RNAi-Based Approaches to Control Mycotoxin Producers: Challenges and Perspectives
Abstract
1. Introduction
2. RNAi Principles and Its Current Applications
2.1. Mechanism of RNAi and Key Components of the Process
2.2. Cross-Kingdom RNAi and dsRNA Uptake
2.3. HIGS and SIGS
| Fungal Species | Method(s) | Host Plant(s) | Target Gene(s) | Effect on Mycotoxin Production | Reference |
|---|---|---|---|---|---|
| F. graminearum | SIGS | Barley | CYP51A + CYP51B + CYP51C | - | [76] |
| F. asiaticum | SIGS | Wheat | Myo5 (myosin 5) | - | [77] |
| F. verticillioides | HIGS | Tobacco | GUS (β-glucuronidase) | - | [83] |
| F. graminearum | HIGS | Wheat | Chs3b (chitin synthase 3b) | 78–85% reduction (DON) | [84] |
| F. culmorum | HIGS, VIGS | Wheat | FcFg1 (secreted lipase); FcFmk1 (MAP-kinase); FcGls1 (β-1,3-Glucan synthase); FcChsV (chitin synthase V) | - | [85] |
| A. flavus | HIGS | Maize | Amy1 (alpha-amylase) | Drastically reduced (AFB1, AFB2) | [86] |
| A. flavus | HIGS | Maize | AFLR | 14-fold reduction (AFB1, AFB2) | [87] |
| A. flavus | HIGS | Maize | Alk (alkaline protease) | 84–87% reduction (aflatoxin) | [88] |
| A. flavus | HIGS | Maize | AFLM (versicolorin dehydrogenase) | 54.2–95.3% reduction (AFB1) | [89] |
| A. flavus | HIGS | Maize | p2c (polygalacturonase) | 30.3–93.7% reduction (aflatoxin) | [90] |
| A. flavus, A. parasiticus | HIGS | Maize | AFLC | 100% reduction (AFB1) | [91] |
| F. graminearum | HIGS | Arabidopsis, barley | CYP51A + CYP51B + CYP51C (sterol 14α-demethylase; single product) | - | [92] |
| F. graminearum | HIGS, SIGS | Arabidopsis, barley | CYP51A; CYP51B; CYP51C | - | [93] |
| A. flavus | HIGS | Peanut | AFLS (transcriptional regulator) + AFLR (transcriptional regulator) + AFLC (polyketide synthase) + Pes1 (nonribosomal peptide synthetase) + AFLep (efflux pump) | 100% reduction (AFB1, AFB2) | [94] |
| A. flavus | HIGS | Groundnut | NsdC (Cys2His2 zinc finger transcriptional regulator) + VeA (development and secondary metabolism regulator) + AFLR + AFLM | >1000-fold reduction (AFB1) | [95] |
| F. culmorum | SIGS, VIGS | Wheat | TRI5 (trichodiene synthase) | 53–85% reduction (DON, in vitro) | [102] |
| F. asiaticum | SIGS | Wheat | β2-tubulin | - | [106] |
| F. graminearum | SIGS | Wheat | TRI6 (transcriptional regulator) | 72% reduction (DON) | [107] |
| F. graminearum | SIGS | Wheat | Chs7 (chitin synthase 7); Gls (glucan synthase); Pkc (protein kinase C) | 25–50% reduction (DON) | [108] |
| F. graminearum | SIGS | Barley | AGO (ARGONAUTE); DCL (DICER) | - | [109] |
3. The Ways to Improve RNAi Efficiency and Facilitate Its Transition to the Field-Scale Application
3.1. Selection of Target Gene(s)
3.2. Design and Optimization of dsRNAs
3.3. dsRNA Production: Increasing Quantities and Lowering Costs
3.4. Improving Host Plants’ dsRNA Uptake
3.5. dsRNA Formulation: Improving Delivery Efficiency and Overcoming Degradation
3.6. Nanoparticle-Free Approaches for RNAi-Based Systems
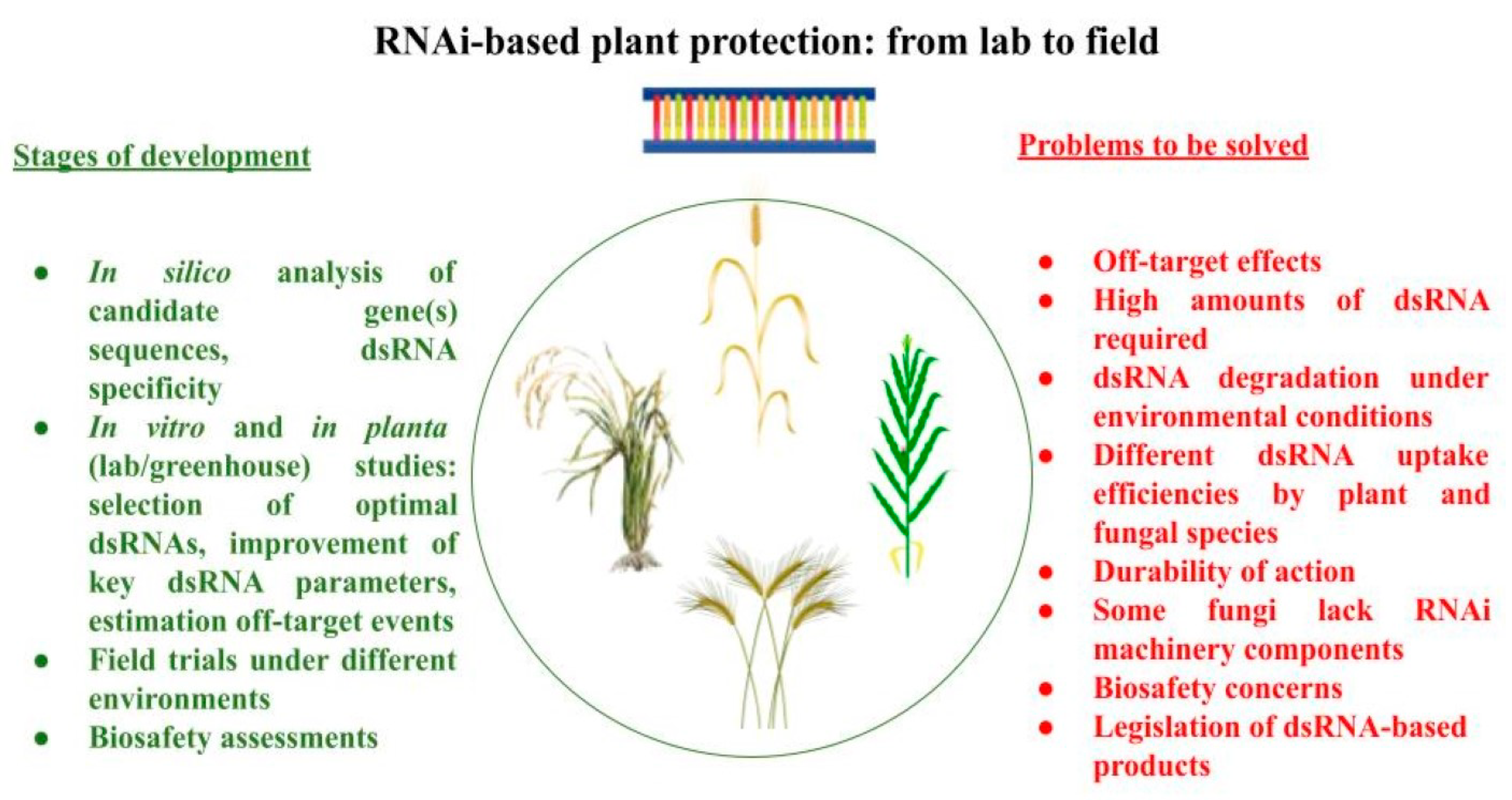
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almeida, F.; Rodriguez, M.L.; Coelho, C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.N.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ciegler, A. Do mycotoxins function in ecological processes? J. Food Saf. 1983, 5, 23–30. [Google Scholar] [CrossRef]
- Latham, R.L.; Boyle, J.T.; Barbano, A.; Loveman, W.G.; Brown, N.A. Diverse mycotoxin threats to safe food and feed cereals. Essays Biochem. 2023, 67, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Bin-Umer, M.A.; McLaughlin, J.; Basu, D.; McCormick, S.; Tumer, N.E. Trichothecene mycotoxins inhibit mitochondrial translation—Implication for the mechanism of toxicity. Toxins 2011, 3, 1484–1501. [Google Scholar] [CrossRef] [PubMed]
- Cundliffe, E.; Cannon, M.; Davies, J. Mechanism of inhibition of eukaryotic protein synthesis by trichothecene fungal toxins. Proc. Natl. Acad. Sci. USA 1974, 71, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of Fusarium mycotoxins in the last decade: A review. Food Chem Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V. Aflatoxins: Properties, toxicity and detoxification. Nutr. Food Sci. 2018, 6, 555696. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins 2021, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and its metabolites—General overview, occurrence, and toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; McCormick, S.; Tiley, H.; Gutiérrez, S.; Yulfo-Soto, G.; Vaughan, M.M.; Ward, T.J. NX trichothecenes are required for Fusarium graminearum infection of wheat. Mol. Plant-Microbe Interact. 2023, 36, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.H.; Desjardins, A.E.; Plattner, R.D. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2002, 153, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Langevin, F.; Eudes, F.; Comeau, A. Effect of trichothecenes produced by Fusarium graminearum during Fusarium head blight development in six cereal species. Eur. J. Plant Pathol. 2004, 110, 735–746. [Google Scholar] [CrossRef]
