Application of Quantitative Magnetic Resonance Imaging (QMRI) to Evaluate the Effectiveness of Ultrasonic Atomization of Water in Truffle Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ascoma Selection and Preparation
2.2. QMRI Acquisitions
2.3. Statistical Analysis
3. Results
3.1. Ascoma Characteristics
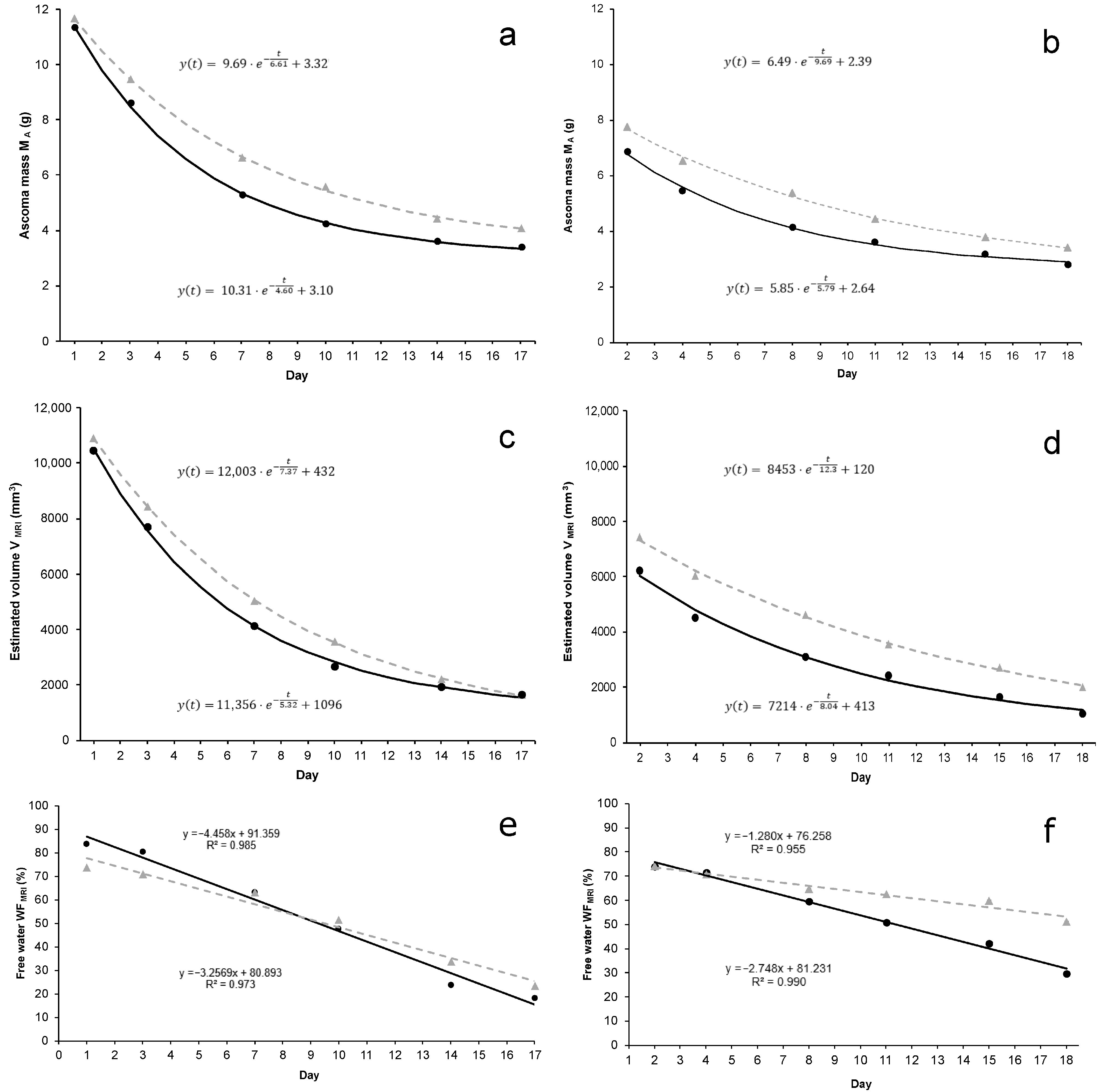
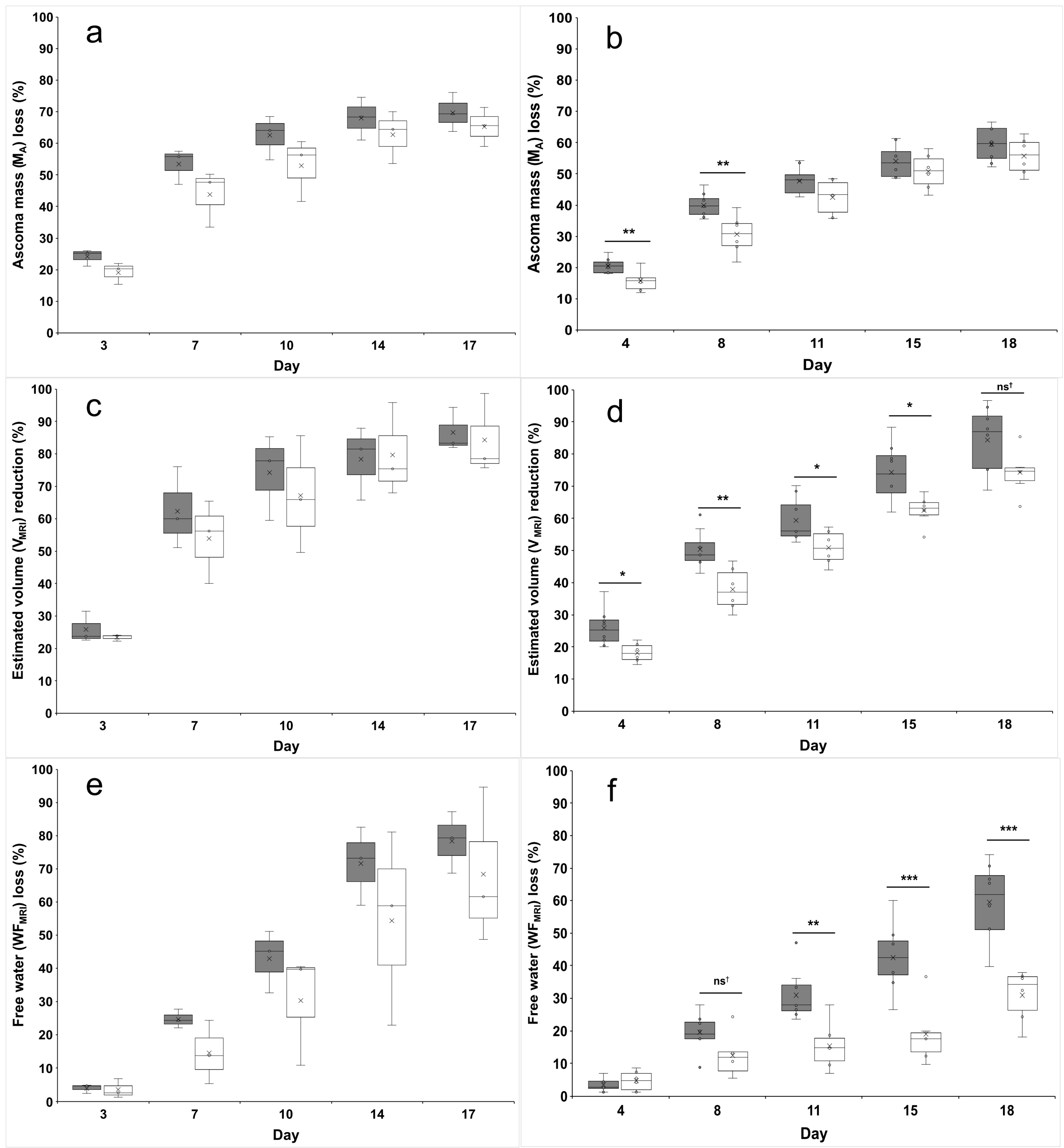
3.2. Ascoma Volume (V)
3.3. Ascoma Mass (MA)
3.4. Free-Water Fraction (WF) and Residual Mass (Mresidual)
3.5. ADC, T1, and T2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, I.; Brown, G.; Zambonelli, A. Taming the Truffle. The History, Lore, and Science of the Ultimate Mushroom; Timber Press: Portland, OR, USA, 2007. [Google Scholar]
- Bonito, G.M.; Gryganskyi, A.P.; Trappe, J.M.; Vilgalys, R.A. Global Meta-Analysis of Tuber ITS rDNA Sequences: Species Diversity, Host Associations and Long-Distance Dispersal. Mol. Ecol. 2010, 19, 4994–5008. [Google Scholar] [CrossRef] [PubMed]
- Reyna, S.; Garcia-Barreda, S. Black Truffle Cultivation: A Global Reality. For. Syst. 2014, 23, 317–328. [Google Scholar] [CrossRef]
- Hall, I.; Fitzpatrick, N.; Miros, P.; Zambonelli, A. Counter-Season Cultivation of Truffles in the Southern Hemisphere: An Update. Ital. J. Mycol. 2017, 46, 21–36. [Google Scholar] [CrossRef]
- Zambonelli, A.; Iotti, M.; Hall, I. Current Status of Truffle Cultivation: Recent Results and Future Perspectives. Ital. J. Mycol. 2015, 44, 31–40. [Google Scholar] [CrossRef]
- Loewe-Muñoz, V.; Delard, C.; del Río, R.; Gregori, G.; Balzarini, M. Effects of Tuber borchii Inoculation on Pinus pinea 3 Years after Establishment along a Latitudinal Gradient in the Southern Hemisphere. Agrofor. Syst. 2024, 98, 369–381. [Google Scholar] [CrossRef]
- Pacioni, G. La Coltivazione Moderna e Redditizia del Tartufo; De Vecchi: Milano, Italy, 1985; 144p. [Google Scholar]
- Bellesia, F.; Pinetti, A.; Bianchi, A.; Tirillini, B. Volatile Compounds of the White Truffle (Tuber magnatum Pico) from Middle Italy. Flavour. Frag. J. 1996, 11, 239–243. [Google Scholar] [CrossRef]
- Campo, E.; Marco, P.; Oria, R.; Blanco, D.; Venturini, M.E. What Is the Best Method for Preserving the Genuine Black Truffle (Tuber melanosporum) Aroma? An Olfactometric and Sensory Approach. LWT-Food Sci. Technol. 2017, 80, 84–91. [Google Scholar] [CrossRef]
