Analysis of Mycorrhization Trends and Undesired Fungi Species in Three- and Six-Year-Old Tuber aestivum Plantations in Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Plantations and the Host Plant Species
2.2. Soil Sampling and pH Measurement
2.3. Root Sampling Techniques, Mycorrhizal Assessment Methods and Analysis
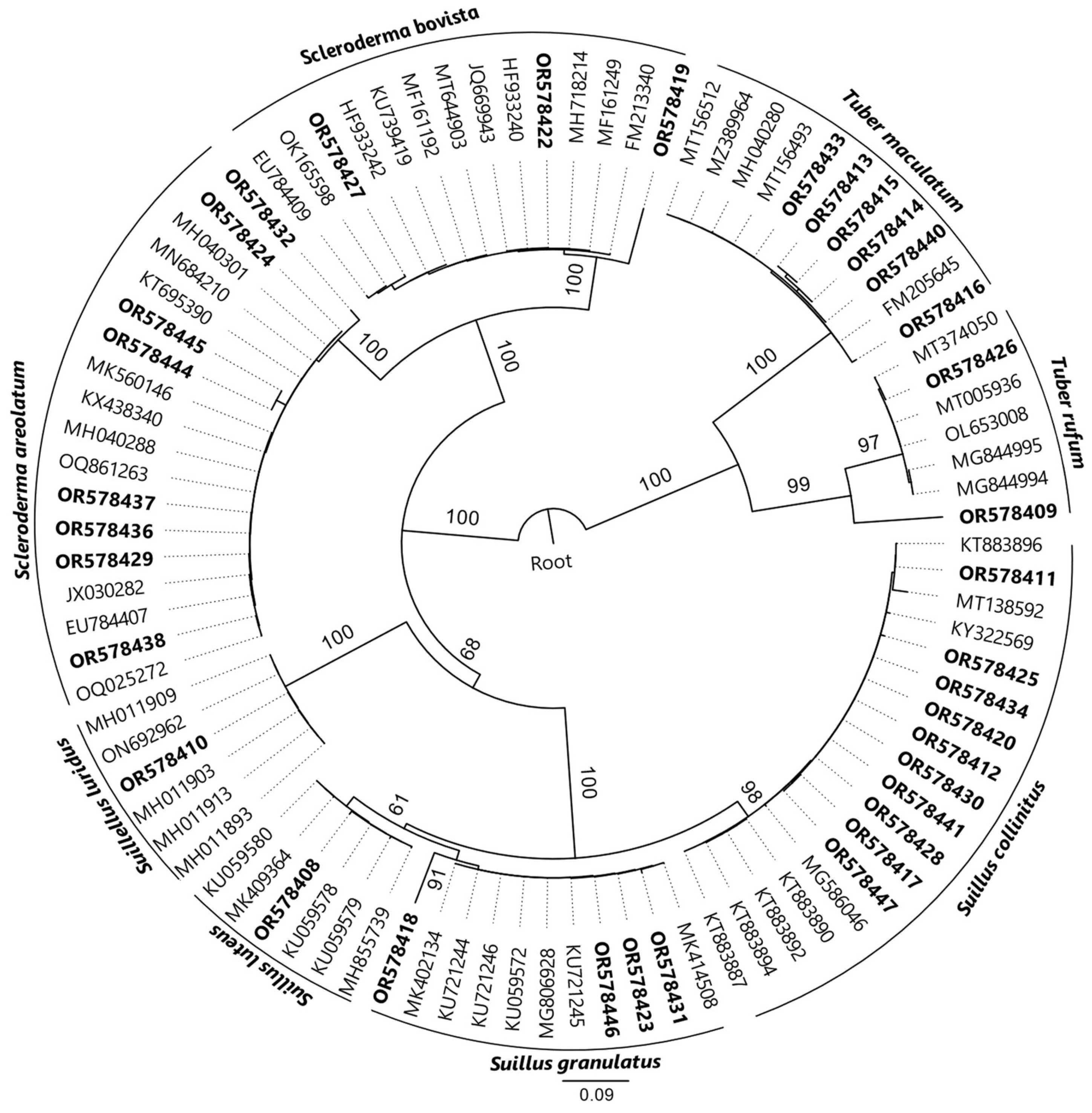
2.4. Morphological and Molecular Studies and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
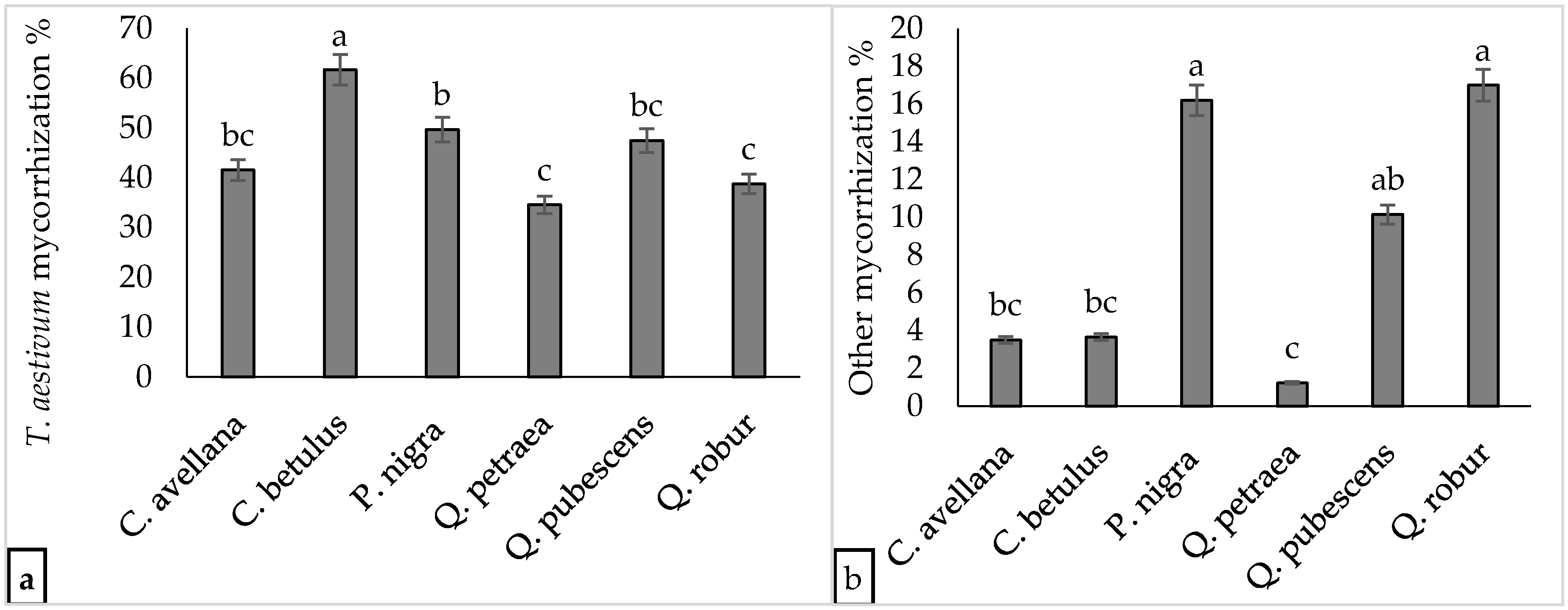
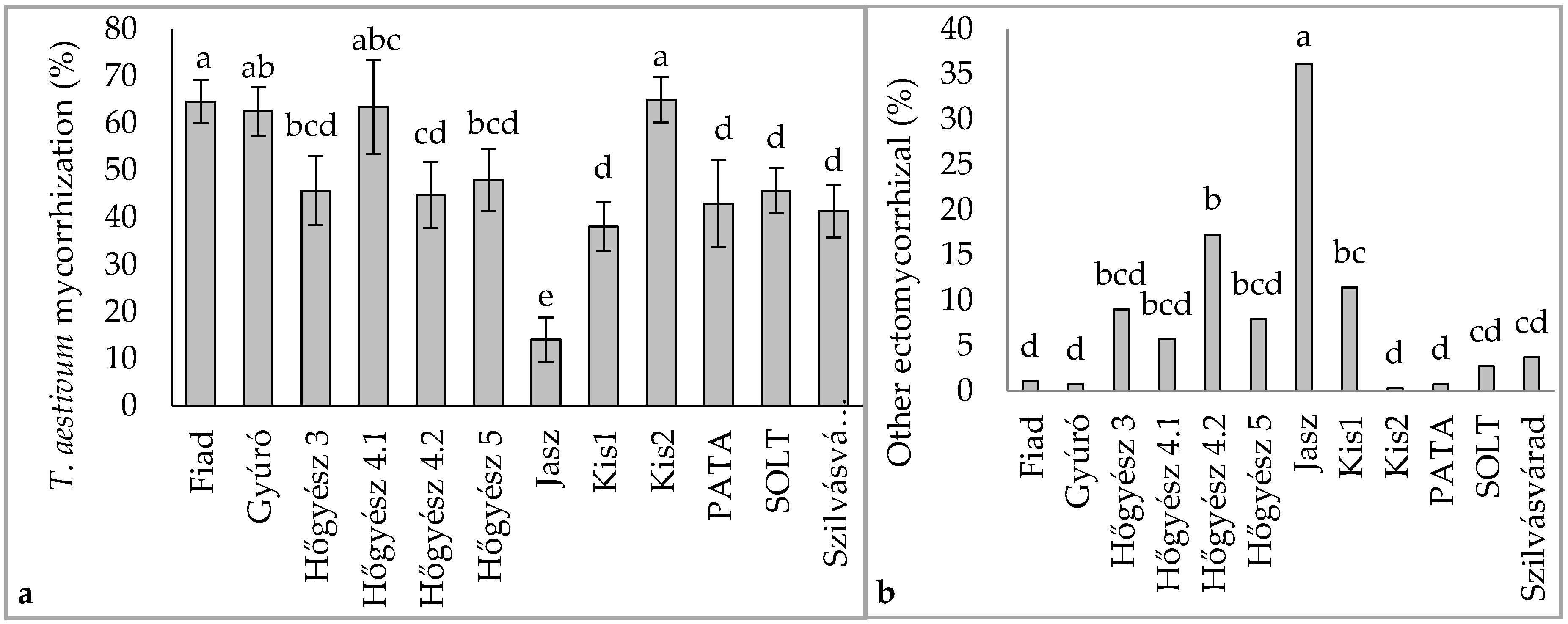
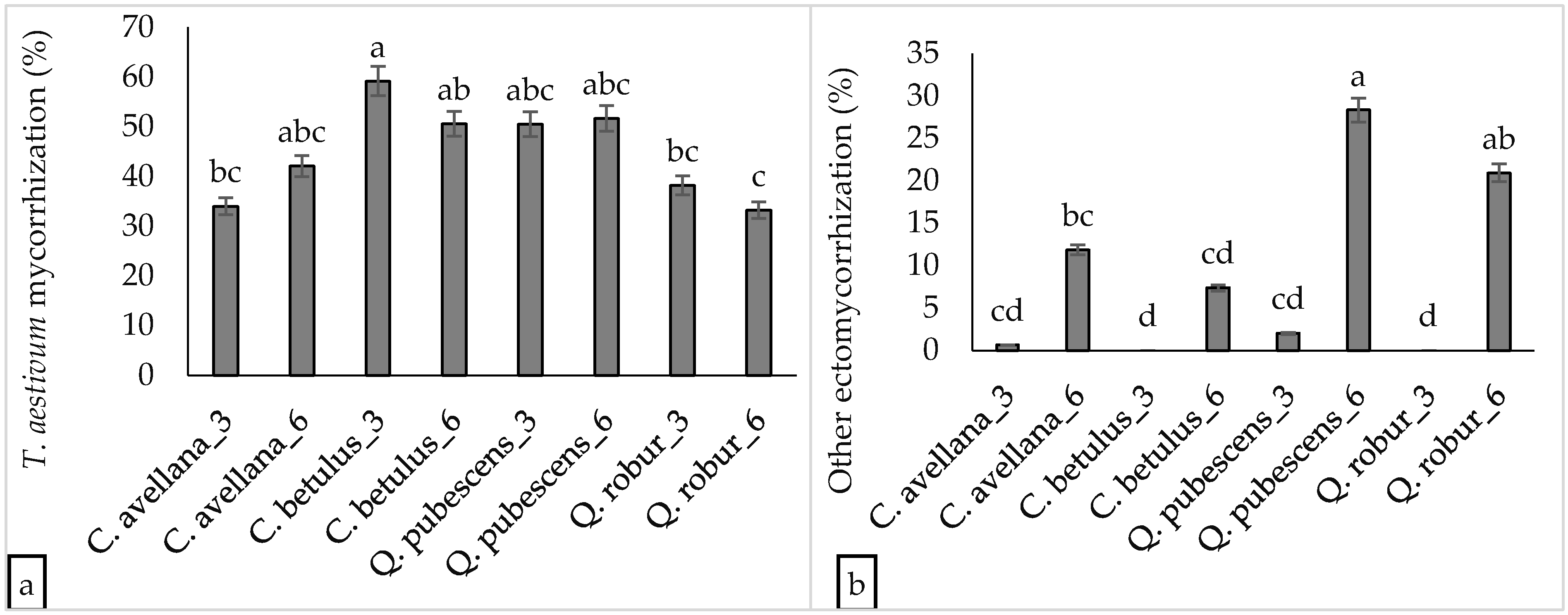
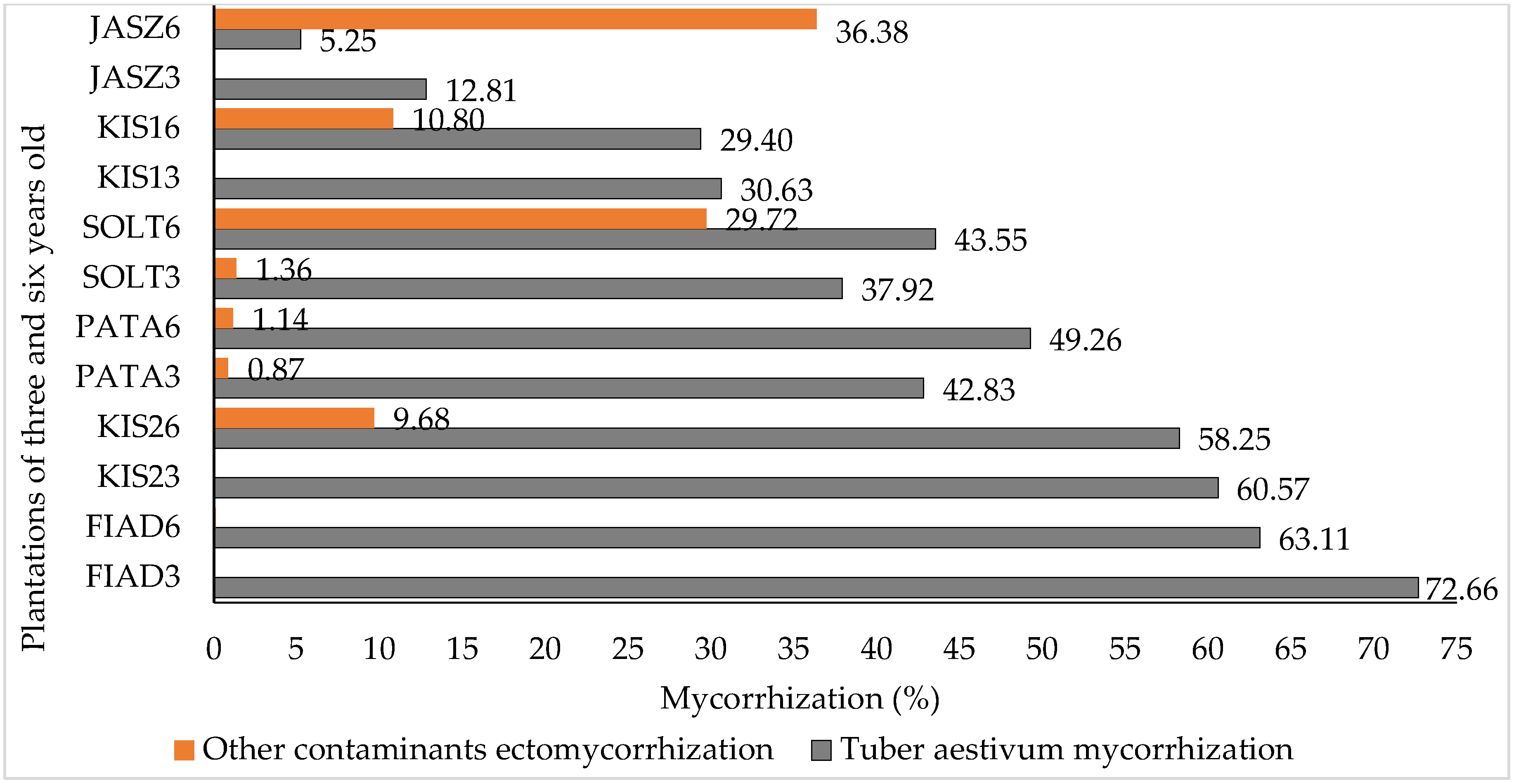
3.1. Tuber Aestivum Plantations and Overview of Ectomycorrhizal Colonization
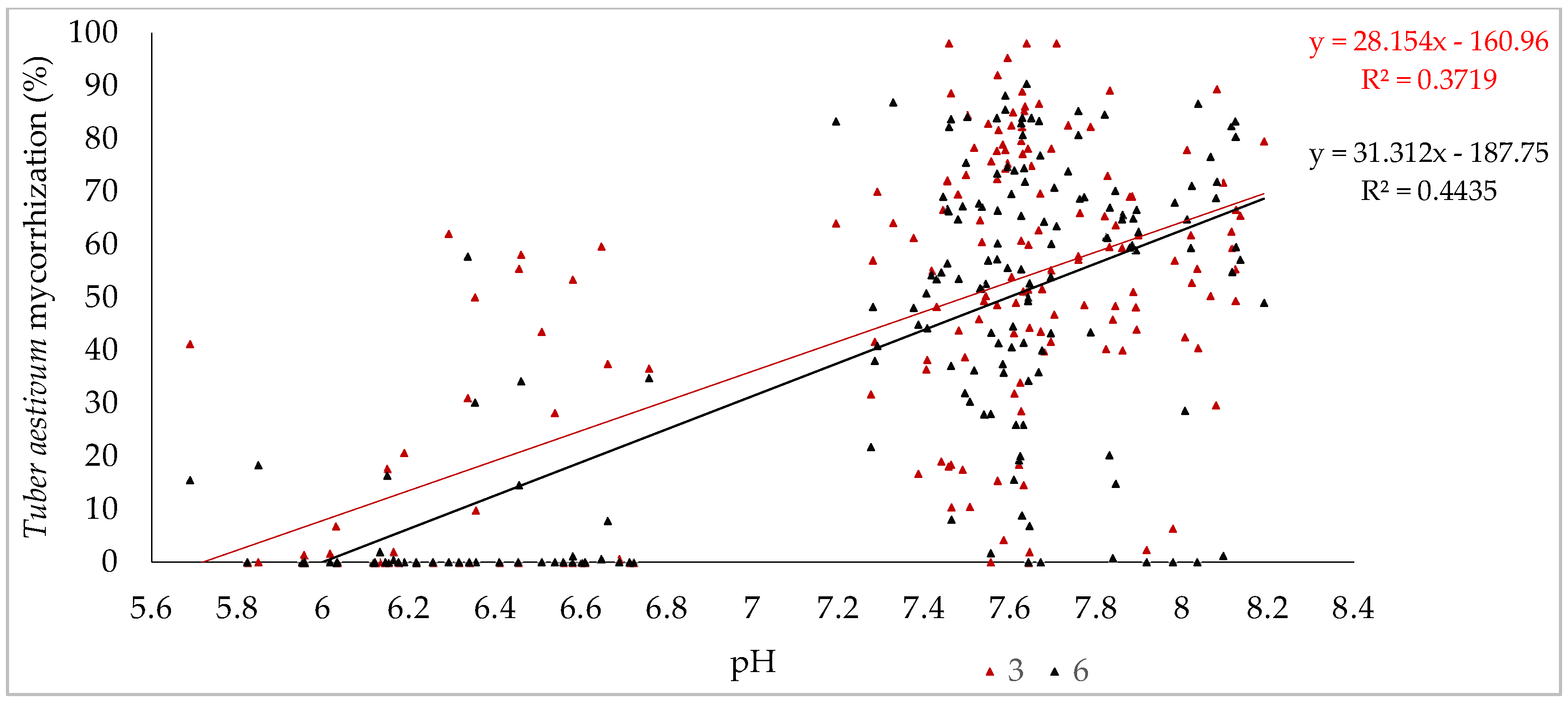
3.2. Effect of Plantation Age and Soil pH on Mycorrhizal Colonization Levels
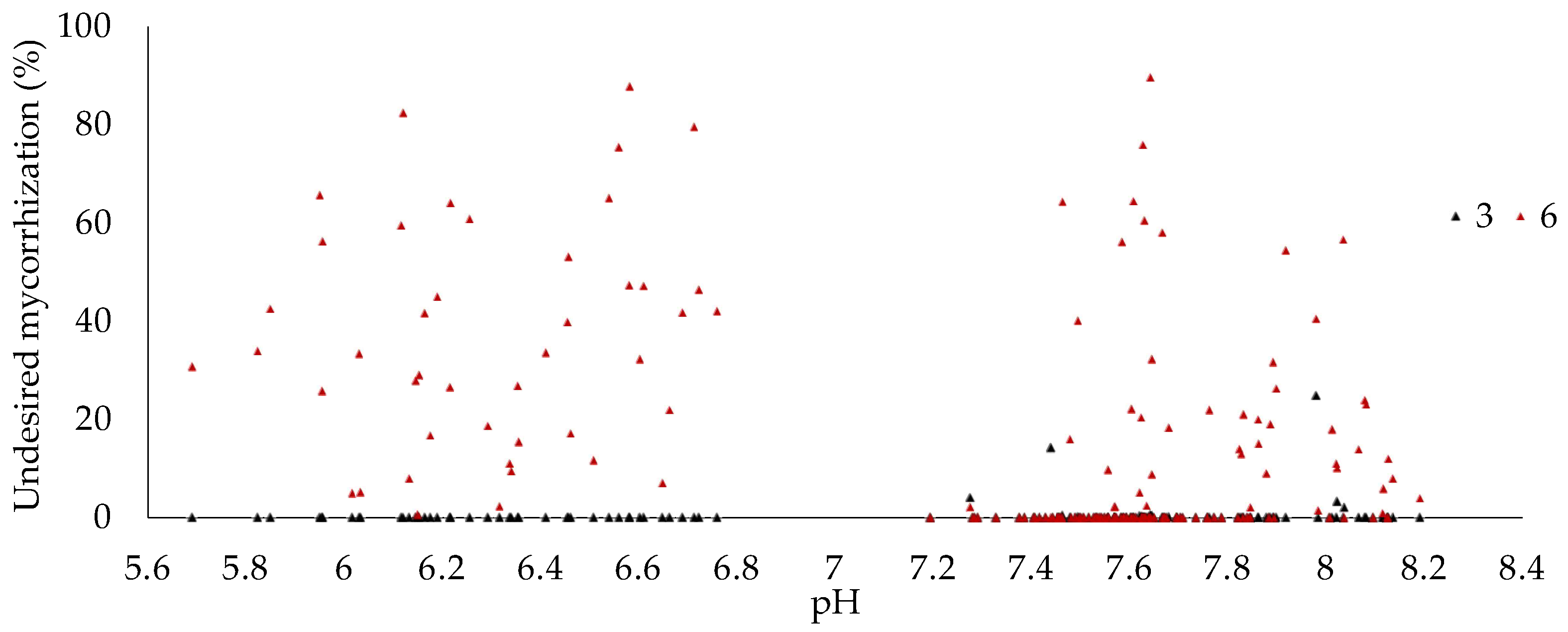
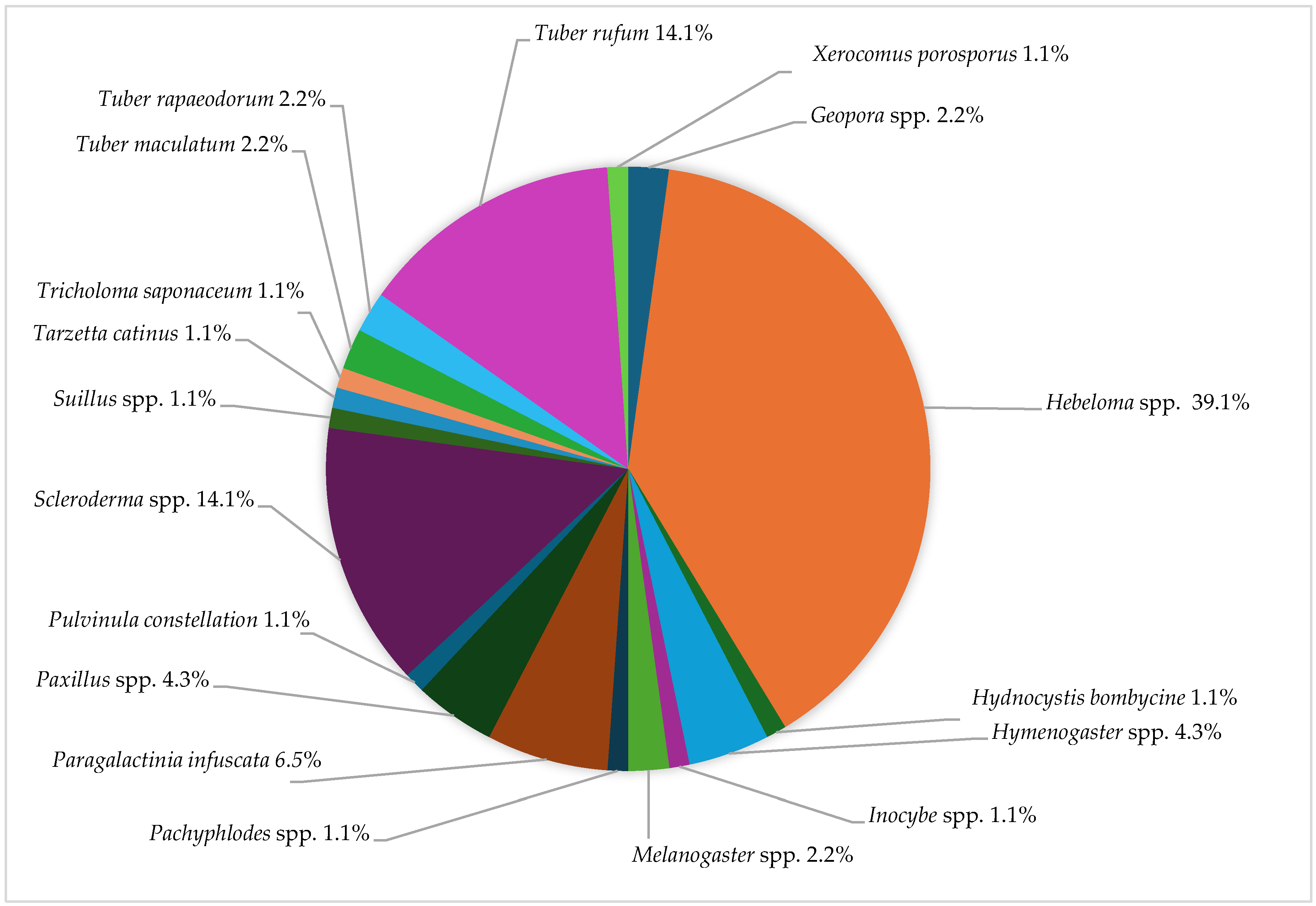
3.3. Undesired Ectomycorrhizal Fungi and Fungal Fruit Bodies in T. aestivum Plantations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Original Name of the plantations | Abbreviations used in the article |
| Kiskunfélegyháza I | KIS 1 |
| Kiskunfélegyháza II | KIS 2 |
| Gyöngyöspata | PATA |
| Jászszentandrás | JASZ |
References
