Nerol as a Novel Antifungal Agent: In Vitro Inhibitory Effects on Fusarium oxysporum, Pestalotiopsis neglecta, and Valsa mali and Its Potential Mechanisms against F. oxysporum
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Microbiological Media
2.2. Pathogens
2.3. Effects of Nerol on Mycelial Growth
2.4. F. oxysporum and Nerol Interaction
2.4.1. Mycelial Biomass
2.4.2. Microconidia Germination
2.4.3. Scanning Electron Microscopy Observations
2.4.4. Membrane Integrity
Staining of Microconidia with Propidium Iodide (PI)
Measurement of Extracellular Relative Conductivity, Release of Soluble Proteins, and Na+/K+-ATPase Activity
Determination of H2O2 and MDA Concentration
2.4.5. Evaluation of Nerol’s Effect on Activity of F. oxysporum Enzyme
2.5. Data Analysis
3. Results
3.1. Effect of Nerol on Mycelial Growth of F. oxysporum, P. neglecta, and V. mali
3.2. F. oxysporum and Nerol Interaction
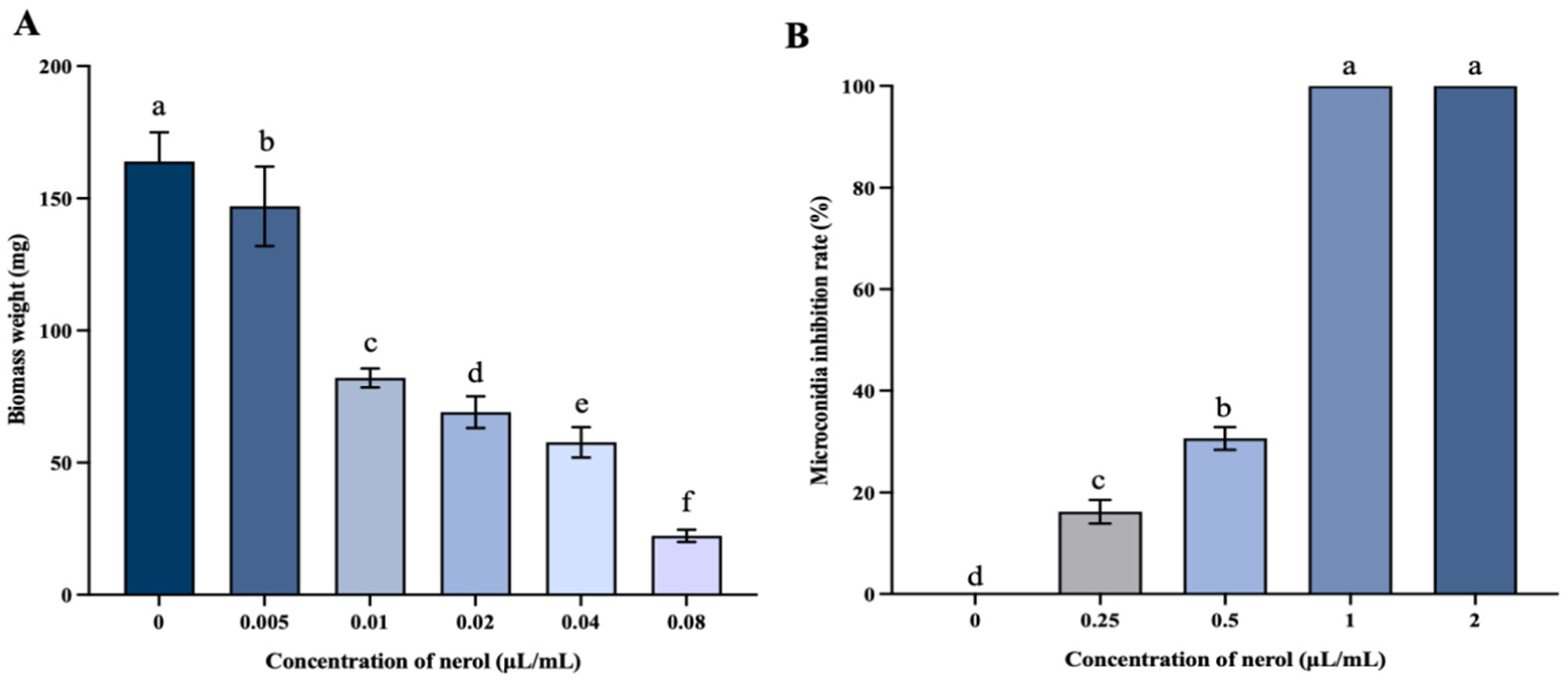
3.2.1. Mycelial Biomass and Microconidia Germination
3.2.2. Nerol Induces Morphological Changes and Abnormal Growth in F. oxysporum Mycelia
3.2.3. Nerol Affected the Permeability of Membrane in F. oxysporum
3.2.4. Disruption of Membrane Integrity by Nerol in F. oxysporum
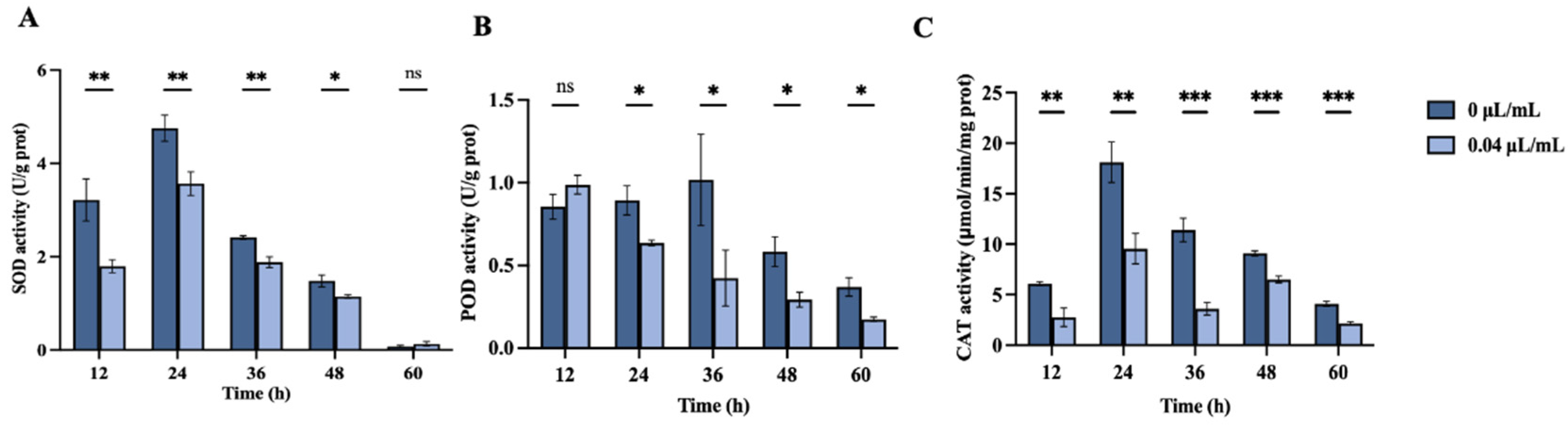
3.2.5. Impact of Nerol on Antioxidant Enzyme Activity in F. oxysporum
3.2.6. Effect of Nerol on PG, PL, and EG Activities of F. oxysporum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jangir, P.; Mehra, N.; Sharma, K.; Singh, N.; Rani, M.; Kapoor, R. Secreted in Xylem Genes: Drivers of Host Adaptation in Fusarium oxysporum. Front. Plant Sci. 2021, 12, 628611. [Google Scholar] [CrossRef] [PubMed]
- Edel-Hermann, V.; Lecomte, C. Current Status of Fusarium oxysporum Formae Speciales and Races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhai, L.; Jiang, Y.; Wang, Z.; He, L.; Song, F.; Wu, L. First report of Fusarium oxysporum and Fusarium solani causing root rot on trifoliate orange rootstock in China. Plant Dis. 2022, 107, 944, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, Y.X.; Zhang, Y.D.; Wang, S.R.; Zhang, G.C.; Yang, J. Phytic acid is a new substitutable plant-derived antifungal agent for the seedling blight of Pinus sylvestris var. mongolica caused by Fusarium oxysporum. Pestic. Biochem. Physiol. 2023, 191, 105341. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pinto, P.; Pajares, J.; Díez, J. Pathogenicity of Fusarium verticillioides and Fusarium oxysporum on Pinus nigra seedlings in northwest Spain. For. Pathol. 2008, 38, 78–82. [Google Scholar] [CrossRef]
