Morphological and Phylogenetic Analyses Reveal Three New Species of Phyllosticta (Botryosphaeriales, Phyllostictaceae) in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Morphology
2.2. DNA Extraction and Amplification
2.3. Phylogenetic Analyses
3. Results
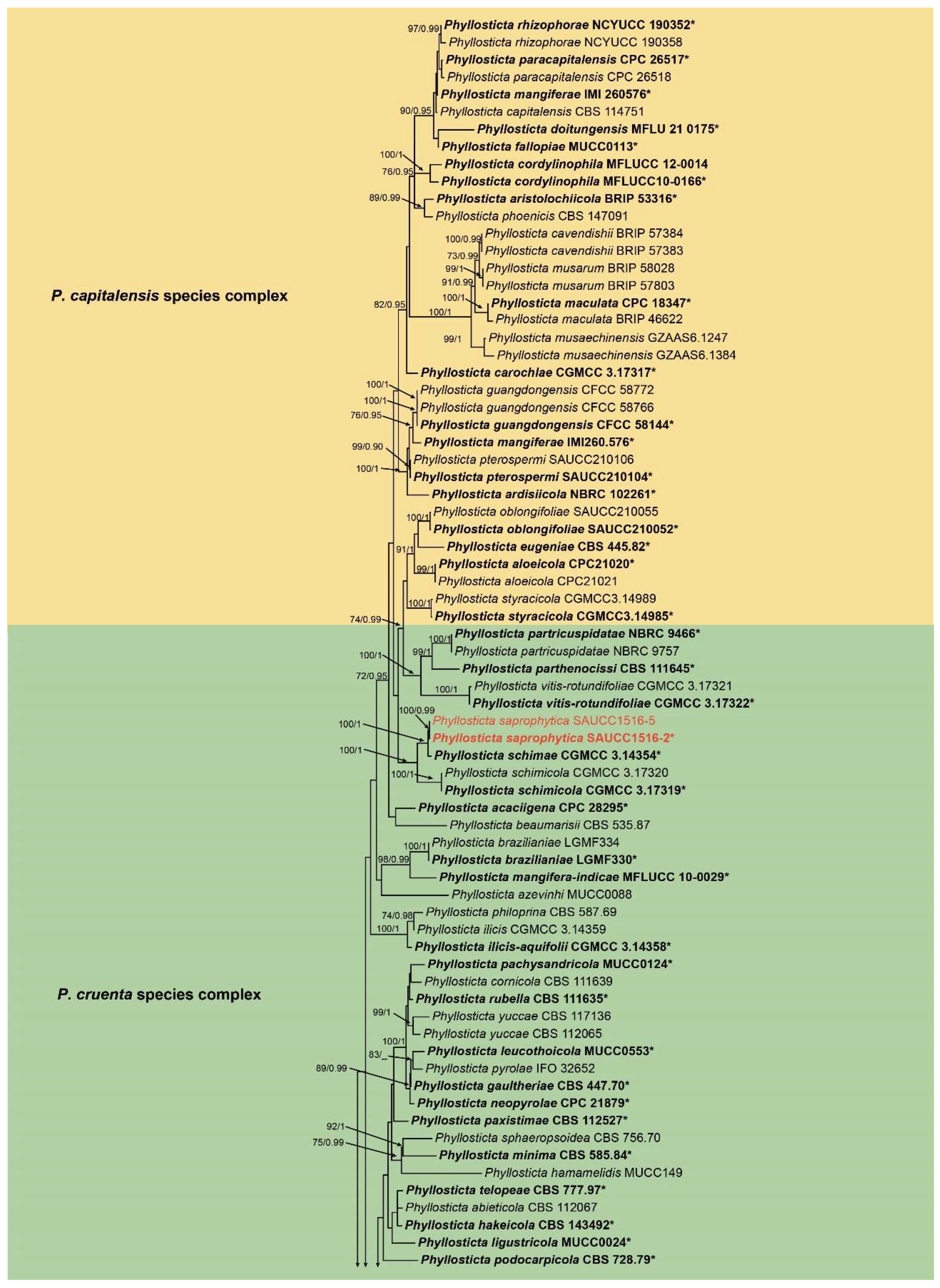
3.1. Phylogenetic Analyses
3.2. Taxonomy
3.2.1. Phyllosticta fujianensis Y. Jiang, Z.X. Zhang & X.G. Zhang, sp. nov.
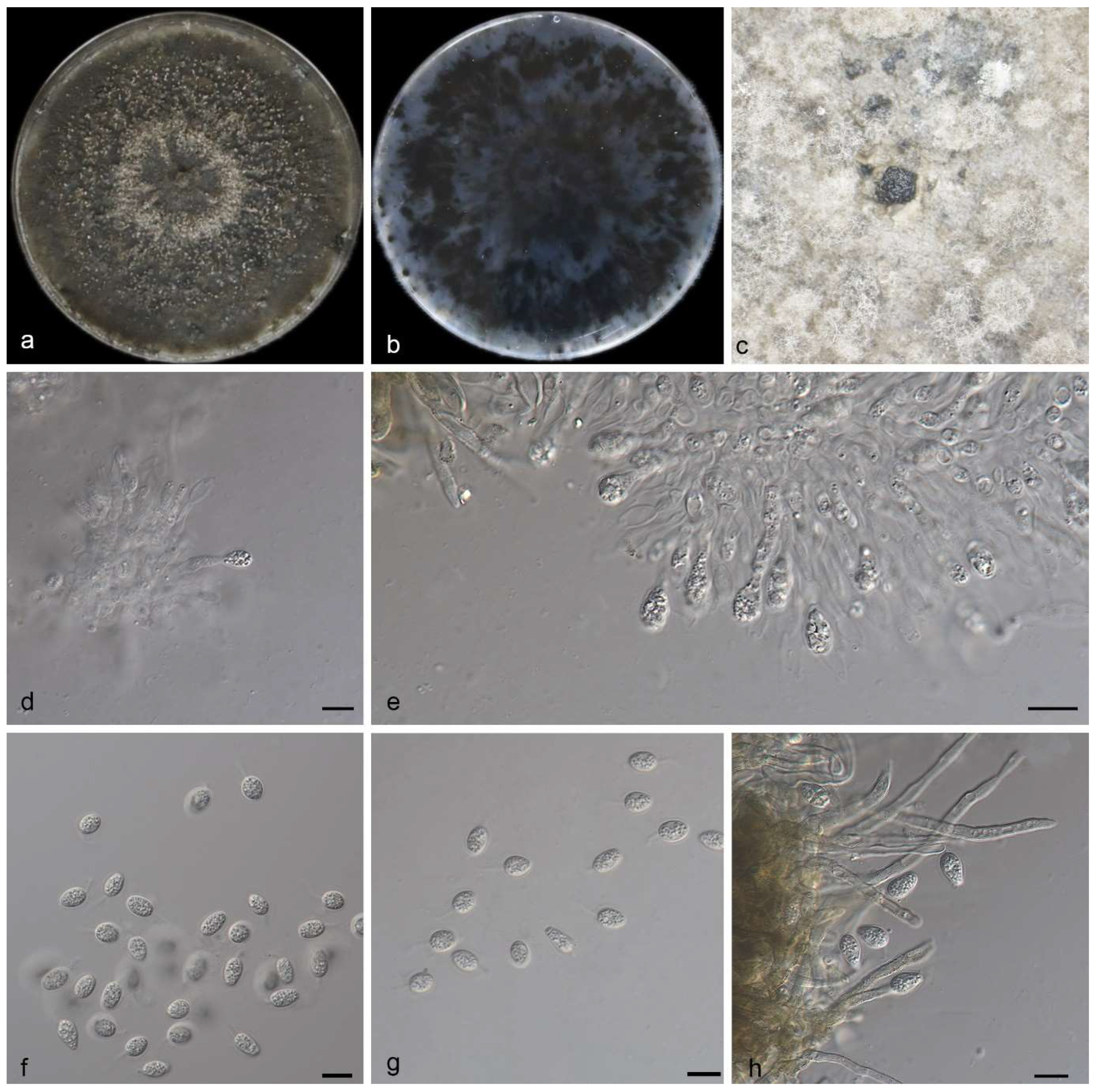
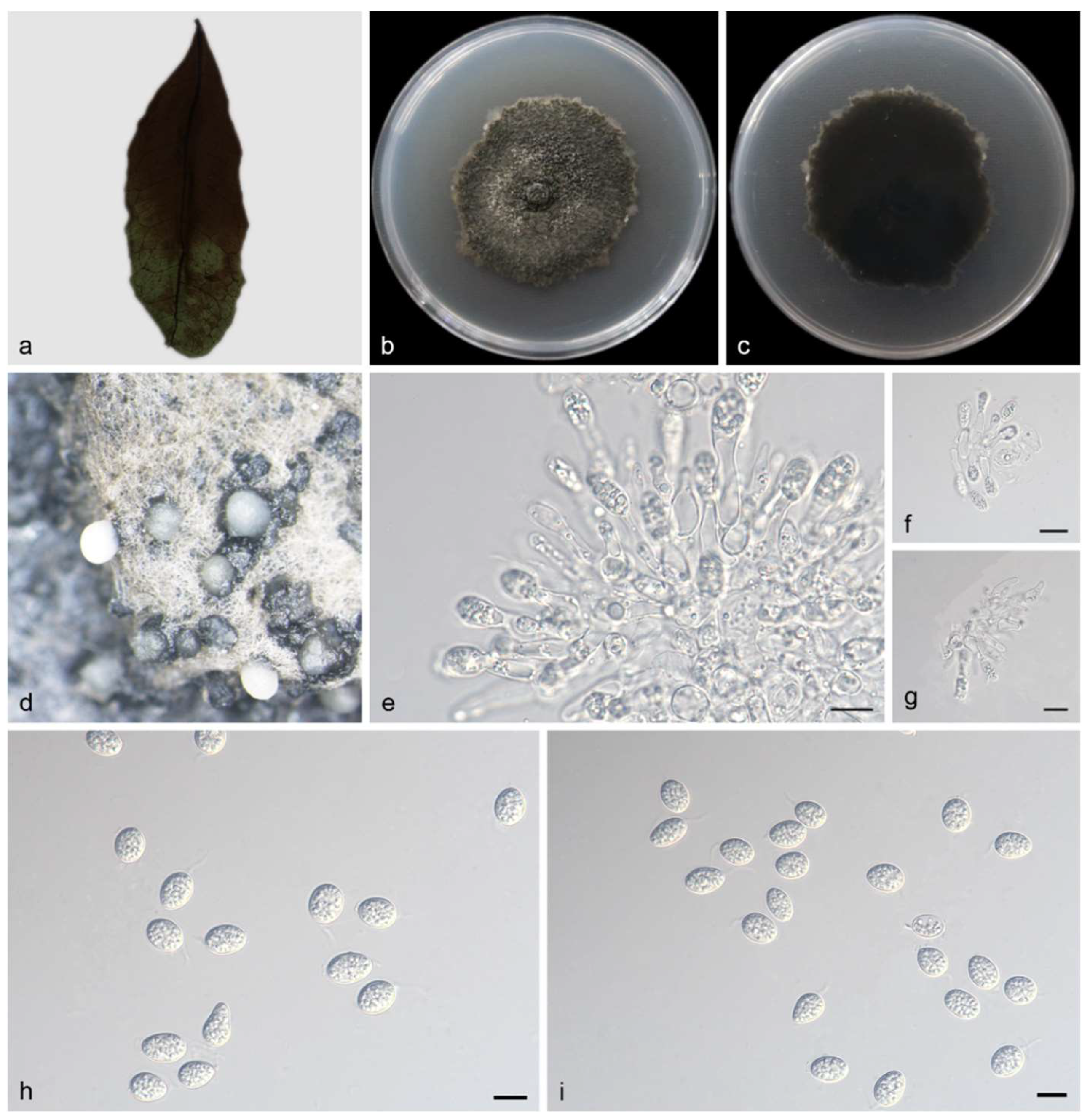
3.2.2. Phyllosticta saprophytica Y. Jiang, Z.X. Zhang & X.G. Zhang, sp. nov.
3.2.3. Phyllosticta turpiniae Y. Jiang, Z.X. Zhang & X.G. Zhang, sp. nov.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Persoon, C.H. Traité sur les Champignons Comestibles, Contenant L’indication des Espèces Nuisibles; A L’histoire des Champignons; Belin-Leprieur: Paris, France, 1818. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Hyde, K.D.; Alves, A.; Liu, J.K. Families in Botryosphaeriales: A phylogenetic, morphological and evolutionary perspective. Fungal Divers. 2019, 94, 1–22. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Al-Ani, L.K.T.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.C.; Zhao, R.L.; Aptroot, A.; Leontyev, D.V.; Saxena, R.K.; et al. Outline of Fungi and fungus-like taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Viala, P.; Ravaz, L. Sur la Dénomination Botanique (Guignardia bidwellii) du Black-Rot. Semanticscholar 1982. Available online: https://api.semanticscholar.org/CorpusID:90730616 (accessed on 25 October 2023).
- Norphanphoun, C.; Hongsanan, S.; Gentekaki, E.; Chen, Y.J.; Kuo, C.H.; Hyde, K.D. Differentiation of species complexes in Phyllosticta enables better species resolution. Mycosphere 2020, 11, 2542–2628. [Google Scholar] [CrossRef]
- Slippers, B.; Boissin, E.; Phillips, A.J.L.; Groenewald, J.Z.; Lombard, L.; Wingfield, M.J.; Postma, A.; Burgess, T.; Crous, P.W. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Stud. Mycol. 2013, 76, 31–49. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Liu, X.Y.; Zhang, X.G.; Meng, Z. Morphological and Phylogenetic Analyses Reveal Two New Species and a New Record of Phyllosticta (Botryosphaeriales, Phyllostictaceae) from Hainan, China. MycoKeys 2022, 91, 1–23. [Google Scholar] [CrossRef]
- Wang, C.B.; Yang, J.; Li, Y.; Xue, H.; Piao, C.G.; Jiang, N. Multi-Gene Phylogeny and Morphology of Two New Phyllosticta (Phyllostictaceae, Botryosphaeriales) Species from China. MycoKeys 2023, 95, 189–207. [Google Scholar] [CrossRef]
- van der Aa, H.A. Studies in Phyllosticta. Stud. Mycol. 1973, 5, 1–110. [Google Scholar]
- van der Aa, H.A.; Vanev, S. A Revision of the Species Described in Phyllosticta; Centraalbureau voor Schimmelcultures (CBS): Utrecht, The Netherlands, 2002. [Google Scholar]
- Wulandari, N.F.; Bhat, D.J.; To-anun, C. A modern account of the genus Phyllosticta. Plant Pathol. Quar. 2013, 3, 145–159. [Google Scholar] [CrossRef]