- Saltarelli, R.; Ceccaroli, P.; Cesari, P.; Barbieri, E.; Stocchi, V. Effect of Storage on Biochemical and Microbiological Parameters of Edible Truffle Species. Food Chem. 2008, 109, 8–16. [Google Scholar] [CrossRef]
- Reale, A.; Sorrentino, E.; Iacumin, L.; Tremonte, P.; Manzano, M.; Maiuro, L.; Comi, G.; Coppola, R.; Succi, M. Irradiation Treatments to Improve the Shelf Life of Fresh Black Truffles (Truffles Preservation by Gamma-Rays). J. Food Sci. 2009, 74, M196–M200. [Google Scholar] [CrossRef]
- Rivera, C.S.; Blanco, D.; Marco, P.; Oria, R.; Venturini, M.E. Effects of Electron-Beam Irradiation on the Shelf Life, Microbial Populations and Sensory Characteristics of Summer Truffles (Tuber aestivum) Packaged under Modified Atmospheres. Food Microbiol. 2011, 28, 141–148. [Google Scholar] [CrossRef]
- Sorrentino, E.; Reale, A.; Tremonte, P.; Maiuro, L.; Succi, M.; Tipaldi, L.; Di Renzo, T.; Pannella, G.; Coppola, R. Lactobacillus plantarum Inhibits Penicillium spp. Involved in the Spoilage of Black Truffles (Tuber aestivum). J. Food Sci. 2013, 78, M1188–M1194. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.; Tremonte, P.; Reale, A.; Tipaldi, L.; Pannella, G.; Di Renzo, T.; Sorrentino, E. Modified Atmosphere Packaging, Ultrasound and Chitosan: Effect of Co-Treatments on the Shelf-Life of Black Truffle (Tuber aestivum). In Proceedings of the XI International Controlled and Modified Atmosphere Research Conference, Trani, Italy, 3 June 2013; pp. 471–475. [Google Scholar] [CrossRef]
- Culleré, L.; Ferreira, V.; Venturini, M.E.; Marco, P.; Blanco, D. Chemical and Sensory Effects of the Freezing Process on the Aroma Profile of Black Truffles (Tuber melanosporum). Food Chem. 2013, 136, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Calvo, E.; Baquero-Aznar, V.; Vega-Diez, S.; Salvador, M.L.; Sanz, M.Á.; Sánchez, S.; Marco, P.; García-Barreda, S.; González-Buesa, J. Potential of a Smart Gelatine Hydrogel-Based Package to Extend Fresh Black Truffle (Tuber melanosporum) Shelf-Life Preserving Its Aroma Profile. Food Hydrocolloid 2024, 151, 109874. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Venturini, M.E.; Blanco, D.; Soler-Rivas, C. Effects of Combining Electron-Beam or Gamma Irradiation Treatments with Further Storage under Modified Atmospheres on the Bioactive Compounds of Tuber melanosporum Truffles. Postharvest Biol. Technol. 2019, 155, 149–155. [Google Scholar] [CrossRef]