- Dita, M.; Waalwijk, C.; Buddenhagen, I.; Souza Junior, M.; Kema, G. A molecular diagnostic for tropical race 4 of the banana fusarium wilt pathogen. Plant Pathol. 2010, 59, 348–357. [Google Scholar] [CrossRef]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The Consequence of Tree Pests and Diseases for Ecosystem Services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Zhang, F.; Zheng, D.; Chang, Y.; Xu, L.; Huang, L. Biocontrol activity of Bacillus velezensis D4 against apple Valsa canker. Biol. Control 2021, 163, 104760. [Google Scholar] [CrossRef]
- Hernández-Aparicio, F.; Lisón, P.; Rodrigo, I.; Bellés, J.M.; López-Gresa, M.P. Signaling in the Tomato Immunity against Fusarium oxysporum. Molecules 2021, 26, 1818. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Zhang, Y.; Chen, Y.; Zhou, H.; Zhang, G. Identification, Culture Characteristics and Whole-Genome Analysis of Pestalotiopsis neglecta Causing Black Spot Blight of Pinus sylvestris var. mongolica. J. Fungi 2023, 9, 564. [Google Scholar] [CrossRef]
- Tagne, A.; Mathur, S.B. First report of chlorotic spot of maize caused by Pestalotiopsis neglecta. Plant Pathol. 2001, 50, 791. [Google Scholar] [CrossRef]
- Bessho, H.; Tsuchiya, S.; Soejima, J. Screening methods of apple trees for resistance to Valsa canker. Euphytica 1994, 77, 15–18. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. Chapter Two—The Evolution of Fungicide Resistance. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 90, pp. 29–92. [Google Scholar]
- Percival, G.C.; Graham, S. The potential of resistance inducers and synthetic fungicide combinations for management of foliar diseases of nursery stock. Crop Prot. 2021, 145, 105636. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, R.; Gu, S.; Chen, K.; Li, J.; He, X.; Shang, S.; Song, Z.; Song, J. Discovery of Natural Rosin Derivatives Containing Oxime Ester Moieties as Potential Antifungal Agents to Control Tomato Gray Mold Caused by Botrytis cinerea. J. Agric. Food Chem. 2022, 70, 5551–5560. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.; Gurr, S.J. Fungi, fungicide discovery and global food security. Fungal Genet. Biol. 2020, 144, 103476. [Google Scholar] [CrossRef]
- Ali, A.; AlHussaini, K.I. Pesticides: Unintended Impact on the Hidden World of Gut Microbiota. Metabolites 2024, 14, 155. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a potential natural compound to manage plant diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef]