- Crous, P.W.; Carnegie, A.J.; Wingfield, M.J.; Sharma, R.; Mughini, G.; Noordeloos, M.E.; Santini, A.; Shouche, Y.S.; Bezerra, J.D.P.; Dima, B.; et al. Fungal Planet description sheets: 868–950. Persoonia 2019, 39, 291–473. [Google Scholar] [CrossRef]
- Crous, P.W.; Hernández-Restrepo, M.; Schumacher, R.K.; Cowan, D.A.; Maggs-Kölling, G.; Marais, E.; Wingfield, M.J.; Yilmaz, N.; Adan, O.C.G.; Akulov, A.; et al. New and interesting fungi. 4. Fungal Syst. Evol. 2021, 7, 255–343. [Google Scholar] [CrossRef]
- Lin, S.; Sun, X.; He, W.; Zhang, Y. Two new endophytic species of Phyllosticta (Phyllostictaceae, Botryosphaeriales) from southern China. Mycosphere 2017, 8, 1273–1288. [Google Scholar] [CrossRef]
- Wikee, S.; Lombard, L.; Crous, P.W. Phyllosticta capitalensis, a widespread endophyte of plants. Fungal Divers. 2013, 60, 91–105. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, F.J.R.M.; Lee, S.H.; Taylor, L.; Shawe-Taylor, J. Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Rehner, A.; Samuels Gary, J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequence. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA from Several Cryptococcus Species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple Evolutionary Origins of the Fungus Causing Panama Disease of Banana: Concordant Evidence from Nuclear and Mitochondrial Gene Genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Myllys, L.; Stenroos, S.; Thell, A. New genes for phylogenetic studies of lichenized fungi, glyceraldehyde-3-phosphate dehydrogenase and beta-tubulin genes. Lichenologist 2002, 34, 237–246. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Niskanen, T.; Suwannarach, N.; Wannathes, N.; Chen, Y.J.; McKenzie, E.H.C.; Maharachchikumbura, S.S.N.; Buyck, B.; Zhao, C.L.; Fan, Y.G.; et al. The numbers of fungi: Are the most specious genera truly diverse? Fungal Divers. 2022, 114, 387–462. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Lim, H.J.; Chu, S.J.; Lee, H.B. Two new species and three new records of Ascomycetes in Korea. Mycobiology 2022, 50, 30–45. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.M.; Xiong, C.L.; Gao, S.S.; Xu, W.M.; Zhao, L.L.; Song, C.Y.; Liu, X.Y.; James, T.Y.; Li, Z.; et al. ASF1 regulates asexual and sexual reproduction in Stemphylium eturmiunum by DJ-1 stimulation of the PI3K/AKT signaling pathway. Fungi Divers. 2023. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest Version 2. Program Distributed by the Author Evolutionary Biology Centre; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: Enabling high-impact science for phylogenetics researchers with limited resources. In Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the Extreme to the Campus and Beyond, Chicago, IL, USA, 16–20 July 2012; Association for Computing Machinery: San Diego, CA, USA, 2012; p. 8. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 312–1313. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Le Roux, J.J.; Richardson, D.M.; Strasberg, D.; Shivas, R.G.; Alvarado, P.; Edwards, J.; Moreno, G.; Sharma, R.; et al. Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot. Persoonia 2011, 26, 47–56. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Schumacher, R.K.; Summerell, B.A.; Giraldo, A.; Gené, J.; Guarro, J.; Wanasinghe, D.N.; Hyde, K.D.; Camporesi, E.; et al. Fungal Planet description sheets: 107–127. Persoonia 2012, 28, 212–289. [Google Scholar] [CrossRef]
- Su, Y.Y.; Cai, L. Polyphasic characterisation of three new Phyllosticta spp. Persoonia 2012, 28, 76–84. [Google Scholar] [CrossRef]
- Crous, P.W.; Cowan, D.A.; Maggs-Kölling, G. Fungal Planet description sheets: 469–557. Persoonia 2016, 37, 313–528. [Google Scholar] [CrossRef]
- Motohashi, K.; Araki, I.; Nakashima, C. Four new species of Phyllosticta, one new species of Pseudocercospora, and one new combination in Passalora from Japan. Mycoscience 2008, 49, 138–146. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Richardson, D.M.; Leroux, J.J.; Strasberg, D.; Edwards, J.; Roets, F.; Hubka, V.; Taylor, P.W.J.; Heykoop, M.; et al. Fungal Planet description sheets: 128–153. Persoonia 2012, 29, 316–458. [Google Scholar] [CrossRef]
- Paul, A.P.; Blackburn, M.D. Phyllosticta beaumarisii sp. nov.: A cause of leafspot on Muehlenbeckia adpressa. Australas. Plant Pathol. 1986, 15, 40–41. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, Q.; Carroll, G.; Zhang, N.; Shivas, R.G.; Cai, L. Polyphasic characterization of four new plant pathogenic Phyllosticta species from China, Japan, and the United States. Fungal Biol. 2015, 119, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Crous, P.W.; Henderson, J. Phyllosticta species associated with freckle disease of banana. Fungal Divers. 2012, 56, 173–187. [Google Scholar] [CrossRef]
- Young, Y. Studies in Porto Rican Parasitic Fungi–I. Mycologia 1915, 7, 143–150. [Google Scholar] [CrossRef]
- Wikee, S.; Lombard, L.; Nakashima, C.; Motohashi, K.; Chukeatirote, E.; Cheewangkoon, R.; McKenzie, E.H.C.; Hyde, K.D.; Crous, P.W. A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Stud. Mycol. 2013, 76, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Liu, Y.X.; Yuan, J.; Wang, Y.; Hyde, K.D.; Liu, Z.Y. Phyllosticta species from banana (Musa sp.) in Chongqing and Guizhou Provinces, China. Phytotaxa 2014, 188, 135–144. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Groenewald, J.Z.; Li, H.; Glienke, C.; Carstens, E.; Hattingh, V.; Fourie, P.H.; Crous, P. First report of Phyllosticta citricarpa and description of two new species, P. paracapitalensis and P. paracitricarpa, from citrus in Europe. Stud. Mycol. 2017, 87, 161–185. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, N.; Cai, L. Typification and phylogenetic study of Phyllosticta ampelicida and P. vaccinii. Mycologia 2013, 105, 1030–1042. [Google Scholar] [CrossRef]