- Haidukowski, M.; Pascale, M.; Perrone, G.; Pancaldi, D.; Campagna, C.; Visconti, A. Effect of fungicides on the development of Fusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum. J. Sci. Food Agric. 2005, 85, 191–198. [Google Scholar] [CrossRef]
- Cendoya, E.; Nichea, M.J.; del Pilar Monge, M.; Zachetti, V.G.L.; Chiacchiera, S.M.; Ramirez, M.L. Effect of fungicides commonly used for Fusarium head blight management on growth and fumonisin production by Fusarium proliferatum. Rev. Argent. Microbiol. 2021, 53, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Chulze, S.; Magan, N. Impact of environmental factors on growth and deoxynivalenol production by Fusarium graminearum isolates from Argentinian wheat. Crop Prot. 2004, 23, 117–125. [Google Scholar] [CrossRef]
- Tao, H.; Bao, Z.; Jin, C.; Miao, W.; Fu, Z.; Jin, Y. Toxic effects and mechanisms of three commonly used fungicides on the human colon adenocarcinoma cell line Caco-2. Environ. Pollut. 2020, 263, 114660. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Arroyo, T.; Valero, E. A long term field study of the effect of fungicides penconazole and sulfur on yeasts in the vineyard. Int. J. Food Microbiol. 2014, 189, 189–194. [Google Scholar] [CrossRef] [PubMed]
- de Chaves, M.A.; Reginatto, P.; da Costa, B.S.; de Paschoal, R.I.; Teixeira, M.L.; Feuntefria, A.A. Fungicide resistance in Fusarium graminearum species complex. Curr. Microbiol. 2022, 79, 62. [Google Scholar] [CrossRef]
- Fraaije, B.; Atkins, S.; Hanley, S.; Macdonald, A.; Lucas, J. The multi-fungicide resistance status of Aspergillus fumigatus populations in arable soils and the wider European environment. Front. Microbiol. 2020, 11, 599233. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Wright, C.W.; El-Sawi, N.; Yli-Mattila, T.; Malinen, A.M. A methanolic extract of Zanthoxylum bungeanum modulates secondary metabolism regulator genes in Aspergillus flavus and shuts down aflatoxin production. Sci. Rep. 2022, 12, 5995. [Google Scholar] [CrossRef] [PubMed]
- Dzhavakhiya, V.G.; Voinova, T.M.; Popletaeva, S.B.; Statsyuk, N.V.; Limantseva, L.A.; Shcherbakova, L.A. Effect of various compounds blocking the colony pigmentation on the aflatoxin B1 production by Aspergillus flavus. Toxins 2016, 8, 313. [Google Scholar] [CrossRef]
- Liang, D.; Xing, F.; Selvaraj, J.N.; Liu, X.; Wang, L.; Hua, H.; Zhou, L.; Zhao, Y.; Wang, Y.; Liu, Y. Inhibitory effect of cinnamaldehyde, citral, and eugenol on aflatoxin B1 biosynthesis in Aspergillus flavus. J. Food Sci. 2015, 80, M2917–M2924. [Google Scholar] [CrossRef] [PubMed]
- Stakheev, A.A.; Erokhin, D.V.; Meleschuk, E.A.; Mikityuk, O.D.; Statsyuk, N.V. Effect of compactin on the mycotoxin production and expression of related biosynthetic and regulatory genes in toxigenic Fusarium culmorum. Microorganisms 2022, 10, 1347. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Billmyre, R.B.; Calo, S.; Feretzaki, M.; Wang, X.; Heitman, J. RNAi function, diversity, and loss in the Fungal Kingdom. Chromosome Res. 2013, 21, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Billmyre, R.B.; Lee, S.C.; Heitman, J. Broad antifungal resistance mediated by RNAi-dependent epimutation in the basal human fungal pathogen Mucor circinelloides. PLoS Genet. 2019, 15, e1007957. [Google Scholar] [CrossRef]
- Covey, S.N.; Al-Kaff, N.S.; Lángara, A.; Turner, D.S. Plants combat infection by gene silencing. Nature 1997, 385, 781–782. [Google Scholar] [CrossRef]
- Son, H.; Park, A.R.; Lim, J.Y.; Shin, C.; Lee, Y.-W. Genome-wide exonic small interference RNA-mediated gene silencing regulates sexual reproduction in the homothallic fungus Fusarium graminearum. PLoS Genet. 2017, 13, e1006595. [Google Scholar] [CrossRef]
- Wang, X.-B.; Wu, Q.; Ito, T.; Cillo, F.; Li, W.-X.; Chen, X.; Yu, J.-L.; Ding, S.-W. RNAi-mediated viral immunity requires amplification of virus-derived siRNAs in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 484–489. [Google Scholar] [CrossRef]
- Yu, R.; Wang, X.; Moazed, D. Epigenetic inheritance mediated by coupling of RNAi and histone H3K9 methylation. Nature 2018, 558, 615–619. [Google Scholar] [CrossRef]
- Lax, C.; Tahiri, G.; Patiño-Medina, J.A.; Cánovas-Márquez, J.T.; Pérez-Ruiz, J.A.; Osorio-Concepción, M.; Navarro, E.; Calo, S. The evolutionary significance of RNAi in the fungal kingdom. Int. J. Mol. Sci. 2020, 21, 9348. [Google Scholar] [CrossRef]
- Romano, N.; Macino, G. Quelling: Transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol. Microbiol. 1992, 6, 3343–3353. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Desmukh, R.; Purohit, H.J. siRNA mediated gene silencing in Fusarium sp. HKF15 for overproduction of bikaverin. Bioresour. Technol. 2014, 157, 368–371. [Google Scholar] [CrossRef]
- Ma, C.; Liu, J.; Tang, J.; Sun, Y.; Jiang, X.; Zhang, T.; Feng, Y.; Liu, Q.; Wang, L. Current genetic approaches to investigate gene functions in Trichoderma reesei. Microb. Cell Factories 2023, 22, 97. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; de Vries, R.P.; Garrigues, S. Strategies for the development of industrial fungal producing strains. J. Fungi 2023, 9, 834. [Google Scholar] [CrossRef]