- Gógán, A.C.; Nagy, Z.; Dégi, Z.; Bagi, I.; Dimény, J. Ecological characteristic of Hungarian summer truffle (Tuber aestivum Vittad.) producing area. Acta Mycol. 2012, 47, 133–138. [Google Scholar] [CrossRef]
- Benucci GM, N.; Bonito, G.; Falini, L.B.; Bencivenga, M. Mycorrhization of Pecan trees (Carya illinoinensis) with commercial truffle species: Tuber aestivum Vittad. and Tuber borchii Vittad. Mycorrhiza 2012, 22, 383–392. [Google Scholar] [CrossRef]
- Garcia-Montero, L.G.; Moreno, D.; Monleon, V.J.; Arredondo-Ruiz, F. Natural production of Tuber aestivum in central Spain: Pinus spp. versus Quercus spp. brûles. For. Syst. 2014, 23, 394–399. [Google Scholar] [CrossRef]
- Chevalier, G. The truffle of Europe (Tuber aestivum): Geographic limits, ecology and possibility of cultivation. Osterr. Z. Pilzkd. 2010, 19, 249–259. [Google Scholar]
- Shamekh, S.; Grebenc, T.; Leisola, M.; Turunen, O. The cultivation of oak seedlings inoculated with Tuber aestivum Vittad. in the boreal region of Finland. Mycol. Prog. 2014, 13, 373–380. [Google Scholar] [CrossRef]
- Bratek, Z.; Merényi, Z.; Varga, T. Changes of hypogeous fungi in the Carpathian Pannonian region in the past centuries. Acta Mycol. 2013, 48, 33–39. [Google Scholar] [CrossRef]