- Zhang, K.; Su, Y.Y.; Cai, L. Morphological and phylogenetic characterisation of two new species of Phyllosticta from China. Mycol Prog. 2012, 12, 547–556. [Google Scholar] [CrossRef]
- Crous, P.W.; Cowan, D.A.; Maggs-Kölling, G.; Yilmaz, N.; Thangavel, R.; Wingfield, M.J. Fungal Planet description sheets: 625–715. Persoonia 2017, 39, 313–528. [Google Scholar] [CrossRef]
- Wang, X.H.; Chen, G.Q.; Huang, F.; Zhang, J.Z.; Hyde, K.D.; Li, H.Y. Phyllosticta species associated with citrus diseases in China. Fungal Divers. 2012, 52, 209–224. [Google Scholar] [CrossRef]
- Weidemann, G.J.; Boone, D.M.; Burdsall, H.H. Taxonomy of Phyllosticta vaccinii (Coelomycetes) and a New Name for the True Anamorph of Botryosphaeria vaccinii (Dothideales, Dothioraceae). Mycologia 1982, 74, 59–65. [Google Scholar] [CrossRef]
- Crous, P.W.; Summerell, B.A.; Shivas, R.G.; Romberg, M.; Mel’nik, V.A.; Verkley, G.J.; Groenewald, J.Z. Fungal Planet description sheets: 92–106. Persoonia 2011, 27, 130–162. [Google Scholar] [CrossRef] [PubMed]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Wingfield, M.J.; Akulov, A.; Carnegie, A.J.; Cheewangkoon, R.; Gramaje, D.; Groenewald, J.Z.; Guarnaccia, V.; Halleen, F.; et al. Genera of phytopathogenic fungi: GOPHY 2. Stud. Mycol. 2019, 92, 47–133. [Google Scholar] [CrossRef] [PubMed]
- Wikee, S.; Wulandari, N.F.; McKenzie, E.H.; Hyde, K.D. Phyllosticta ophiopogonis sp. nov. from Ophiopogon japonicus (Liliaceae). Saudi J. Biol. Sci. 2011, 9, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Okane, I.; Lumyong, S.; Ito, T.; Nakagiri, A. Extensive host range of an endophytic fungus, Guignardia endophyllicola (anamorph, Phyllosticta capitalensis). Mycoscience 2003, 44, 353–363. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Jeewon, R.; Ghobad-Nejhad, M.; Wanasinghe, D.N.; Liu, N.G.; Phillips, A.J.L.; Oliveira-Filho, J.R.C.; da Silva, G.A.; Gibertoni, T.B.; et al. One stop shop II: Taxonomic update with molecular phylogeny for important phytopathogenic genera: 26–50. Fungal Divers. 2019, 94, 41–129. [Google Scholar] [CrossRef]
- Tran, N.T.; Miles, A.K.; Dietzgen, R.G.; Drenth, A. Phyllosticta capitalensis and P. paracapitalensis are endophytic fungi that show potential to inhibit pathogenic P. citricarpa on Citrus. Australas. Plant Pathol. 2019, 48, 281–296. [Google Scholar] [CrossRef]
- Hattori, Y.; Motohashi, K.; Tanaka, K.; Nakashima, C. Taxonomical re-examination of the genus Phyllosticta–Parasitic fungi on Cupressaceae trees in Japan. For. Pathol. 2020, 50, 2542–2628. [Google Scholar] [CrossRef]
- Nasehi, A.; Sathyapriya, H.; Wong, M.Y. First report of leaf spot on oil palm caused by Phyllosticta capitalensis in Malaysia. Plant Dis. 2019, 103, 2964. [Google Scholar] [CrossRef]
- Liao, Y.M.; Wang, Z.X.; Wei, M.C.; Wang, C. First report of Phyllosticta capitalensis causing black spot disease on Psidium guajava in mainland China. Plant Dis. 2020, 104, e3252. [Google Scholar] [CrossRef]