- Savini, S.; Longo, E.; Servili, A.; Murolo, S.; Mozzon, M.; Romanazzi, G.; Boselli, E. Hypobaric Packaging Prolongs the Shelf Life of Refrigerated Black Truffles (Tuber melanosporum). Molecules 2020, 25, 3837. [Google Scholar] [CrossRef]
- Choo, K.S.; Bollen, M.; Dykes, G.A.; Coorey, R. Aroma-Volatile Profile and Its Changes in Australian Grown Black Périgord Truffle (Tuber melanosporum) During Storage. Int. J. Food Sci. Technol. 2021, 56, 5762–5776. [Google Scholar] [CrossRef]
- Phong, W.N.; Payne, A.D.; Dykes, G.A.; Coorey, R. Postharvest Decontamination of Fresh Black Truffle (Tuber melanosporum): Effects on Microbial Population and Organoleptic Qualities. Postharvest Biol. Technol. 2023, 197, 112191. [Google Scholar] [CrossRef]
- Palacios, I.; Guillamón, E.; García-Lafuente, A.; Villares, A. Effects of Freeze-Drying Treatment on the Aromatic Profile of Tuber spp. Truffles. J. Food Process Pres. 2014, 38, 768–773. [Google Scholar] [CrossRef]
- Cheng, S.; Li, R.; Yang, H.; Wang, S.; Tan, M. Water Status and Distribution in Shiitake Mushroom and the Effects of Drying on Water Dynamics Assessed by LF-NMR and MRI. Dry. Technol. 2020, 38, 1001–1010. [Google Scholar] [CrossRef]
- Galante, A.; Marino, A.; Bianchi, S.; Leonardi, M.; Zambonelli, A.; Iotti, M.; Alecci, M. Quantitative Magnetic Resonance Imaging Technology as an Effective Tool for Monitoring Post-Harvest Decay of Tuber aestivum Ascomata. Postharvest Biol. Technol. 2022, 194, 112069. [Google Scholar] [CrossRef]
- Bonito, G. Fast DNA-Based Identification of the Black Truffle Tuber melanosporum with Direct PCR and Species-Specific Primers. FEMS Microbiol. Lett. 2009, 301, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Amicucci, A.; Zambonelli, A.; Giomaro, G.; Potenza, L.; Stocchi, V. Identification of Ectomycorrhizal Fungi of the Genus Tuber by Species-Specific ITS Primers. Mol. Ecol. 1998, 7, 273–277. [Google Scholar] [CrossRef]