- Elshafie, H.; Mancini, E.; Camele, I.; De Martino, L.; De Feo, V. In vivo antifungal activity of two essential oils from Mediterranean plants against postharvest brown rot disease of peach fruit. Ind. Crops Prod. 2015, 66, 11–15. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sonker, N.; Singh, P. Efficacy of Some Essential Oils Against Aspergillus flavus with Special Reference to Lippia alba Oil an Inhibitor of Fungal Proliferation and Aflatoxin B1 Production in Green Gram Seeds during Storage. J. Food Sci. 2016, 81, M928–M934. [Google Scholar] [CrossRef]
- Quin, M.B.; Flynn, C.M.; Schmidt-Dannert, C. Traversing the fungal terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Stoyanova, A.S.; Denkova, Z.; Nikolova, R.; Geissler, M. Purity, Antimicrobial Activities and Olfactoric Evaluations of Geraniol/Nerol and Various of Their Derivatives. J. Essent. Oil Res. 2007, 19, 288–291. [Google Scholar] [CrossRef]
- Li, R.; Yao, B.; Zeng, H. Identification and Characterization of a Nerol Synthase in Fungi. J. Agric. Food Chem. 2024, 72, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Lapczynski, A.; Foxenberg, R.J.; Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on nerol. Food Chem. Toxicol. 2008, 46, S241–S244. [Google Scholar] [CrossRef] [PubMed]
- Coêlho, M.L.; Islam, M.T.; Laylson da Silva Oliveira, G.; Oliveira Barros de Alencar, M.V.; Victor de Oliveira Santos, J.; Campinho Dos Reis, A.; Oliveira Ferreira da Mata, A.M.; Correia Jardim Paz, M.F.; Docea, A.O.; Calina, D.; et al. Cytotoxic and Antioxidant Properties of Natural Bioactive Monoterpenes Nerol, Estragole, and 3,7-Dimethyl-1-Octanol. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 8002766. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Zhou, Z.; Xing, K.; Tessema, A.; Zeng, H.; Tian, J. Inhibitory effect of nerol against Aspergillus niger on grapes through a membrane lesion mechanism. Food Control 2015, 55, 54–61. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, K.; Chen, L.; Yan, R.; Qu, S.; Li, Y.-X.; Liu, M.; Zeng, H.; Tian, J. Activities of Nerol, a natural plant active ingredient, against Candida albicans in vitro and in vivo. Appl. Microbiol. Biotechnol. 2020, 104, 5039–5052. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Huang, T.; Yang, K.; Zhou, S.; Li, Y.; Tian, J. Antifungal effect of nerol via transcriptome analysis and cell growth repression in sweet potato spoilage fungi Ceratocystis fimbriata. Postharvest Biol. Technol. 2021, 171, 111343. [Google Scholar] [CrossRef]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Naturforschung. C J. Biosci. 2007, 62, 507–513. [Google Scholar] [CrossRef]
- Jing, Y.; Lian-Nan, L.; Xiao-Bo, Z.; Yue, W.; Bing, B.; Guo-Cai, Z.; Chuan-Shan, Z. Sodium pheophorbide a has photoactivated fungicidal activity against Pestalotiopsis neglecta. Pestic. Biochem. Physiol. 2019, 158, 25–31. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Wang, S.-R.; Li, T.; Zhang, G.-C.; Yang, J. Antifungal Activity of 6-Methylcoumarin against Valsa mali and Its Possible Mechanism of Action. J. Fungi 2023, 9, 5. [Google Scholar] [CrossRef]