- Lee, H.; Nam, K.; Zahra, Z.; Farooqi, M.Q.U. Potentials of truffles in nutritional and medicinal applications: A review. Fungal Biol. Biotechnol. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Vijay, V.; Pandey, A.K.; Semwal, A.D. Biochemical and Health Properties of Truffles. Def. Life Sci. J. 2021, 6, 251–258. [Google Scholar] [CrossRef]
- Vetter, J.; Kruzselyi, D. Complex chemical evaluation of the summer truffle (Tuber aestivum Vittad.) fruit bodies. J. Appl. Bot. Food Qual. 2014, 87, 291–295. [Google Scholar]
- Robin, C.; Goutal-Pousse, N.; Le Tacon, F. Soil Characteristics for Tuber aestivum (Syn. T. uncinatum). In True Truffle (Tuber spp.) in the World; Zambonelli, A., Iotti, M., Murat, C., Eds.; Soil Biology; Springer: Cham, Switzerland, 2016; Volume 47, pp. 211–231. [Google Scholar] [CrossRef]
- Büntgen, U.; Bagi, I.; Fekete, O.; Molinier, V.; Peter, M.; Splivallo, R.; Egli, S. New insights into the complex relationship between weight and maturity of Burgundy truffles (Tuber aestivum). PLoS ONE 2017, 12, e0170375. [Google Scholar] [CrossRef]