- Tang, J.R.; Liu, Y.L.; Yin, X.G.; Lu, J.N.; Zhou, Y.H. First report of castor dark leaf spot caused by Phyllosticta capitalensis in Zhanjiang, China. Plant Dis. 2020, 104, 1856. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, L.; Chen, X.R.; Lin, L.; Chen, H.G. Phyllosticta ephedricola sp. nov. on Ephedra intermedia. Mycotaxon 2013, 125, 165–167. [Google Scholar] [CrossRef]
- Zhang, K.; Shivas, R.G.; Cai, L. Synopsis of Phyllosticta in China. Mycology 2015, 6, 50–75. [Google Scholar] [CrossRef]


| Loci | PCR Primers | Sequence (5′–3′) | PCR Cycles | References |
|---|---|---|---|---|
| ITS | ITS5 | GGA AGT AAA AGT CGT AAC AAG G | (94 °C: 30 s, 55 °C: 30 s, 72 °C: 1 min) × 35 cycles | [16] |
| ITS4 | TCC TCC GCT TAT TGA TAT GC | |||
| LSU | LR0R | GTA CCC GCT GAA CTT AAG C | (94 °C: 30 s, 51 °C: 30 s, 72 °C: 1 min) × 35 cycles | [17,18] |
| LR7 | TAC TAC CAC CAA GAT CT | |||
| TEF1α | EF1-728F | CAT CGA GAA GTT CGA GAA GG | (94 °C: 30 s, 48 °C: 30 s, 72 °C: 1 min) × 35 cycles | [19,20] |
| EF2 | GGA RGT ACC AGT SAT CAT GTT | |||
| ACT | ACT-512F | ATG TGC AAG GCC GGT TTC GC | (94 °C: 30 s, 52 °C: 30 s, 72 °C: 1 min) × 35 cycles | [20] |
| ACT-783R | TAC GAG TCC TTC TGG CCC AT | |||
| GPDH | Gpd1-LM | ATT GGC CGC ATC GTC TTC CGC AA | (94 °C: 30 s, 52 °C: 30 s, 72 °C: 1 min) × 35 cycles | [21] |
| Gpd2-LM | CCC ACT CGT TGT CGT ACC A |
| Species | Conidiogenous Cells | Size of Conidiogenous Cells (μm) | Conidia | Size of Conidia (μm) | References |
|---|---|---|---|---|---|
| Phyllosticta rhizophorae | Reduced to conidiogenous cells, subcylindrical to ampulliform | 13.0–25.0 × 3.0–5.0 μm | Terminal, subcylindrical, hyaline, smooth | 10.0–17.0 × 3.0–5.0 μm | [5] |
| P. oblongifoliae | Indistinct | 9.0–14.0 × 2.5–4.5 μm | Hyaline, aseptate, ovoid, ampulliform, ellipsoidal to subglobose | 8.0–13.0 × 6.0–8.0 μm | [7] |
| P. pterospermi | Cylindrical, hyaline, smooth | 7.5–11.0 × 2.5–4.5 μm | Hyaline, aseptate, thin- and smooth-walled, obovoid, ellipsoidal to subglobose | 8.0–12.0 × 4.5–8.5 μm | [7] |
| P. anhuiensis | Phialidic, hyaline, thin-walled, smooth, subcylindrical to ampulliform | 10.0–16.0 × 2.5–4.5 μm | Solitary, hyaline, aseptate, thin- and smooth-walled, coarsely guttulate, globose or ellipsoid to obvoid | 8.5–12.0×5.5–9.0 μm | [8] |
| P. guangdongensis | Subcylindrical to ampulliform, hyaline, smooth | 10.0–15.0 × 2.5–4.0 μm | Solitary, hyaline, aseptate, thin and smooth-walled, ellipsoid to obovoid | 10.0–14.0 × 6.0–8.0 μm | [8] |
| P. musarum | Cylindrical or conical | 4.0–11.0 × 2.5–5.0 µm | One-celled, obovoidal, ellipsoidal or short cylindrical, pyriform when young | 15.0–18.0 × 9.0–10.0 µm | [9] |
| P. phoenicis | Subcylindrical to ampulliform, terminal, hyaline, smooth, coated in a mucoid layer | 9.0–15.0 × 3.0–4.0 μm | Solitary, hyaline, aseptate, thin- and smooth-walled, granular | 10.0–15.0 × 7.0–9.0 μm | [13] |