- Badawy, M.E.; Rabea, E.I.; Taktak, N.E. Antimicrobial and inhibitory enzyme activity of N-(benzyl) and quaternary N-(benzyl) chitosan derivatives on plant pathogens. Carbohydr. Polym. 2014, 111, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Chen, T.; Ma, D.; Liu, J.; Xu, Y.; Tian, S. Inhibitory effects of methyl thujate on mycelial growth of Botrytis cinerea and possible mechanisms. Postharvest Biol. Technol. 2018, 142, 46–54. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- González-Ramírez, A.E.; González-Trujano, M.E.; Orozco-Suárez, S.A.; Alvarado-Vásquez, N.; López-Muñoz, F.J. Nerol alleviates pathologic markers in the oxazolone-induced colitis model. Eur. J. Pharmacol. 2016, 776, 81–89. [Google Scholar] [CrossRef] [PubMed]
- de Menezes-Filho, J.E.R.; de Souza, D.S.; Santos-Miranda, A.; Cabral, V.M.; Santos, J.N.A.; Cruz, J.D.S.; de Araujo, A.M.; de Vasconcelos, C.M.L. Nerol Attenuates Ouabain-Induced Arrhythmias. Evid. Based Complement. Altern. Med. 2019, 2019, 5935921. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Quispe, C.; Islam, M.A.; Ali, E.S.; Saha, S.; Asha, U.H.; Mondal, M.; Razis, A.F.A.; Sunusi, U.; Kamal, R.M.; et al. Effects of nerol on paracetamol-induced liver damage in Wistar albino rats. Biomed. Pharmacother. 2021, 140, 111732. [Google Scholar] [CrossRef]
- Pan, J.; Hao, X.; Yao, H.; Ge, K.; Ma, L.; Ma, W. Matrine inhibits mycelia growth of Botryosphaeria dothidea by affecting membrane permeability. J. For. Res. 2019, 30, 1105–1113. [Google Scholar] [CrossRef]
- Perveen, K.; Bukhari, N.A.; Al Masoudi, L.M.; Alqahtani, A.N.; Alruways, M.W.; Alkhattaf, F.S. Antifungal potential, chemical composition of Chlorella vulgaris and SEM analysis of morphological changes in Fusarium oxysporum. Saudi J. Biol. Sci. 2022, 29, 2501–2505. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Wang, W.; Feng, G.; Li, X.; Ruan, C.; Ming, J.; Zeng, K. Inhibition of Three Citrus Pathogenic Fungi by Peptide PAF56 Involves Cell Membrane Damage. Foods 2021, 10, 2031. [Google Scholar] [CrossRef]
- Ghosh, P.; Roy, A.; Hess, D.; Ghosh, A.; Das, S. Deciphering the mode of action of a mutant Allium sativum Leaf Agglutinin (mASAL), a potent antifungal protein on Rhizoctonia solani. BMC Microbiol. 2015, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Long, Y.; Yin, X.; Wang, W.; Zhang, R.; Mo, F.; Zhang, Z.; Chen, T.; Chen, J.; Wang, B.; et al. Antifungal activity and mechanism of tetramycin against Alternaria alternata, the soft rot causing fungi in kiwifruit. Pestic. Biochem. Physiol. 