- Bragato, G.; Fornasier, F.; Bagi, I.; Egli, S.; Marjanović, Ž. Soil parameters explain short-distance variation in production of Tuber aestivum Vittad. in an oak plantation in the central-northern part of the Great Hungarian Plain (Jászság region, Hungary). For. Ecol. Manag. 2021, 479, 118578. [Google Scholar] [CrossRef]
- Payen, T.; Murat, C.; Bonito, G. Truffle phylogenomics: New insights into truffle evolution and truffle life cycle. Adv. Bot. Res. 2014, 70, 211–234. [Google Scholar]
- Oliach, D.; Colinas, C.; Castaño, C.; Fischer, C.R.; Bolaño, F.; Bonet, J.A.; Oliva, J. The influence of forest surroundings on the soil fungal community of black truffle (Tuber melanosporum) plantations. For. Ecol. Manag. 2020, 470, 118212. [Google Scholar] [CrossRef]
- Mello, A.; Murat, C.; Bonfante, P. Truffles: Much more than a prized and local fungal delicacy. FEMS Microbiol. Lett. 2006, 260, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, P.; Lugli, F.; Iotti, M.; Puliga, F.; Piana, F.; Gallo, M.; Chiarantini, L. Effects of bio generated ferric hydroxides nanoparticles on truffle mycorrhized plants. Mycorrhiza 2020, 30, 211–219. [Google Scholar] [CrossRef]
- Thomas, P.; Büntgen, U. A risk assessment of Europe’s black truffle sector under predicted climate change. Sci. Total Environ. 2019, 655, 27–34. [Google Scholar] [CrossRef]