| P. doitungensis | Terminal, subcylindrical, hyaline, smooth | 6.0–8.0 × 2.0–4.0 μm | Solitary, hyaline, aseptate, thin and smooth-walled, with a single, large central guttulate, tapering | 5.0–8.0 × 3.5–6.0 μm | [22] |
| P. gwangjuensis | Subcylindrical, ampulliform, hyaline | 8.5–22.5 × 3.5–5.5 μm | Ovoid to ellipsoid shape, rounded at both ends | 10.0–13.5 × 7.0–9.0 μm | [23] |
| P. citribraziliensis | Subcylindrical to doliiform, hyaline, smooth, coated in a mucoid layer | 7.0–20.0 × 3.0–4.0 μm | Solitary, hyaline, aseptate, thin- and smooth-walled, coarsely guttulate, ellipsoid to obovoid | 10.0–12.0 × 6.0–8.0 μm | [31] |
| P. bifrenariae | Subcylindrical to ampulliform, hyaline, smooth | 7.0–10.0 × 4.0–5.0 μm | Solitary, hyaline, aseptate, thin- and smooth-walled, ellipsoid to ovoid or obovoid, tapering toward a narrowly truncate base | 11.0–13.0 × 8.0–9.0 μm | [31] |
| P. ericarum |
Subcylindrical, hyaline, smooth, coated in a mucoid layer | 12.0–20.0 × 3.0–4.0 μm |
Solitary, hyaline, aseptate, thin- and smooth walled, coarsely guttulate | 8.0–12.0 × 6.0–7.0 μm | [32] |
| P. hostae |
Holoblastic, phialidic, cylindrical, subcylindrical to ampulliform, hyaline, thin-walled, smooth | 7.0–22.0 × 2.0–5.0 μm |
Unicellular, thin- and smooth-walled, ellipsoid, subglobose to obovoid, with a large central guttule | 8.0–15.0 × 5.0–9.0 μm | [33] |
| P. ilicis-aquifolii |
Holoblastic, phialidic cylindrical, subcylindrical to ampulliform, hyaline, thin-walled, smooth | 12.0–17.0 × 3.0–4.0 μm, | Unicellular, thin-and smooth-walled, globose ellipsoid to obovoid | 12.0–17.0 × 2.0–3.0 μm | [33] |
| P. schimae | Holoblastic, phialidic, short cylindrical, subcylindrical to ampulliform, hyaline, thin-walled, smooth | 8.0–30.0 × 2.0–4.0 μm | Unicellular, thin- and smooth-walled, globose, ellipsoid to obovoid | 7.0–13.0 × 4.0–7.0 μm | [33] |
| P acaciigena | Terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer | 7.0–15.0 × 3.0–5.0 μm | Solitary, hyaline, aseptate, thin and smooth-walled, coarsely guttulate, ellipsoid to obovoid | 12.0–15.0 × 7.0–8.0 μm | [34] |
| P. ardisiicola | Holoblasticae, hyalinae, cylindricae vel conicae | 5.0–12.5 × 1.2–2.5 μm | Globosa, elliptica vel obovata, primo basi truncata, posterius utrinque rotundata | 7.0–11.0 × 5.0–7.5 μm | [35] |
| P. aspidistricola | Hyalinae, cylindricae, conicae vel lageniformes | 7.0–12.5 × 1.2–2.5 μm | Globosa, elliptica vel obovata, primo basi truncata, posterius utrinque rotundata | 9.5–12.5 × 8.5–10.0 μm | [35] |
| P. fallopiae | Holoblasticae, hyalinae, cylindricae vel conicae | 5.0–10.0 × 1.2–2.5 μm | globosa, elliptica vel obovata, primo basi truncata, posterius utrinque rotundata | 8.5–12.5 × 6.0–7.5 μm | [35] |
| P. aristolochiicola | Hyaline, smooth, subcylindrical to ampulliform | 10.0–20.0 × 2.0–4.0 μm | Subglobose, bovoid, rounded apex, hyaline | 7.0–16.0 × 6.5–11.0 μm | [36] |
| P. beaumarisii | Distinctive | Distinctive | One-celled, hyaline, ovoid–ellipsoidal | 7.5–15.0 × 6.5–8.7 μm | [37] |