- Hutvagner, G.; Simard, M.J. Argonaute proteins: Key players in RNA silencing. Nat. Rev. Cell Biol. 2008, 9, 22–32. [Google Scholar] [CrossRef]
- Makeyev, E.V.; Bamford, D.H. Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes. Mol. Cell 2002, 10, 1417–1427. [Google Scholar] [CrossRef]
- Tijsterman, M.; Plasterk, R.H.A. Dicers at RISC: The mechanism of RNAi. Cell 2004, 117, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Fire, A. Distinct populations of primary and secondary effectors during RNAi in C. elegans. Science 2007, 315, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Q.; Huang, M.; Liu, Y.; Liu, Z.; Liu, X.; Ma, Z. Characterization of RNA silencing components in the plant pathogenic fungus Fusarium graminearum. Sci. Rep. 2015, 5, 12500. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, S.; Ruiz-Vázquez, R.M. The RNAi universe in fungi: A varied landscape of small RNAs and biological functions. Annu. Rev. Microbiol. 2017, 71, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.C.; Doudna, J.A. Molecular mechanisms of RNA interference. Annu. Rev. Biophys. 2013, 42, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Laurie, J.D.; Ali, S.; Linning, R.; Mannhaupt, G.; Wong, P.; Güldener, U.; Münsterkötter, M.; Moore, R.; Kahmann, R.; Bakkeren, G.; et al. Genome comparison of barley and maize smut fungi reveals targeted loss of RNA silencing components and species-specific presence of transposable elements. Plant Cell 2012, 24, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Laurie, J.D.; Linning, R.; Bakkeren, G. Hallmarks of RNA silencing are found in the smut fungus Ustilago hordei but not in its close relative Ustilago maydis. Curr. Genet. 2008, 53, 49–58. [Google Scholar] [CrossRef]
- Nicolás, F.E.; Torres-Martínez, S.; Ruiz-Vázquez, R.M. Loss and retention of RNA interference in fungi and parasites. PLoS Pathog. 2013, 9, e1003089. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, R.; Ishida, F.; Yamaguchi, M.; Tanaka, S. The production and secretion of tRNA-derived RNA fragments in the corn smut fungus Ustilago maydis. Front. Fungal. Biol. 2022, 3, 958798. [Google Scholar] [CrossRef]
- Lee, H.-C.; Li, L.; Gu, W.; Xue, Z.; Crosthwaite, S.K.; Pertsemlidis, A.; Lewis, Z.A.; Freitag, M.; Selker, E.U.; Mello, C.C.; et al. Diverse pathways generate microRNA-like RNAs and Dicer-independent small interfering RNAs in fungi. Mol. Cell 2010, 38, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wang, J.; Wang, Y.; Lin, J.; Fu, Y.; Xie, J.; Jiang, D.; Chen, T.; Liu, H.; Cheng, J. Dicer-like proteins regulate sexual development via the biogenesis of perithecium-specific microRNA in a plant pathogenic fungus Fusarium graminearum. Front. Microbiol. 2018, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Gaffar, F.Y.; Imani, J.; Karlovsky, P.; Koch, A.; Kogel, K.-H. Different components of the RNA interference machinery are required for conidiation, ascosporogenesis, virulence, deoxynivalenol production, and fungal inhibition by exogenous double-stranded RNA in the head blight pathogen Fusarium graminearum. Front. Microbiol. 2019, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-S.; Gu, K.-X.; Duan, X.-X.; Xiao, X.-M.; Hou, Y.-P.; Duan, Y.-B.; Wang, J.-X.; Yu, N.; Zhou, M.-G. Secondary amplification of siRNA machinery limits the application of spray-induced gene silencing. Mol. Plant Pathol. 2018, 19, 2543–2560. [Google Scholar] [CrossRef]
- Weiberg, A.; Bellinger, M.; Jin, H. Conversations between kingdoms: Small RNAs. Curr. Opin. Biotechnol. 2015, 32, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Weiberg, A.; Jin, H. Small RNAs—The secret agents in the plant-pathogen interactions. Curr. Opin. Plant Biol. 2015, 26, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Lin, F.-M.; Thomma, B.P.H.J.; Huang, H.-D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef] [PubMed]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Dellota, E., Jr.; Yamane, D.; Jin, H. Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol. 2017, 14, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Kim, G.; Johnson, N.R.; Wafula, E.; Wang, F.; Coruh, C.; Bernal-Galeano, V.; Phifer, T.; dePamphilis, C.W.; Westwood, J.H.; et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 2018, 553, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Y.-L.; Zhao, J.-H.; Wang, S.; Jin, Y.; Chen, Z.-Q.; Fang, Y.-Y.; Hua, C.L.; Ding, S.-W.; Guo, H.-S. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef]