- Čejka, T.; Trnka, M.; Krusic, P.J.; Stobbe, U.; Oliach, D.; Václavík, T.; Tegel, W. Predicted climate change will increase the truffle cultivation potential in central Europe. Sci. Rep. 2020, 10, 21281. [Google Scholar] [CrossRef]
- Habtemariam, A.A.; Cseh, P.; Bratek, Z. European Tuber melanosporum plantations: Adaptation status in Hungary, mycorrhizal level, and first ascocarp detection in two truffle orchards. Biol. Futura 2023, 74, 507–517. [Google Scholar] [CrossRef]
- Bratek, Z.; Merényi, Z.; Illyés, Z.; Völcz, G.; Tamasko, G.; Orczán, K.Á.; Viktor, J.; Chevalier, G. First results from experimental truffle orchards established in Hungary in the framework of INRA-ELTE cooperation. Osterr. Z. Pilzkd. 2010, 19, 231–237. [Google Scholar]
- Stobbe, U.; Egli, S.; Tegel, W.; Peter, M.; Sproll, L.; Büntgen, U. Potential and limitations of Burgundy truffle cultivation. Appl. Microbiol. Biotechnol. 2013, 97, 5215–5224. [Google Scholar] [CrossRef]
- Gógán, A.C.; Bratek, Z.; Dimény, J. A new tool for rural development: Truffle cultivation. Cereal Res. Commun. 2007, 35, 413–416. [Google Scholar] [CrossRef]