| P. carochlae | Holoblastic, hyaline, cylindrical | 2.0–6.5 × 5.5–13.0 μm | Unicellular, ovoid, obovoid, ellipsoidal to subglobose | 6.0–8.5 × 10.0–12.0 μm | [38] |
| P. partricuspidatae | Holoblastic, hyaline, long cylindrical | 2.0–6.0 × 3.5–12.0 μm | Unicellular, thin- and smooth-walled, ampulliform, ovoid, obovoid, ellipsoidal to subglobose | 5.0–8.5 × 8.0–12.0 μm | [38] |
| P. schimicola | Holoblastic, hyaline, long cylindrical, subcylindrical to ampulliform | 1.5–4.5 × 5.0–12.0 μm | Unicellular, smooth-walled, ovoid to long ovoid, ampulliform, ellipsoidal to subglobose | 5.0–8.0 × 8.0–11.0 μm | [38] |
| P. vitis-rotundifoliae | Holoblastic, hyaline, long cylindrical, subcylindrical to ampulliform | 5.0–11.0 × 2.0–5.5 μm | Unicellular, thin- and smooth-walled, ampulliform, ovoid, obovoid, ellipsoidal to subglobose, truncate at the base | 6.0–9.5 × 9.0–13.0 μm | [38] |
| P. cavendishii | Doliiform or subcylindrical, solitary, hyaline | 8.0–12.0 × 4.0–5.0 μm, | Solitary, hyaline, aseptate, coarsely guttulate, thin and smooth-walled | 13.0–16.0 × 8.0–9.0 μm | [39] |
| P. maculata | Subcylindrical to doliiform, solitary, hyaline | 9.0–12.0 × 4.0–5.0 μm | Oblong or obovoid to subclavate, apex solitary, hyaline | 16.0–19.0 × 10.0–12.0 μm | [39] |
| P. eugeniae | Distinctive | Distinctive | Ovate, hyaline, granular | 9.6–16.8 × 4.8–6.0 μm | [40] |
| P. kerriae | Hyalinae, cylindricae vel conicae | 5.0–7.5 × 1.2–2.5 μm | Obovata, primo basi truncata, posterius utrinque rotundata | 9.5–12.5 × 6.0–7.5 μm | [40] |
| P. citrimaxima | Phialidic, cylindrical, hyaline | 3.0–5.0 × 1.0–2.0 μm | Ellipsoidal, hyaline, one-celled, smooth | 5.0–8.0 × 3.0–7.0 μm | [41] |
| P. mangifera-indicae | Lining the inner wall, phialidic, cylindrical, hyaline | 3.0–5.0 × 3.0–4.0 μm | Ellipsoidal, hyaline, aseptate, smooth | 6.0–13.0 × 4.0–6.0 μm | [41] |
| P. musaechinensis | Hyaline, aseptate, coarsely guttulate, ellipsoidal or clavate, thin- and smooth-walled | 14.0–18.0 × 8.0–12.0 μm | Hyaline, aseptate, coarsely guttulate, ellipsoidal, clavate or irregular, thin- and smooth-walled | 5.5–22.5 × 8.5–13.0 μm | [42] |
| P. paracapitalensis | Terminal, subcylindrical, hyaline, smooth, coated in a mucoid layer | 7.0–15.0 × 3.0–4.0 μm | Solitary, hyaline, aseptate, thin and smooth-walled, granular | 3.0–14.0 × 6.0–7.0 μm | [43] |
| P. paracitricarpa | Subcylindrical, hyaline, smooth, coated in a mucoid layer | 12.0–17.0 × 3.0–4.0 μm | Solitary, hyaline, aseptate, thin- and smooth-walled, granular | 11.0–15.0 × 7.0–9.0 μm | [43] |
| P. parthenocissi | Cylindrical or conical | 7.5–12.5 × 2.5–3.5 μm | One-celled, globose or subglobose | 7.5–10.0 × 6.0–9.0 μm | [44] |
| P. styracicola | Holoblastic, hyaline, cylindrical | 2.0–3.5 × 8.0–12.5 μm |
One-celled, ellipsoidal to subglobose, surrounded by a thick mucilaginous layer | 9.5–13.0 × 6.5–9.0 μm | [45] |