- Derbyshire, M.; Mbengue, M.; Barascud, M.; Navaud, O.; Raffaele, S. Small RNAs from the plant pathogenic fungus Sclerotinia sclerotiorum highlight host candidate genes associated with quantitative disease resistance. Mol. Plant Pathol. 2019, 20, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-M.; Mao, H.-Y.; Li, S.-J.; Feng, T.; Zhang, Z.-Y.; Cheng, L.; Luo, S.-J.; Borkovich, K.A.; Ouyang, S.-Q. Fol-milR1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses. New Phytol. 2021, 232, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Liang, X. One small RNA of Fusarium graminearum targets and silences CEBiP gene in common wheat. Microorganisms 2019, 7, 425. [Google Scholar] [CrossRef] [PubMed]
- Pyott, D.E.; Molnar, A. Going mobile: Non-cell-autonomous small RNAs shape the genetic landscape of plants. Plant Biotechnol. J. 2015, 13, 306–318. [Google Scholar] [CrossRef]
- Tamiru, M.; Hardcastle, T.J.; Lewsey, M.G. Regulation of genome-wide DNA methylation by mobile small RNAs. New Phytol. 2018, 217, 540–546. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Weiberg, A.; Buck, A.H.; Jin, H. Small RNAs and extracellular vesicles: New mechanisms of cross-specific communication and innovative tools for disease control. PLoS Pathog. 2019, 15, e1008090. [Google Scholar] [CrossRef]
- Cheng, A.-P.; Kwon, S.; Adeshara, T.; Göhre, V.; Feldbrügge, M.; Weiberg, A. Extracellular RNAs released by plant-associated fungi: From fundamental mechanisms to biotechnological applications. Appl. Microbiol. Biotechnol. 2023, 107, 5935–5945. [Google Scholar] [CrossRef]
- Kwon, S.; Rupp, O.; Brachmann, A.; Blum, C.F.; Kraege, A.; Goesmann, A.; Feldbrügge, M. mRNA inventory of extracellular vesicles from Ustilago maydis. J. Fungi 2021, 7, 562. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, T.; Barth, P.; Weipert, L.; Preußer, C.; Hardt, M.; Möbus, A.; Busche, T.; Koch, A. Isolation and characterization of barley (Hordeum vulgare) extracellular vesicles to assess their role in RNA spray-based crop protection. Int. J. Mol. Sci. 2021, 22, 7212. [Google Scholar] [CrossRef]
- Schlemmer, T.; Lischka, R.; Wegner, L.; Ehlers, K.; Biedenkopf, D.; Koch, A. Extracellular vesicles isolated from dsRNA-sprayed barley plants exhibit no growth inhibition or gene silencing in Fusarium graminearum. Fungal Biol. Biotechnol. 2022, 9, 14. [Google Scholar] [CrossRef]
- Karimi, H.Z.; Baldrich, P.; Rutter, B.D.; Borniego, L.; Zajt, K.K.; Meyers, B.C.; Innes, R.W. Arabidopsis apoplastic fluid contains sRNA- and circular RNA-protein complexes that are located outside extracellular vesicles. Plant Cell 2022, 34, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino-Sánchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-S.; Gu, K.-X.; Duan, X.-X.; Xiao, X.-M.; Hou, Y.-P.; Duan, Y.-B.; Wang, J.-X.; Zhou, M.-G. A myosin5 dsRNA that reduces the fungicide resistance and pathogenicity of Fusarium asiaticum. Pestic. Biochem. Physiol. 2018, 150, 1–9. [Google Scholar] [CrossRef]
- Kettles, G.J.; Hofinger, B.J.; Hu, P.; Bayon, C.; Rudd, J.J.; Balmer, D.; Courbot, M.; Hammond-Kosack, K.E.; Scalliet, G.; Kanyuka, K. sRNA profiling combined with gene function analysis reveals a lack of evidence for cross-kingdom RNAi in the wheat—Zymoseptoria tritici pathosystem. Front. Plant Sci. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Wytinck, N.; Sullivan, D.S.; Biggar, K.T.; Crisostomo, L.; Pelka, P.; Belmonte, M.F.; Whyard, S. Clathrin mediated endocytosis is involved in the uptake of exogenous double-stranded RNA in the white mold phytopathogen Sclerotinia sclerotiorum. Sci. Rep. 2020, 10, 12773. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hadi, A.M.; Caley, D.P.; Carter, D.R.F.; Magan, N. Control of aflatoxin production of Aspergillus flavus and Aspergillus parasiticus using RNA silencing technology by targeting aflD (nor-1) gene. Toxins 2011, 3, 647–659. [Google Scholar] [CrossRef]
- McDonald, T.; Brown, D.; Keller, N.P.; Hammond, T.M. RNA silencing of mycotoxin production in Aspergillus and Fusarium species. Mol. Plant-Microbe Interact. 2005, 18, 539–545. [Google Scholar] [CrossRef]
- Nowara, D.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J.; Schweizer, P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 2010, 22, 3130–3141. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, M.L.P.; Dias, B.B.A.; Dall’Astta, R.C.; Pamphile, J.A.; Aragão, F.J.L. In vivo trans-specific gene silencing in fungal cells by in planta expression of a double-stranded RNA. BMC Biol. 2010, 8, 27. [Google Scholar] [CrossRef]
- Cheng, W.; Song, X.-S.; Li, H.-P.; Cao, L.-H.; Sun, K.; Qiu, X.-L.; Xu, Y.-B.; Yang, P.; Huang, T.; Zhang, J.-B.; et al. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 2015, 13, 1335–1345. [Google Scholar] [CrossRef]
- Chen, W.; Kastner, C.; Nowara, D.; Oliveira-Garcia, E.; Rutten, T.; Zhao, Y.; Deising, H.B.; Kumlehn, J.; Schweizer, P. Host-induced silencing of Fusarium culmorum genes protects wheat from infection. J. Exp. Bot. 2016, 67, 4979–4991. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.K.; Majumdar, R.; Rajasekaran, K.; Chen, Z.-Y.; Wei, Q.; Sickler, C.M.; Lebar, M.D.; Cary, J.W.; Frame, B.R.; Wang, K. RNA interference-based silencing of the alpha-amylase (amy1) gene in Aspergillus flavus decreases fungal growth and aflatoxin production in maize kernels. Planta 2018, 247, 1465–1473. [Google Scholar] [CrossRef]
