y.; Ji, S.; Wang, Y.; Sun, B. Construction and Activity Evaluation of Novel Bifunctional Inhibitors and a COF Carrier Based on a Fungal Infection Microenvironment. J. Med. Chem. 2024, 67, 8420–8444. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, L.; Cong, Z.; Jiang, W.; Xiao, X.; Xie, J.; Luo, Z.; Chen, S.; Wu, Y.; Xue, X.; et al. A dual-targeting antifungal is effective against multidrug-resistant human fungal pathogens. Nat. Microbiol. 2024, 9, 1325–1339. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Li, X.; Abutaleb, N.S.; Mohammad, H.; Seleem, M.N. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int. J. Antimicrob. Agents 2018, 52, 754–761. [Google Scholar] [CrossRef]
- Eyler, R.F.; Shvets, K. Clinical Pharmacology of Antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Salama, E.A.; Lanman, N.A.; Hazbun, T.R.; Seleem, M.N. Potent Synergistic Interactions between Lopinavir and Azole Antifungal Drugs against Emerging Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2020, 65, e00684-20. [Google Scholar] [CrossRef]
- Salama, E.A.; Eldesouky, H.E.; Elgammal, Y.; Abutaleb, N.S.; Seleem, M.N. Lopinavir and ritonavir act synergistically with azoles against Candida auris in vitro and in a mouse model of disseminated candidiasis. Int. J. Antimicrob. Agents 2023, 62, 106906. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, L.; Yao, Z.; Gao, L.; Yang, J.; Zeng, T. In Vitro and In Vivo Interactions of TOR Inhibitor AZD8055 and Azoles against Pathogenic Fungi. Microbiol. Spectr. 2022, 10, e0200721. [Google Scholar] [CrossRef] [PubMed]
- Chresta, C.M.; Davies, B.R.; Hickson, I.; Harding, T.; Cosulich, S.; Critchlow, S.E.; Vincent, J.P.; Ellston, R.; Jones, D.; Sini, P.; et al. AZD8055 Is a Potent, Selective, and Orally Bioavailable ATP-Competitive Mammalian Target of Rapamycin Kinase Inhibitor with In Vitro and In Vivo Antitumor Activity. Cancer Res. 2010, 70, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Eldesouky, H.E.; Lanman, N.A.; Hazbun, T.R.; Seleem, M.N. Aprepitant, an antiemetic agent, interferes with metal ion homeostasis of Candida auris and displays potent synergistic interactions with azole drugs. Virulence 2020, 11, 1466–1481. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Salama, E.A.; Hazbun, T.R.; Mayhoub, A.S.; Seleem, M.N. Ospemifene displays broad-spectrum synergistic interactions with itraconazole through potent interference with fungal efflux activities. Sci. Rep. 2020, 10, 6089. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Mellado, E.; Garcia-Effron, G.; Alcázar-Fuoli, L.; Melchers, W.J.; Verweij, P.E.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother 2007, 51, 1897–1904. [Google Scholar] [CrossRef]
- Sharma, C.; Hagen, F.; Moroti, R.; Meis, J.F.; Chowdhary, A. Triazole-resistant Aspergillus fumigatus harbouring G54 mutation: Is it de novo or environmentally acquired? J. Glob. Antimicrob. Resist. 2015, 3, 69–74. [Google Scholar] [CrossRef]
- van der Linden, J.W.; Camps, S.M.; Kampinga, G.A.; Arends, J.P.; Debets-Ossenkopp, Y.J.; Haas, P.J.; Rijnders, B.J.; Kuijper, E.J.; van Tiel, F.H.; Varga, J.; et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, 513–520. [Google Scholar] [CrossRef]