- Merényi, Z.; Illyés, Z.; Völcz, G.; Bratek, Z. A database and its application for the development of truffle cultivation methods. Osterr. Z. Pilzkd. 2010, 19, 239–244. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; International Soil Reference and Information Centre (ISRIC): Wageningen, The Netherlands, 2002. [Google Scholar]
- Bohus, G. Studies on the pH requirement of soil-inhabiting mushrooms: The R-spectra of mushroom assemblages in deciduous forest communities. Acta Bot. Hung. 1984, 30, 155–171. [Google Scholar]
- Alvarado, P.; Manjon, J.L. A quantitative and molecular examination of Tuber melanosporum mycorrhizae in Quercus ilex seedlings from different suppliers in Spain. For. Syst. 2013, 22, 159–169. [Google Scholar] [CrossRef]
- Sánchez, S.; Ágreda, T.; Águeda, B.; Martín, M.; de Miguel, A.M.; Barriuso, J. Persistence and detection of black truffle ectomycorrhizas in plantations: Comparison between two field detection methods. Mycorrhiza 2014, 24, 39–46. [Google Scholar] [CrossRef]
- Habtemariam, A.A.; Bratek, Z.; Gyulavári, P. Observations on mycorrhization of pecan seedlings with a European truffle. Rhizosphere 2021, 19, 100409. [Google Scholar] [CrossRef]
- Agerer, R.; Rambold, G. DEEMY–An Information System for Characterization and Determination of Ectomycorrhizae; München, Germany, 2004–2021. Available online: http://www.deemy.de (accessed on 19 August 2024).