- Masanga, J.O.; Matheka, J.M.; Omer, R.A.; Ommeh, S.C.; Monda, E.O.; Alakonya, A.E. Downregulation of transcription factor aflR in Aspergillus flavus confers reduction of aflatoxin accumulation in transgenic maize with alteration of host plant architecture. Plant Cell Rep. 2015, 34, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Omolehin, O.; Raruang, Y.; Hu, D.; Han, Z.-Q.; Wei, Q.; Wang, K.; Rajasekaran, K.; Cary, J.W.; Chen, Z.-Y. Resistance to aflatoxin accumulation in maize mediated by host-induced silencing of the Aspergillus flavus alkaline protease (alk) gene. J. Fungi 2021, 7, 904. [Google Scholar] [CrossRef] [PubMed]
- Raruang, Y.; Omolehin, O.; Hu, D.; Wei, Q.; Han, Z.-Q.; Rajasekaran, K.; Cary, J.W.; Wang, K.; Chen, Z.-Y. Host induced gene silencing targeting Aspergillus flavus aflM reduced aflatoxin contamination in transgenic maize under field condition. Front. Microbiol. 2020, 11, 754. [Google Scholar] [CrossRef]
- Raruang, Y.; Omolehin, O.; Hu, D.; Wei, Q.; Promyou, S.; Parekattil, L.J.; Rajasekaran, K.; Cary, J.W.; Wang, K.; Chen, Z.-Y. Targeting the Aspergillus flavus p2c gene through host-induced gene silencing reduces A. flavus infection and aflatoxin contamination in transgenic maize. Front. Plant Sci. 2023, 14, 1150086. [Google Scholar] [CrossRef]
- Thakare, D.; Zhang, J.; Wing, R.A.; Cotty, P.J.; Schmidt, M.A. Aflatoxin-free transgenic maize using host-induced gene silencing. Sci. Adv. 2017, 3, e1602382. [Google Scholar] [CrossRef]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.-H. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Höfle, L.; Werner, B.T.; Imani, J.; Schmidt, A.; Jelonek, L.; Kogel, K.-H. SIGS vs HIGS: A study on the efficacy of two dsRNA delivery strategies to silence Fusarium FgCYP51 genes in infected host and non-host plants. Mol. Plant Pathol. 2019, 20, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Arias, R.; Dang, P.M.; Sobolev, V.S. RNAi-mediated control of aflotoxins in peanut: Method to analyze mycotoxin production and transgene expression in the peanut/Aspergillus pathosystem. J. Vis. Exp. 2015, 106, e53398. [Google Scholar] [CrossRef]
- Prasad, K.; Yogendra, K.; Sanivarapu, H.; Rajasekaran, K.; Cary, J.W.; Sharma, K.K.; Bhatnagar-Mathur, P. Multiplexed host-induced gene silencing of Aspergillus flavus genes confers aflatoxin resistance in groundnut. Toxins 2023, 15, 319. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; McCallum, B.; Jordan, M.; Loewen, M.; Fobert, P.; McCartney, C.; Bakkeren, G. RNA silencing approaches for identifying pathogenicity and virulence elements towards engineering crop resistance to plant pathogenic fungi. CAB Rev. 2016, 11, 027. [Google Scholar] [CrossRef]
- Mishra, R.; Zhao, K. Genome editing technologies and their applications in crop improvement. Plant Biotechnol. Rep. 2018, 12, 57–68. [Google Scholar] [CrossRef]
- Jang, G.; Joung, Y.H. CRISPR/Cas-mediated genome editing for crop improvement: Current applications and future prospects. Plant Biotechnol. Rep. 2019, 13, 1–10. [Google Scholar] [CrossRef]
- Herman, R.A.; Storer, N.P.; Anderson, J.A.; Amijee, F.; Cnudde, F.; Raybould, A. Transparency in risk-disproportionate regulation of regulation of modern crop-breeding techniques. GM Crops Food 2021, 12, 376–381. [Google Scholar] [CrossRef]
- Scofield, S.R.; Huang, L.; Brandt, A.S.; Gill, B.S. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiol. 2005, 138, 2165–2173. [Google Scholar] [CrossRef]
- Yuan, C.; Li, C.; Yan, L.; Jackson, A.O.; Liu, Z.; Han, C.; Yu, J.; Li, D. A high throughput barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS ONE 2011, 6, e26468. [Google Scholar] [CrossRef]
- Tretiakova, P.; Voegele, R.T.; Soloviev, A.; Link, T.I. Successful silencing of the mycotoxin synthesis gene TRI5 in Fusarium culmorum and observation of reduced virulence in VIGS and SIGS experiments. Genes 2022, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Jurgenson, J.E.; Hulbert, S.H. Development of a host-induced RNAi system in the wheat stripe rust fungus Puccinia striiformis f. sp. tritici. Mol. Plant-Microbe Interact. 2011, 24, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.; Ruan, S.; Nzabanita, C.; Wang, Y.; Guo, L. A mycovirus VIGS vector confers hypovirulence to a plant pathogenic fungus to control wheat FHB. Adv. Sci. 2023, 10, 2302606. [Google Scholar] [CrossRef] [PubMed]
- Biedenkopf, D.; Will, T.; Knauer, T.; Jelonek, L.; Furch, A.C.U.; Busche, T.; Koch, A. Systemic spreading of exogenous applied RNA biopesticides in the crop plant Hordeum vulgare. ExRNA 2020, 2, 12. [Google Scholar] [CrossRef]
- Gu, K.-X.; Song, X.-S.; Xiao, X.-M.; Duan, X.-X.; Wang, J.-X.; Duan, Y.-B.; Hou, Y.-P.; Zhou, M.-G. A β2-tubulin dsRNA derived from Fusarium asiaticum confers plant resistance to multiple phytopathogens and reduces fungicide resistance. Pestic. Biochem. Physiol. 2019, 153, 36–46. [Google Scholar] [CrossRef]