2023, 192, 105409. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shao, X.; Wei, Y.; Dai, K.; Xu, J.; Xu, F.; Wang, H. Transcriptome analysis of Botrytis cinerea in response to tea tree oil and its two characteristic components. Appl. Microbiol. Biotechnol. 2020, 104, 2163–2178. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, X.; Zhao, Y.; Xie, Y. The antifungal activity of o-vanillin against Aspergillus flavus via disrupting ergosterol biosynthesis and promoting oxidative stress, and an RNA-seq analysis thereof. LWT 2022, 164, 113635. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Y.; Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Quek, S.Y.; Yao, W. Antifungal effects of thymol and salicylic acid on cell membrane and mitochondria of Rhizopus stolonifer and their application in postharvest preservation of tomatoes. Food Chem. 2019, 285, 380–388. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.G. Role of calcium in reactive oxygen species-induced apoptosis in Candida albicans: An antifungal mechanism of antimicrobial peptide, PMAP-23. Free Radic. Res. 2019, 53, 8–17. [Google Scholar] [CrossRef]
- Lin, W.; Yuan, D.; Deng, Z.; Niu, B.; Chen, Q. The cellular and molecular mechanism of glutaraldehyde-didecyldimethylammonium bromide as a disinfectant against Candida albicans. J. Appl. Microbiol. 2019, 126, 102–112. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Li, W.; Yuan, S.; Sun, J.; Li, Q.; Jiang, W.; Cao, J. Ethyl p-coumarate exerts antifungal activity in vitro and in vivo against fruit Alternaria alternata via membrane-targeted mechanism. Int. J. Food Microbiol. 2018, 278, 26–35. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Xu, S.; Liu, L.; Xu, Y.; Feng, X.; Zhao, X.; Chen, Y. Inhibitory Effects of the Natural Product Esculetin on Phytophthora capsici and Its Possible Mechanism. Plant Dis. 2021, 105, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, J.; Wei, Y.; Han, P.; Dai, K.; Zou, X.; Jiang, S.; Xu, F.; Wang, H.; Sun, J.; et al. Tea tree oil controls brown rot in peaches by damaging the cell membrane of Monilinia fructicola. Postharvest Biol. Technol. 2021, 175, 111474. [Google Scholar] [CrossRef]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V.R. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kaur, S.; Ahuja, N.; Kohli, R.K. β-Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma 2013, 250, 691–700. [Google Scholar] [CrossRef]