- Paolocci, F.; Rubini, A.; Granetti, B.; Arcioni, S. Rapid Molecular Approach for a Reliable Identification of Tuber spp. ectomycorrhizae. FEMS Microbiol. Ecol. 1999, 28, 23–30. [Google Scholar] [CrossRef]
- Dellarosa, N.; Laghi, L.; Ragni, L.; Dalla Rosa, M.; Galante, A.; Ranieri, B.; Florio, T.M.; Alecci, M. Pulsed Electric Fields Processing of Apple Tissue: Spatial Distribution of Electroporation by means of Magnetic Resonance Imaging and Computer Vision System. Innov. Food Sci. Emerg. 2018, 47, 120–126. [Google Scholar] [CrossRef]
- Abdelaziz, G.B.; Dahab, M.A.; Omara, M.A.; Sharshir, S.W.; Elsaid, A.M.; El-Said, E.M.S. Humidification Dehumidification Saline Water Desalination System Utilizing High Frequency Ultrasonic Humidifier and Solar Heated Air Stream. Therm. Sci. Eng. Prog. 2022, 27, 101144. [Google Scholar] [CrossRef]
- Sung, C.C.; Bai, C.Y.; Chen, J.H.; Chang, S.J. Controllable Fuel Cell Humidification by Ultrasonic Atomization. J. Power Sources 2013, 239, 151–156. [Google Scholar] [CrossRef]
- Naidu, H.; Kahraman, O.; Feng, H. Novel Applications of Ultrasonic Atomization in the Manufacturing of Fine Chemicals, Pharmaceuticals, and Medical Devices. Ultrason. Sonochemistry 2022, 86, 105984. [Google Scholar] [CrossRef]
- Fabbri, S.; Olsen, S.I.; Owsianiak, M. Improving Environmental Performance of Post-Harvest Supply Chains of Fruits and Vegetables in Europe: Potential Contribution from Ultrasonic Humidification. J. Clean. Prod. 2018, 182, 16–26. [Google Scholar] [CrossRef]
- Mohammed, M.; Alqahtani, N.; El-Shafie, H. Development and Evaluation of an Ultrasonic Humidifier to Control Humidity in a Cold Storage Room for Postharvest Quality Management of Dates. Foods 2021, 10, 949. [Google Scholar] [CrossRef]
- Sun, M.; Zhuang, Y.; Gu, Y.; Zhang, G.; Fan, X.; Ding, Y. A Comprehensive Review of the Application of Ultrasonication in the Production and Processing of Edible Mushrooms: Drying, Extraction of Bioactive Compounds, and Post-Harvest Preservation. Ultrason. Sonochemistry 2024, 102, 106763. [Google Scholar] [CrossRef]
- Brown, T.; Corry, J.E.L.; James, S.J. Humidification of Chilled Fruit and Vegetables on Retail Display Using an Ultrasonic Fogging System with Water/Air Ozonation. Int. J. Refrig. 2004, 27, 862–868. [Google Scholar] [CrossRef]