- Hao, G.; McCormick, S.; Vaughan, M.M. Effects of double-stranded RNAs targeting Fusarium graminearum TRI6 on Fusarium head blight and mycotoxins. Phytopathology 2021, 111, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yi, S.-Y.; Nian, J.-N.; Yuan, Q.-S.; He, W.-J.; Zhang, J.-B.; Liao, Y.-C. Application of double-strand RNAs targeting chitin synthase, glucan synthase, and protein kinase reduces Fusarium graminearum spreading in wheat. Front. Microbiol. 2021, 12, 660976. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.T.; Gaffar, F.Y.; Schuemann, J.; Biedenkopf, D.; Koch, A.M. RNA-spray-mediated silencing of Fusarium graminearum AGO and DCL genes improve barley disease resistance. Front. Plant Sci. 2020, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Ui-Tei, K.; Naito, Y.; Takahashi, F.; Haraguchi, T.; Ohki-Hamazaki, H.; Juni, A.; Ueda, R.; Saigo, K. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 2004, 32, 936–948. [Google Scholar] [CrossRef]
- Jackson, A.L.; Bartz, S.R.; Schelter, J.; Kobayashi, S.V.; Burchard, J.; Mao, M.; Li, B.; Cavet, G.; Linsley, P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003, 21, 635–637. [Google Scholar] [CrossRef]
- Stakheev, A.A.; Samokhvalova, L.V.; Mikityuk, O.D.; Zavriev, S.K. Phylogenetic analysis and molecular typing of trichothecene-producing Fusarium fungi from Russian collections. Acta Naturae 2018, 10, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Ghag, S.B.; Shekhawat, U.K.S.; Ganapathi, T.R. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol. J. 2014, 12, 541–553. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Majumdar, R.; Rajasekaran, K.; Cary, J.W. RNA interference (RNAi) as a potential tool for control of mycotoxin contamination in crop plants: Concepts and considerations. Front. Plant Sci. 2017, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yamada, T.; Matsumiya, T.; Ui-Tei, K.; Saigo, K.; Morishita, S. dsCheck: Highly sensitive off-target search software for double-stranded RNA-mediated RNA interference. Nucleic Acids Res. 2005, 33, W589–W591. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Boutros, M. E-RNAi: A web application for the multi-species design of RNAi reagents—2010 update. Nucleic Acids Res. 2010, 38, W332–W339. [Google Scholar] [CrossRef] [PubMed]
- Yilmazel, B.; Hu, Y.; Sigoillot, F.; Smith, J.A.; Shamu, C.E.; Perrimon, N.; Mohr, S.E. Online GESS: Prediction of miRNA-like off-target effects in large-scale RNAi screen data by seed region analysis. BMC Bioinform. 2014, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Lück, S.; Kreszies, T.; Strickert, M.; Schweizer, P.; Kuhlmann, M.; Douchkov, D. siRNA-finder (si-Fi) software for RNAi-target design and off-target prediction. Front. Plant Sci. 2019, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Senthil-Kumar, M.; Dai, X.; Ramu, V.S.; Lee, S.; Mysore, K.S.; Zhao, P.X. pssRNAit: A web server for designing effective and specific plant siRNAs with genome-wide off-target assessment. Plant Physiol. 2020, 184, 65–81. [Google Scholar] [CrossRef]
- Cazares, T.; Higgs, R.E.; Wang, J.; Ozer, H.G. SeedMatchR: Identify off-target effects mediated by siRNA seed regions in RNA-seq experiments. Bioinformatics 2024, 40, btae011. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, Y.; Zhang, H.; Wang, K.; Zhao, C.; Zhu, G.; Reddy Palli, S.; Han, Z. Off-target effects of RNAi correlate with the mismatch rate between dsRNA and non-target mRNA. RNA Biol. 2021, 18, 1747–1759. [Google Scholar] [CrossRef]
- Kamola, P.; Nakano, Y.; Takahashi, T.; Wilson, P.A.; Ui-Tei, K. The siRNA non-seed region and its target sequences are auxiliary determinants of off-target effects. PLoS Comput. Biol. 2015, 11, e1004656. [Google Scholar] [CrossRef]
- Chen, J.; Sheng, C.-W.; Peng, Y.; Wang, K.; Jiao, Y.; Reddy Palli, S.; Cao, H. Transcript level and sequence matching are key determinants of off-target effects in RNAi. J. Agric. Food Chem. 2024, 72, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.T.; Proctor, R.H.; Dunlap, C.A.; Busman, M. Reducing production of fumonisin mycotoxins in Fusarium verticillioides by RNA interference. Mycotoxin Res. 2018, 34, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, T.; Islamovic, E.; Klos, K.; Schwartz, P.; Gillespie, J.; Hunter, S.; Bregitzer, P. Silencing efficiency of dsRNA fragments targeting Fusarium graminearum TRI6 and patterns of small interfering RNA associated with reduced virulence and mycotoxin production. PLoS ONE 2018, 13, e0202798. [Google Scholar] [CrossRef]
- Power, I.L.; Faustinelli, P.C.; Orner, V.A.; Sobolev, V.S.; Arias, R.S. Analysis of small RNA populations generated in peanut leaves after exogenous application of dsRNA and dsDNA targeting aflatoxin synthesis genes. Sci. Rep. 2020, 10, 13820. [Google Scholar] [CrossRef]
- Dahlmann, T.A.; Kück, U. Dicer-dependent biogenesis of small RNAs and evidence for microRNA-like RNAs in the penicillin producing fungus Penicillium chrysogenum. PLoS ONE 2015, 10, e0125989. [Google Scholar] [CrossRef]