- Maqsood, A.; Wu, H.; Kamran, M.; Altaf, H.; Mustafa, A.; Ahmar, S.; Hong, N.T.T.; Tariq, K.; He, Q.; Chen, J.-T. Variations in Growth, Physiology, and Antioxidative Defense Responses of Two Tomato (Solanum lycopersicum L.) Cultivars after Co-Infection of Fusarium oxysporum and Meloidogyne incognita. Agronomy 2020, 10, 159. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, N.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Yan, J.; Wu, H.; Shi, F.; Wang, H.; Chen, K.; Feng, J.; Jia, W. Antifungal activity screening for mint and thyme essential oils against Rhizopus stolonifer and their application in postharvest preservation of strawberry and peach fruits. J. Appl. Microbiol. 2021, 130, 1993–2007. [Google Scholar] [CrossRef]
- Tian, J.; Gan, Y.; Pan, C.; Zhang, M.; Wang, X.; Tang, X.; Peng, X. Nerol-induced apoptosis associated with the generation of ROS and Ca2+ overload in saprotrophic fungus Aspergillus flavus. Appl. Microbiol. Biotechnol. 2018, 102, 6659–6672. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Sun, Y.; Chen, Q.; Zhang, L.; Le, T. The antifungal effects of cinnamaldehyde against Aspergillus niger and its application in bread preservation. Food Chem. 2020, 317, 126405. [Google Scholar] [CrossRef]
- Sun, Q.; Shang, B.; Wang, L.; Lu, Z.; Liu, Y. Cinnamaldehyde inhibits fungal growth and aflatoxin B1 biosynthesis by modulating the oxidative stress response of Aspergillus flavus. Appl. Microbiol. Biotechnol. 2016, 100, 1355–1364. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Zeng, H.; Li, Z.; Zhang, P.; Tessema, A.; Peng, X. Efficacy and possible mechanisms of perillaldehyde in control of Aspergillus niger causing grape decay. Int. J. Food Microbiol. 2015, 202C, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Jaikua, W.; Kueakhai, P.; Chaithirayanon, K.; Tanomrat, R.; Wongwairot, S.; Riengrojpitak, S.; Sobhon, P.; Changklungmoa, N. Cytosolic superoxide dismutase can provide protection against Fasciola gigantica. Acta Trop. 2016, 162, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zheng, L.; Yu, P.; Jiang, Q.; Wu, Y.; Huang, C.; Yin, B. Characterization and Application of Lignin–Carbohydrate Complexes from Lignocellulosic Materials as Antioxidants for Scavenging In Vitro and In Vivo Reactive Oxygen Species. ACS Sustain. Chem. Eng. 2020, 8, 256–266. [Google Scholar] [CrossRef]
- Du, S.-S.; Luo, X.-F.; An, J.-X.; Zhang, Z.-J.; Zhang, S.-Y.; Wang, Y.-R.; Ding, Y.-Y.; Jiang, W.-Q.; Zhang, B.-Q.; Ma, Y.; et al. Exploring boron applications in modern agriculture: Antifungal activities and mechanisms of phenylboronic acid derivatives. Pest. Manag. Sci. 2023, 79, 2748–2761. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-L.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Hu, Y.-S.; Cai, J.-P. The antifungal mechanisms of plant volatile compound 1-octanol against Aspergillus flavus growth. Appl. Microbiol. Biotechnol. 2022, 106, 5179–5196. [Google Scholar] [CrossRef]