- Khodavaisy, S.; Gharehbolagh, S.A.; Abdorahimi, M.; Rezaie, S.; Ahmadikia, K.; Badali, H.; Meis, J.F.; Mahmoudi, S. In vitro combination of antifungal drugs with tacrolimus (FK506) holds promise against clinical Candida species, including Candida auris. Med. Mycol. 2023, 61, myad069. [Google Scholar] [CrossRef]
- Hertogs, K.; de Béthune, M.P.; Miller, V.; Ivens, T.; Schel, P.; Van Cauwenberge, A.; Van Den Eynde, C.; Van Gerwen, V.; Azijn, H.; Van Houtte, M.; et al. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob Agents Chemother 1998, 42, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gamez, S.; Hill, A.L.; Rosenbloom, D.I.; Petrov, D.A.; Nowak, M.A.; Pennings, P.S. Imperfect drug penetration leads to spatial monotherapy and rapid evolution of multidrug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, E2874–E2883. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.F.; Kanyama, C.; Heyderman, R.S.; Loyse, A.; Kouanfack, C.; Chanda, D.; Mfinanga, S.; Temfack, E.; Lakhi, S.; Lesikari, S.; et al. Antifungal Combinations for Treatment of Cryptococcal Meningitis in Africa. N. Engl. J. Med. 2018, 378, 1004–1017. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, Y.; Sun, H.; Ma, J.; Li, X.; Han, X.; Fang, Z.; Tan, J.; Qiu, Y.; Qu, T.; et al. Cryo-EM structures of Candida albicans Cdr1 reveal azole-substrate recognition and inhibitor blocking mechanisms. Nat. Commun. 2024, 15, 7722. [Google Scholar] [CrossRef]
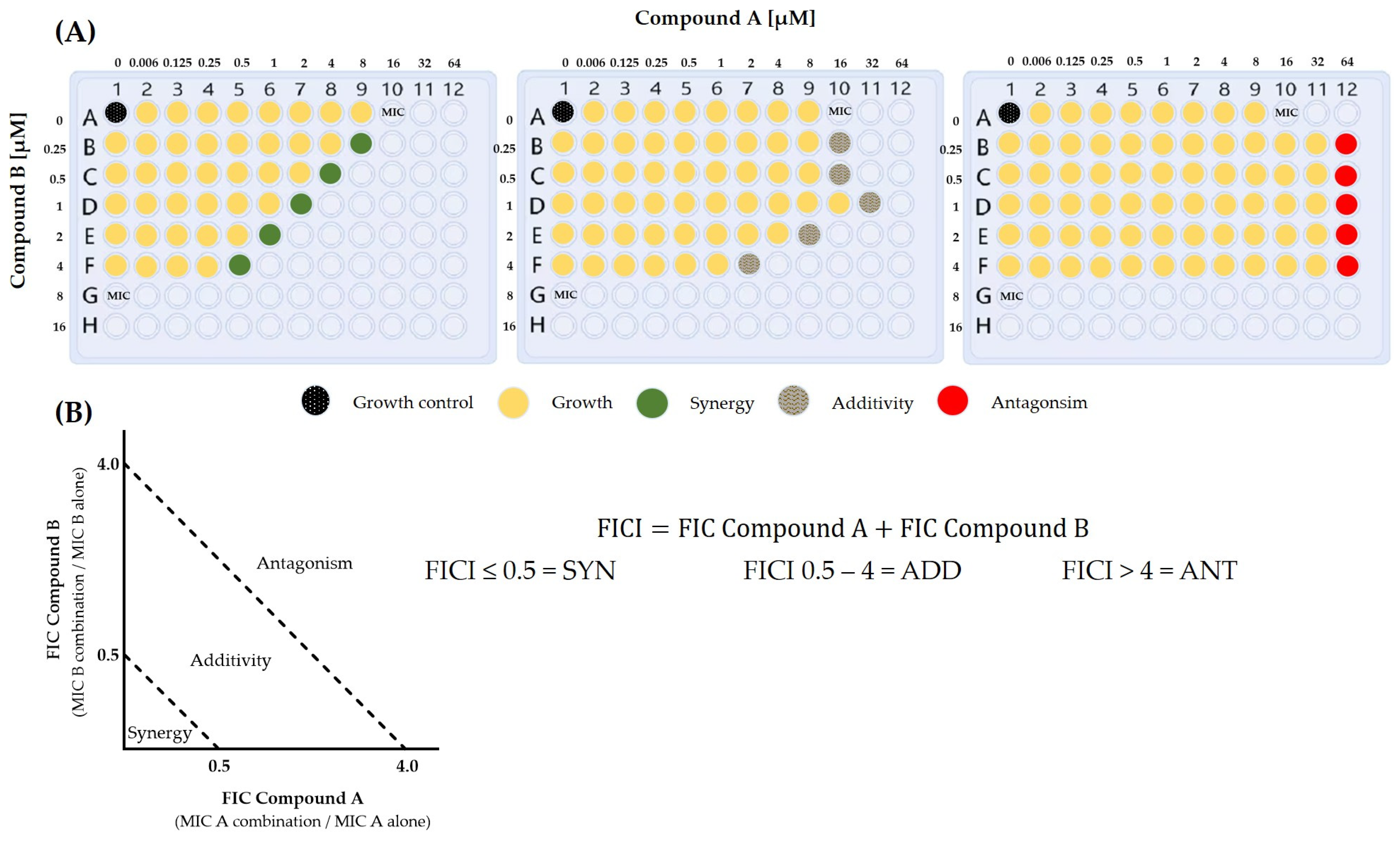
| Antifungal Class | Antifungal | Mode of Action | Information | Source |
|---|---|---|---|---|
| N-phosphonooxymethyl manogepix prodrug | Fosmanogepix (APX001) | The active form manogepix targets the fungal enzyme Gwt1. Cell wall integrity is impaired and fungal growth inhibited | Active against Candida except P. kudriavzevii | [106,107,108] |
| Triterpenoid | Ibrexafungerp (SCY-078, MK-3118) | Inhibitor of β-(1,3)-d-glucan synthase, like the echinocandins, but with different enzyme binding site | Active against Candida including C. auris and N. glabratus | [109,110,111,112,113,114,115] |
| Echinocandin | Rezafungin (CD101) | Inhibitor of β-(1,3)-d-glucan synthase | Active against Candida including C. auris | [116,117,118,119] |
| Tetrazoles | Oteseconazole (VT-1161), VT-1598 *, VT-11134 *, VT-1129 † | Disruption of the sterol biosynthetic pathway by inhibition of CYP51 | Active against Candida including C. auris, and FLC and echinocandin resistant N. glabratus | [120,121,122,123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toepfer, S.; Keniya, M.V.; Lackner, M.; Monk, B.C. Azole Combinations and Multi-Targeting Drugs That Synergistically Inhibit Candidozyma auris. J. Fungi 2024, 10, 698. https://doi.org/10.3390/jof10100698
Toepfer S, Keniya MV, Lackner M, Monk BC. Azole Combinations and Multi-Targeting Drugs That Synergistically Inhibit Candidozyma auris. Journal of Fungi. 2024; 10(10):698. https://doi.org/10.3390/jof10100698
Chicago/Turabian StyleToepfer, Stephanie, Mikhail V. Keniya, Michaela Lackner, and Brian C. Monk. 2024. "Azole Combinations and Multi-Targeting Drugs That Synergistically Inhibit Candidozyma auris" Journal of Fungi 10, no. 10: 698. https://doi.org/10.3390/jof10100698
APA StyleToepfer, S., Keniya, M. V., Lackner, M., & Monk, B. C. (2024). Azole Combinations and Multi-Targeting Drugs That Synergistically Inhibit Candidozyma auris. Journal of Fungi, 10(10), 698. https://doi.org/10.3390/jof10100698