- Mi, S.; Cai, T.; Hu, Y.; Chen, Y.; Hodges, E.; Ni, F.; Wu, L.; Li, S.; Zhou, H.; Long, C.; et al. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 2008, 133, 116–127. [Google Scholar] [CrossRef]
- Qi, X.; Bao, F.S.; Xie, Z. Small RNA deep sequencing reveals role for Arabidopsis thaliana RNA-dependent RNA polymerases in viral siRNA biogenesis. PLoS ONE 2009, 4, e4971. [Google Scholar] [CrossRef]
- Höfle, L.; Biedenkopf, D.; Werner, B.T.; Shrestha, A.; Jelonek, L.; Koch, A. Study on the efficiency of dsRNAs with increasing length in RNA-based silencing of the Fusarium CYP51 genes. RNA Biol. 2020, 17, 463–473. [Google Scholar] [CrossRef]
- Beaucage, S.L.; Reese, C.B. Recent advances in the chemical synthesis of RNA. Curr. Protoc. Nucleic Acid Chem. 2009, 38, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Papić, L.; Rivas, J.; Toledo, S.; Romero, J. Double-stranded RNA production and kinetics of recombinant Escherichia coli HT115 in fed-batch culture. Biotechnol. Rep. 2018, 20, e00292. [Google Scholar] [CrossRef]
- Guan, R.; Chu, D.; Han, X.; Miao, X.; Li, H. Advances in the development of microbial double-stranded RNA production systems for application of RNA interference in agricultural pest control. Front. Bioeng. Biotechnol. 2021, 9, 753790. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-J.; Donahue, K.; Koh, Y.; Martin, R.R.; Choi, M.-Y. Microbial-based double-stranded RNA production to develop cost-effective RNA interference application for insect pest management. Int. J. Insect Sci. 2019, 11, 1179543319840323. [Google Scholar] [CrossRef]
- Prates, L.H.F.; Merlau, M.; Rühl-Teichner, J.; Schetelig, M.F.; Häcker, I. An optimized/scale up-ready protocol for extraction of bacterially produced dsRNA at good yield and low costs. Int. J. Mol. Sci. 2023, 24, 9266. [Google Scholar] [CrossRef] [PubMed]
- Verdonckt, T.-W.; Broeck, J.V. Methods for the cost-effective production of bacteria-derived double-stranded RNA for in vitro knockdown studies. Front. Physiol. 2022, 13, 836106. [Google Scholar] [CrossRef]
- Niehl, A.; Soininen, M.; Poranen, M.M.; Heinlein, M. Synthetic biology approach for plant protection using dsRNA. Plant Biotechnol. J. 2018, 16, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Sánchez, A.R.; Romo-Quinones, C.; Rosas-Quijano, R.; Reyes, A.G.; Barraza, A.; Magallón-Barajas, F.; Angulo, C.; Mejía-Ruíz, C.H. Production of specific dsRNA against white spot syndrome virus in the yeast Yarrowia lipolytica. Aquac. Res. 2018, 49, 480–491. [Google Scholar] [CrossRef]
- Duman-Scheel, M. Saccharomyces cerevisiae (Baker’s yeast) as an interfering RNA expression and delivery system. Curr. Drug Targets 2019, 20, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Holeva, M.C.; Sklavounos, A.; Rajeswaran, R.; Pooggin, M.M.; Voloudakis, A.E. Topical application of double-stranded RNA targeting 2b and CP genes of Cucumber mosaic virus protects plants against local and systemic viral infection. Plants 2021, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Nerva, L.; Sandrini, M.; Gambino, G.; Chitarra, W. Double-stranded RNAs (dsRNAs) as a sustainable tool against gray mold (Botrytis cinerea) in grapevine: Effectiveness of different application methods in an open-air environment. Biomolecules 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Dong, Y.C.; Li, P.; Niu, C.Y. The effect of silencing 20E biosynthesis relative genes by feeding bacterially expressed dsRNA on the larval development of Chilo suppressalis. Sci. Rep. 2016, 6, 28697. [Google Scholar] [CrossRef]
- Hough, J.; Howard, J.D.; Brown, S.; Portwood, D.E.; Kilby, P.M.; Dickman, M.J. Strategies for the production of dsRNA biocontrols as alternatives to chemical pesticides. Front. Bioeng. Biotechnol. 2022, 10, 980592. [Google Scholar] [CrossRef]
- Bennett, M.; Deikman, J.; Hendrix, B.; Iandolino, A. Barriers to efficient foliar uptake of dsRNA and molecular barriers to dsRNA activity in plant cells. Front. Plant Sci. 2020, 11, 816. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; McMillan, J.; Cardoza, V.; Maegele, I.; Dadami, E.; Runne, M.; Krczal, G.; Wassenegger, M. Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front. Plant Sci. 2016, 7, 1327. [Google Scholar] [CrossRef]
- Jibrin, M.O.; Liu, Q.; Jones, J.B.; Zhang, S. Surfactants in plant disease management: A brief review and case studies. Plant Pathol. 2021, 70, 495–510. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Mosa, M.A.; Yuossef, K. Topical delivery of host induced RNAi silencing by layered hydroxide nanosheets: An efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiol. Mol. Plant Pathol. 2021, 115, 101684. [Google Scholar] [CrossRef]
- Chen, X.; Shi, T.; Tang, T.; Chen, C.; Liang, Y.; Zuo, S. Nanosheet-facilitated spray delivery of dsRNAs represents a potential tool to control Rhizoctonia solani infection. Int. J. Mol. Sci. 2022, 23, 12922. [Google Scholar] [CrossRef]