- Lionetti, V.; Métraux, J.-P. Plant cell wall in pathogenesis, parasitism and symbiosis. Front. Plant Sci. 2014, 5, 612. [Google Scholar] [CrossRef]
- Walton, J.D. Deconstructing the Cell Wall. Plant Physiol. 1994, 104, 1113–1118. [Google Scholar] [CrossRef]
- Gibson, D.M.; King, B.C.; Hayes, M.L.; Bergstrom, G.C. Plant pathogens as a source of diverse enzymes for lignocellulose digestion. Curr. Opin. Microbiol. 2011, 14, 264–270. [Google Scholar] [CrossRef]
- Blackman, L.M.; Cullerne, D.P.; Hardham, A.R. Bioinformatic characterisation of genes encoding cell wall degrading enzymes in the Phytophthora parasitica genome. BMC Genom. 2014, 15, 785. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Roy, A.; Jayaprakash, A.; Rajeswary, T.R.; Annamalai, A.; Lakshmi, P. Genome-wide annotation, comparison and functional genomics of carbohydrate-active enzymes in legumes infecting Fusarium oxysporum formae speciales. Mycology 2020, 11, 56–70. [Google Scholar] [CrossRef]
- Perincherry, L.; Urbaniak, M.; Pawłowicz, I.; Kotowska, K.; Waśkiewicz, A.; Stępień, Ł. Dynamics of Fusarium Mycotoxins and Lytic Enzymes during Pea Plants’ Infection. Int. J. Mol. Sci. 2021, 22, 9888. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Ma, W.; An, J.; Zhang, B.; Sun, W.; Zhang, G. Nerol as a Novel Antifungal Agent: In Vitro Inhibitory Effects on Fusarium oxysporum, Pestalotiopsis neglecta, and Valsa mali and Its Potential Mechanisms against F. oxysporum. J. Fungi 2024, 10, 699. https://doi.org/10.3390/jof10100699
Ji J, Ma W, An J, Zhang B, Sun W, Zhang G. Nerol as a Novel Antifungal Agent: In Vitro Inhibitory Effects on Fusarium oxysporum, Pestalotiopsis neglecta, and Valsa mali and Its Potential Mechanisms against F. oxysporum. Journal of Fungi. 2024; 10(10):699. https://doi.org/10.3390/jof10100699
Chicago/Turabian StyleJi, Jingyu, Weihu Ma, Jiyuan An, Bowen Zhang, Wenzhuo Sun, and Guocai Zhang. 2024. "Nerol as a Novel Antifungal Agent: In Vitro Inhibitory Effects on Fusarium oxysporum, Pestalotiopsis neglecta, and Valsa mali and Its Potential Mechanisms against F. oxysporum" Journal of Fungi 10, no. 10: 699. https://doi.org/10.3390/jof10100699
APA StyleJi, J., Ma, W., An, J., Zhang, B., Sun, W., & Zhang, G. (2024). Nerol as a Novel Antifungal Agent: In Vitro Inhibitory Effects on Fusarium oxysporum, Pestalotiopsis neglecta, and Valsa mali and Its Potential Mechanisms against F. oxysporum. Journal of Fungi, 10(10), 699. https://doi.org/10.3390/jof10100699