| P. catimbauensis | Terminal, subcylindrical to ampulliform, hyaline, smooth | 9.5–10.5 × 3–3.5 μm | Solitary, hyaline, aseptate, thin- and smooth-walled, granular, ellipsoid, globose, subglobose | 8.5–10.5 × 5.5–6 μm | [46] |
| P. citrichinensis | Holoblasticae, phialidicae, breviter cylindricae, lageniformes, singulae, hyalina, tenui-muratas, laevia | 6.0–12.0 × 2.0–5.0 μm | Elliptica vel ovoidea, utrinque rotundata, singulae, muratis-levi, strato mucoso circumdantia | 8.0–12.0 × 6.0–9.0 μm | [47] |
| P. elongata | Distinctive | Distinctive | Anguste obovatis | 9.0–14.0 × 5.0–8.0 μm | [48] |
| P. hymenocallidicola | Terminal, subcylindrical to doliiform, hyaline, smooth, coated with a mucoid layer | 7.0–15.0 × 3.0–4.0 μm | Solitary, hyaline, aseptate, thin- and smooth-walled, coarsely guttulate, or with large, single, central guttule, ellipsoid to obovoid | 8.0–11.0 × 6.0–7.0 μm | [49] |
| P. iridigena | Doliiform, hyaline, smooth, proliferating percurrently at apex | 4.0–7.0 × 4.0–6.0 μm | Solitary, ellipsoid to obovoid, aseptate, smooth, hyaline, guttulate, granular | 10.0–15.0 × 7.0–9.0 μm | [50] |
| P. ophiopogonis | Holoblastic, determinate, discrete, hyaline, sometimes, rarely integrated | 7.0–12.0 × 2.0–4.0 μm | Hyaline, one-celled, coarse-guttulate, smooth-walled, globose, ellipsoidal, clavate or obclavate | 10.0–14.0 × 7.0–8.0 μm | [51] |
| P. saprophytica | Oval to ampule, smooth, clear | 11.0–15.0 × 2.5–3.5 μm | Transparent, without diaphragm, with thin, smooth walls, ovoid, ampulliform | 13.0–15.0 × 5.5–7.0 μm | This study |
| P. fujianensis | Subcylindrical, ampulliform, hyaline, smooth | 10.0–14.0 × 1.5–2.0 μm | Without diaphragm, with thin smooth walls, ovoid, ampoule-shaped | 10.0–11.0 × 3.5–5.0 μm | This study |
| P. turpiniae | Terminal, subcylindrical, ampulliform, hyaline, smooth | 9.0–20.0 × 2.5–5.0 μm | Hyaline, aseptate, thin- and smooth-walled | 17.0–22.0 × 8.0–10.0 μm | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhang, Z.; Zhang, J.; Wang, S.; Zhang, X. Morphological and Phylogenetic Analyses Reveal Three New Species of Phyllosticta (Botryosphaeriales, Phyllostictaceae) in China. J. Fungi 2024, 10, 7. https://doi.org/10.3390/jof10010007
Jiang Y, Zhang Z, Zhang J, Wang S, Zhang X. Morphological and Phylogenetic Analyses Reveal Three New Species of Phyllosticta (Botryosphaeriales, Phyllostictaceae) in China. Journal of Fungi. 2024; 10(1):7. https://doi.org/10.3390/jof10010007
Chicago/Turabian StyleJiang, Yang, Zhaoxue Zhang, Jie Zhang, Shi Wang, and Xiuguo Zhang. 2024. "Morphological and Phylogenetic Analyses Reveal Three New Species of Phyllosticta (Botryosphaeriales, Phyllostictaceae) in China" Journal of Fungi 10, no. 1: 7. https://doi.org/10.3390/jof10010007
APA StyleJiang, Y., Zhang, Z., Zhang, J., Wang, S., & Zhang, X. (2024). Morphological and Phylogenetic Analyses Reveal Three New Species of Phyllosticta (Botryosphaeriales, Phyllostictaceae) in China. Journal of Fungi, 10(1), 7. https://doi.org/10.3390/jof10010007