- White, T.J.; Bruns, T.; Lee SJ, W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Vidal, J.M.; Cseh, P.; Merényi, Z.; Bóna, L.; Rudnóy, S.; Bratek, Z.; Paz, A.; Mleczko, P.; Kozak, M.; Chachuła, P.; et al. The genus Gautieria (Gomphales) in Europe and the Mediterranean Basin: A morphological and phylogenetic taxonomic revision. Persoonia-Mol. Phylogeny Evol. Fungi 2023, 50, 48–122. [Google Scholar] [CrossRef]
- Hilszczańska, D.; Sierota, Z.; Leśnej, B.; Stary, S. First attempt towards cultivation of Tuber aestivum in Poland. Austrian J. Mycol. 2010, 19, 209–212. [Google Scholar]
- Donnini, D.; Benucci, G.M.; Bencivenga, M.; Falini, L.B. Quality assessment of truffle-inoculated seedlings in Italy: Proposing revised parameters for certification. For. Syst. 2014, 23, 385–393. [Google Scholar] [CrossRef]
- Todesco, F.; Belmondo, S.; Guignet, Y.; Laurent, L.; Fizzala, S.; Le Tacon, F.; Murat, C. Soil temperature and hydric potential influences the monthly variations of soil Tuber aestivum DNA in a highly productive orchard. Sci. Rep. 2019, 9, 12964. [Google Scholar] [CrossRef] [PubMed]
- Salerni, E.; d‘Aguanno, M.; Leonardi, P.; Perini, C. Ectomycorrhizal communities above and below ground and truffle productivity in a Tuber aestivum orchard. For. Syst. 2014, 23, 329–338. [Google Scholar] [CrossRef]
- Thomas, P.W. The role of pH in Tuber aestivum syn. uncinatum mycorrhiza development within commercial orchards. Acta Mycol. 2012, 47, 2. [Google Scholar] [CrossRef]
- Gajos, M.; Hilszczańska, D. Research on truffles: Scientific journals analysis. Sci. Res. Essays 2013, 8, 1837–1847. [Google Scholar]
- Hilszczańska, D.; Szmidla, H.; Sikora, K.; Rosa-Gruszecka, A. Soil properties conducive to the formation of Tuber aestivum vitt. fruiting bodies. Pol. J. Environ. Stud. 2019, 28, 1713–1718. [Google Scholar] [CrossRef]
- De Miguel, A.M.; Águeda, B.; Sánchez, S.; Parladé, J. Ectomycorrhizal fungus diversity and community structure with natural and cultivated truffle hosts: Applying lessons learned to future truffle culture. Mycorrhiza 2014, 24 (Suppl. S1), 5–18. [Google Scholar] [CrossRef]
- Hilszczańska, D.; Szmidla, H.; Horak, J.; Rosa-Gruszecka, A. Ectomycorrhizal communities in a Tuber aestivum Vittad. orchard in Poland. Open Life Sci. 2016, 11, 348–357. [Google Scholar] [CrossRef]
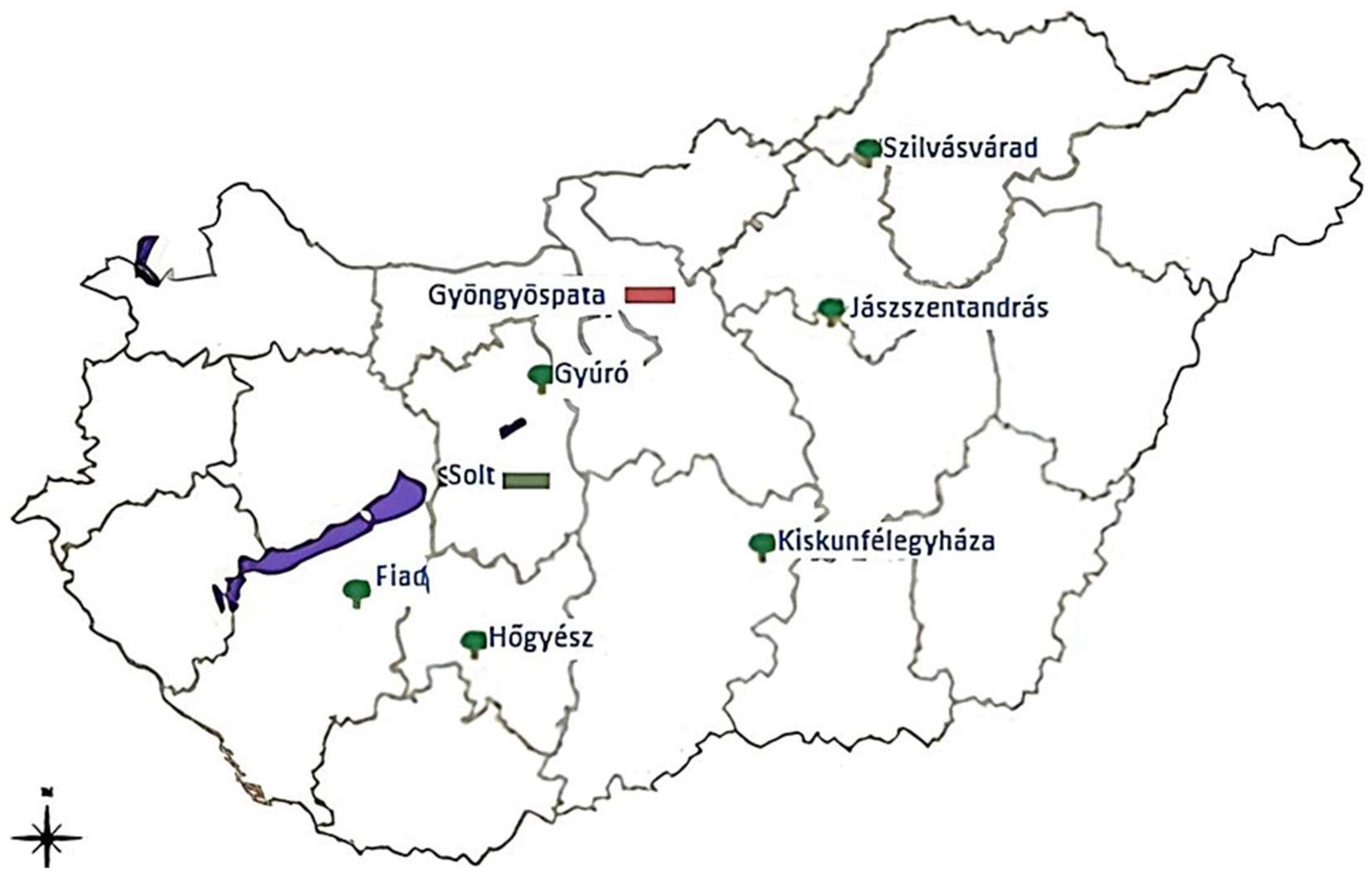
| Plantation | Soil Composition | Soil Physical Characteristics in the Plantation | |||
|---|---|---|---|---|---|
| CaCO3 (m/m%) | Ca (mg/kg) | Salt | pH (H2O) | ||
| Fiad | 10.39 | 2.58 | <0.02 | 7.8 | Brown with light brown loam |
| Gyöngyöspata | 1.6 | 1.15 | <0.02 | 7.5 | Clay |
| Gyúró | 8.52 | 2.86 | <0.02 | 7.68 | Brown silt from 10 cm |
| Jászszentandrás | 0 | 0.4 | <0.02 | 6.7 | Brown sand with humus |
| Hőgyész 4.1 | 4.35 | 1.73 | <0.02 | 7.56 | Dark brown humus layer, clay loam |
| Hőgyész 4.2 | 17.25 | 4.38 | <0.02 | 7.66 | - |