- Lichtenberg, S.S.; Nuti, K.; DeRouchey, J.E.; Tsyuko, O.V.; Unrine, J.M. Efficacy of chitosan/double-stranded RNA polyplex nanoparticles for gene silencing under variable environmental conditions. Environ. Sci. Nano 2020, 7, 1582–1592. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Kolge, H.; Kadam, K.; Galande, S.; Lanjekar, V.; Ghormade, V. New frontiers in pest control: Chitosan nanoparticles-shielded dsRNA as an effective topical RNAi spray for gram podborer biocontrol. ACS Appl. Bio Mater. 2021, 4, 5145–5157. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, Y.; Chen, J.J.; Peng, Y.; Wang, X.; Shen, Z.; Han, Z. Comparison of efficacy of RNAi mediated by various nanoparticles in the rice striped stem borer (Chilo supressalis). Pestic. Biochem. Physiol. 2020, 165, 104467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, Q.; Lan, C.; Tang, T.; Wang, K.; Shen, J.; Niu, D. Nanoparticle carriers enhance RNA stability and uptake efficiency and prolong the protection against Rhizoctonia solani. Phytopathol. Res. 2023, 5, 2. [Google Scholar] [CrossRef]
- Qiao, L.; Niño-Sánchez, J.; Hamby, R.; Capriotti, L.; Chen, A.; Mezzetti, B.; Jin, H. Artificial nanovesicles for dsRNA delivery in spray-induced gene silencing for crop protection. Plant Biotechnol. J. 2023, 21, 854–865. [Google Scholar] [CrossRef]
- Islam, M.T.; Davis, Z.; Chen, L.; Englaender, J.; Zomorodi, S.; Frank, J.; Bartlett, K.; Somers, E.; Carballo, S.M.; Kester, M.; et al. Minicell-based fungal RNAi delivery for sustainable crop protection. Microb. Biotechnol. 2021, 14, 1847–1856. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Chen, L.-H.; De Souza, J.T.; Mosquera, S.; Stergiopoulos, I. Targeted delivery of gene silencing in fungi using genetically engineered bacteria. J. Fungi 2021, 7, 125. [Google Scholar] [CrossRef]
- Mujtaba, M.; Wang, D.; Carvalho, L.B.; Oliveira, J.L.; do Espirito Santo Pereira, A.; Sharif, R.; Jogaiah, S.; Paidi, M.K.; Wang, L.; Ali, Q.; et al. Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: Current trends and future directions. ACS Agric. Sci. Technol. 2021, 1, 417–435. [Google Scholar] [CrossRef]
- Ray, P.; Sahu, D.; Aminedi, R.; Chandran, D. Concepts and considerations for enhancing RNAi efficiency in phytopathogenic fungi for RNAi-based crop protection using nanocarrier-mediated dsRNA delivery systems. Front. Fungal Biol. 2022, 3, 977502. [Google Scholar] [CrossRef]
- Ghosh, S.; Patra, S.; Ray, S. A combinational nanobased spray-induced gene silencing technique for crop protection and improvement. ACS Omega 2023, 8, 22345–22351. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.C. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc. Am. Thorac. Soc. 2010, 7, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.C.; Fenart, L.; Chauvet, R.; Pariat, C.; Cecchelli, R.; Couet, W. Indirect evidence that drug brain targeting using polysorbate 80-coated polybutylcyanoacrylate nanoparticles is related to toxicity. Pharm. Res. 1999, 16, 1836–1842. [Google Scholar] [CrossRef]
- Singewar, K.; Fladung, M. Double-stranded RNA (dsRNA) technology to control forest insect pests and fungal pathogens: Challenges and opportunities. Funct. Integr. Genom. 2023, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Corey, D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018, 46, 1584–1600. [Google Scholar] [CrossRef] [PubMed]
- Peacock, H.; Kannan, A.; Beal, P.A.; Burrows, C.J. Chemical modification of siRNA bases to probe and enhance RNA interference. J. Org. Chem. 2011, 76, 7295–7300. [Google Scholar] [CrossRef]
- Evers, M.M.; Toonen, L.J.A.; van Roon-Mom, W.M.C. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv. Drug Deliv. Rev. 2015, 87, 90–103. [Google Scholar] [CrossRef]
- Howard, J.D.; Beghyn, M.; Dewulf, N.; De Vos, Y.; Phillips, A.; Portwood, D.; Kilby, P.M.; Oliver, D.; Maddelein, W.; Brown, S.; et al. Chemically modified dsRNA induces RNAi effects in insects in vitro and in vivo: A potential new tool for improving RNA-based plant protection. J. Biol. Chem. 2022, 298, 102311. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stakheev, A.A.; Taliansky, M.; Kalinina, N.O.; Zavriev, S.K. RNAi-Based Approaches to Control Mycotoxin Producers: Challenges and Perspectives. J. Fungi 2024, 10, 682. https://doi.org/10.3390/jof10100682
Stakheev AA, Taliansky M, Kalinina NO, Zavriev SK. RNAi-Based Approaches to Control Mycotoxin Producers: Challenges and Perspectives. Journal of Fungi. 2024; 10(10):682. https://doi.org/10.3390/jof10100682
Chicago/Turabian StyleStakheev, Alexander A., Michael Taliansky, Natalia O. Kalinina, and Sergey K. Zavriev. 2024. "RNAi-Based Approaches to Control Mycotoxin Producers: Challenges and Perspectives" Journal of Fungi 10, no. 10: 682. https://doi.org/10.3390/jof10100682
APA StyleStakheev, A. A., Taliansky, M., Kalinina, N. O., & Zavriev, S. K. (2024). RNAi-Based Approaches to Control Mycotoxin Producers: Challenges and Perspectives. Journal of Fungi, 10(10), 682. https://doi.org/10.3390/jof10100682