- Granetti, B.; De Angelis, A.; Materozzi, G. Umbria Terra di Tartufo; Regione Umbria: Terni, France, 2005. [Google Scholar]
- Leonardi, M.; Iotti, M.; Mello, A.; Vizzini, A.; Paz-Conde, A.; Trappe, J.; Pacioni, G. Typification of the Four Most Investigated and Valuable Truffles: Tuber aestivum Vittad., T. borchii Vittad., T. magnatum Picco and T. melanosporum Vittad. Cryptogamie Mycol. 2021, 42, 149–170. [Google Scholar] [CrossRef]
- Ragnelli, A.M.; Pacioni, G.; Aimola, P.; Lanza, B.; Miranda, M. Truffle Melanogenesis: Correlation with Reproductive Differentiation and Ascocarp Ripening. Pigment. Cell Res. 1992, 5, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B.; Casadevall, A. Functions of Fungal Melanin beyond Virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yan, Y.; Qu, Y.; Wang, J.; Feng, X.; Liu, X.; Lin, F.; Lu, J. Role Refinement of Melanin Synthesis Genes by Gene Knockout Reveals Their Functional Diversity in Pyricularia oryzae Strains. Microbiol. Res. 2021, 242, 126620. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, G. Scanning Electron Microscopy of Tuber Sporocarps and Associated Bacteria. Mycol. Res. 1990, 94, 1086–1089. [Google Scholar] [CrossRef]
- Barbieri, E.; Guidi, C.; Bertaux, J.; Frey-Klett, P.; Garbaye, J.; Ceccaroli, P.; Saltarelli, R.; Zambonelli, A.; Stocchi, V. Occurrence and Diversity of Bacterial Communities in Tuber magnatum during Truffle Maturation. Environ. Microbiol. 2007, 9, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Antony-Babu, S.; Deveau, A.; Van Nostrand, J.D.; Zhou, J.; Le Tacon, F.; Robin, C.; Frey-Klett, P.; Uroz, S. Black Truffle-Associated Bacterial Communities during the Development and Maturation of Tuber melanosporum Ascocarps and Putative Functional Roles. Environ. Microbiol. 2014, 16, 2831–2847. [Google Scholar] [CrossRef] [PubMed]
- Niimi, J.; Deveau, A.; Splivallo, R. Aroma and Bacterial Communities Dramatically Change with Storage of Fresh White Truffle Tuber magnatum. LWT-Food Sci. Technol. 2021, 151, 112125. [Google Scholar] [CrossRef]
| Ascoma | Preservation Method † | 1st Round of MRI Measurement | Residual Mass | |||
|---|---|---|---|---|---|---|
| MA (g) | V (mm3) | WF (%) | Mresidual (g) ± SD * | (MW, g) | ||
| Tbo1 | SF | 11.90 | 10,520 | 83 | 3.29 ± 0.21 | 3.90 |
| Tbo2 | SF | 13.02 | 12,319 | 86 | 2.67 ± 0.47 | 2.82 |