| Hőgyész 5 | 15.55 | 3.94 | <0.02 | 7.77 | Gray fine to light brown fine sand |
| Kiskunfélegyháza I | 0.07 | 3.01 | <0.02 | 8.27 | Brown sand with humus |
| Kiskunfélegyháza II | 0.24 | 4.98 | <0.02 | 8.14 | Brown sand with humus |
| Solt | 6.87 | 2.82 | <0.02 | 8 | Brown sand with humus |
| Szilvásvárad | 6.11 | 2.82 | 0.05 | 7.82 | Brown, humus level silty clay |
| Plantation | No. | Mycorrhization (%) | Undesired Mycorrhization (%) | pH (H2O) | Host Plants |
|---|---|---|---|---|---|
| FIAD_3 | 45 | 72.7 ± 17.6 | 0.0 | 7.6 ± 0.10 | C. betulus and Q. robur |
| FIAD_6 | 45 | 63.1 ± 18.6 | 0.1 ± 0.5 | ||
| JASZ_3 | 43 | 12.8 ± 21.2 | 0.0 | 6.3 ± 0.27 | C. betulus and Q. robur |
| JASZ_6 | 43 | 5.3 ± 12.4 | 36.4 ± 23.2 | ||
| KIS1_3 | 8 | 30.6 ± 30.4 | 0.0 | 7.6 ± 0.21 | Q. robur and C. avellana |
| KIS1_6 | 8 | 29.4 ± 27.6 | 10.8 ± 19.3 | ||
| KIS2_3 | 33 | 60.6 ± 13.6 | 0.0 | 7.9 ± 0.17 | Q. robur and C. betulus |
| KIS2_6 | 33 | 58.3 ± 22.9 | 9.7 ± 12.5 | ||
| PATA_3 | 21 | 42.8 ± 18.8 | 0.9 ± 3.2 | 7.4 ± 0.19 | C. avellana |
| PATA_6 | 21 | 49.3 ± 15.2 | 1.1 ± 4.8 | ||
| SOLT_3 | 24 | 37.9 ± 23.6 | 1.4 ± 5.1 | 7.7 ± 0.20 | C. avellana and Q. pubescens |
| SOLT_6 | 24 | 43.6 ± 29.4 | 29.7 ± 27.8 |
| Variables 1 | Variables 2 | Correlation Matrix (r) | p-Value |
|---|---|---|---|
| Age of plantations (from three to six years) | Tuber aestivum mycorrhization | −0.061 | 0.257 |
| Undesired mycorrhization | 0.431 | <0.0001 | |
| Tuber aestivum mycorrhization | Undesired mycorrhization | −0.426 | <0.0001 |
| pH | 0.637 | <0.0001 | |
| Undesired mycorrhizations | pH | −0.276 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habtemariam, A.A.; Cseh, P.; Péter, B.; Heller, Á.; Pitlik, P.; Brandt, S.; László, P.; Bratek, Z. Analysis of Mycorrhization Trends and Undesired Fungi Species in Three- and Six-Year-Old Tuber aestivum Plantations in Hungary. J. Fungi 2024, 10, 696. https://doi.org/10.3390/jof10100696
Habtemariam AA, Cseh P, Péter B, Heller Á, Pitlik P, Brandt S, László P, Bratek Z. Analysis of Mycorrhization Trends and Undesired Fungi Species in Three- and Six-Year-Old Tuber aestivum Plantations in Hungary. Journal of Fungi. 2024; 10(10):696. https://doi.org/10.3390/jof10100696
Chicago/Turabian StyleHabtemariam, Akale Assamere, Péter Cseh, Balázs Péter, Ádám Heller, Peter Pitlik, Sára Brandt, Péter László, and Zoltán Bratek. 2024. "Analysis of Mycorrhization Trends and Undesired Fungi Species in Three- and Six-Year-Old Tuber aestivum Plantations in Hungary" Journal of Fungi 10, no. 10: 696. https://doi.org/10.3390/jof10100696
APA StyleHabtemariam, A. A., Cseh, P., Péter, B., Heller, Á., Pitlik, P., Brandt, S., László, P., & Bratek, Z. (2024). Analysis of Mycorrhization Trends and Undesired Fungi Species in Three- and Six-Year-Old Tuber aestivum Plantations in Hungary. Journal of Fungi, 10(10), 696. https://doi.org/10.3390/jof10100696