| Tbo3 | SF | 9.13 | 8467 | 82 | 2.42 ± 0.20 | 2.65 |
| Tbo4 | HDC | 13.30 | 12,457 | 74 | 3.89 ± 0.23 | 4.04 |
| Tbo5 | HDC | 11.87 | 11,365 | 74 | 3.57 ± 0.15 | 3.02 |
| Tbo6 | HDC | 9.83 | 8923 | 73 | 3.01 ± 0.19 | 3.10 |
| Tme1 | SF | 4.99 | 5050 | 68 | 1.58 ± 0.10 | 1.56 |
| Tme2 | SF | 8.79 | 7707 | 74 | 3.16 ± 0.16 | 3.71 |
| Tme3 | SF | 7.14 | 6623 | 68 | 2.46 ± 0.15 | 2.74 |
| Tme4 | SF | 6.52 | 6048 | 75 | 2.21 ± 0.20 | 2.26 |
| Tme5 | SF | 6.31 | 5636 | 72 | 2.34 ± 0.15 | 2.61 |
| Tme6 | SF | 6.15 | 5735 | 75 | 2.12 ± 0.29 | 2.10 |
| Tme7 | SF | 6.78 | 5739 | 75 | 2.48 ± 0.22 | 2.61 |
| Tme8 | SF | 8.12 | 7041 | 85 | 2.33 ± 0.24 | 2.35 |
| Tme9 | HDC | 9.04 | 7930 | 82 | 2.55 ± 0.33 | 2.44 |
| Tme10 | HDC | 8.22 | 8557 | 72 | 2.45 ± 0.32 | 3.06 |
| Tme11 | HDC | 6.64 | 5949 | 74 | 2.12 ± 0.08 | 2.84 |
| Tme12 | HDC | 5.80 | 5068 | 75 | 1.96 ± 0.05 | 2.45 |
| Tme13 | HDC | 8.49 | 8563 | 68 | 2.50 ± 0.22 | 2.62 |
| Tme15 | HDC | 8.23 | 8351 | 74 | 2.08 ± 0.10 | 2.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, A.; Leonardi, M.; Zambonelli, A.; Iotti, M.; Galante, A. Application of Quantitative Magnetic Resonance Imaging (QMRI) to Evaluate the Effectiveness of Ultrasonic Atomization of Water in Truffle Preservation. J. Fungi 2024, 10, 717. https://doi.org/10.3390/jof10100717
Marino A, Leonardi M, Zambonelli A, Iotti M, Galante A. Application of Quantitative Magnetic Resonance Imaging (QMRI) to Evaluate the Effectiveness of Ultrasonic Atomization of Water in Truffle Preservation. Journal of Fungi. 2024; 10(10):717. https://doi.org/10.3390/jof10100717
Chicago/Turabian StyleMarino, Alessia, Marco Leonardi, Alessandra Zambonelli, Mirco Iotti, and Angelo Galante. 2024. "Application of Quantitative Magnetic Resonance Imaging (QMRI) to Evaluate the Effectiveness of Ultrasonic Atomization of Water in Truffle Preservation" Journal of Fungi 10, no. 10: 717. https://doi.org/10.3390/jof10100717
APA StyleMarino, A., Leonardi, M., Zambonelli, A., Iotti, M., & Galante, A. (2024). Application of Quantitative Magnetic Resonance Imaging (QMRI) to Evaluate the Effectiveness of Ultrasonic Atomization of Water in Truffle Preservation. Journal of Fungi, 10(10), 717. https://doi.org/10.3390/jof10100717






