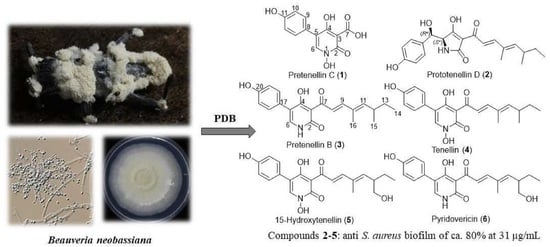
Bioprospection of Tenellins Produced by the Entomopathogenic Fungus Beauveria neobassiana
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungal Material
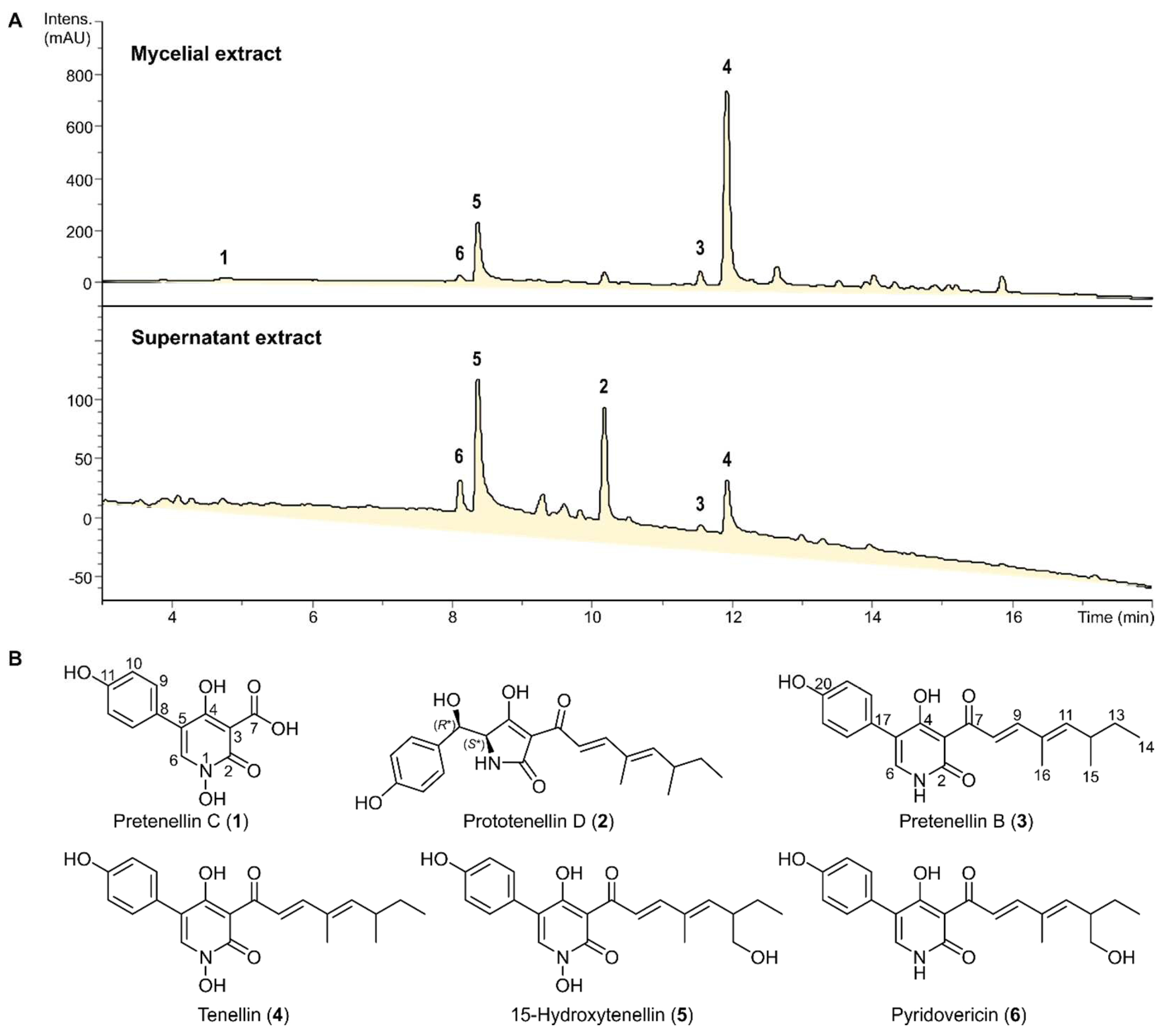
2.3. Cultivation, Extraction and Isolation
2.4. Spectral Data
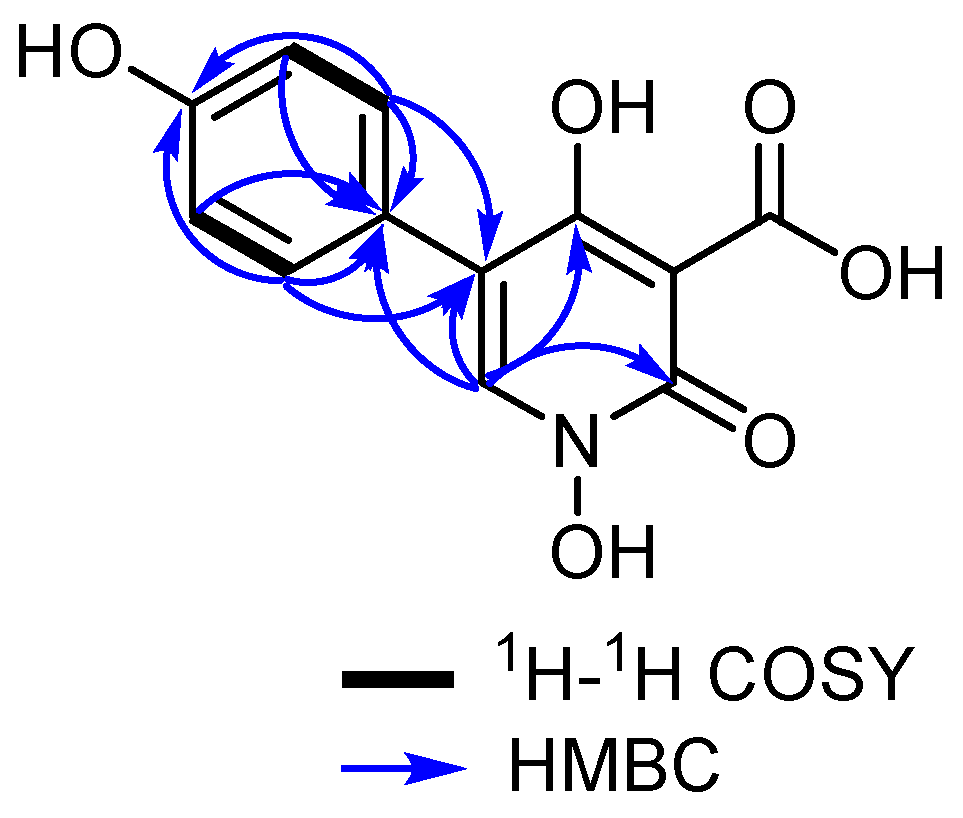
2.4.1. Pretenellin C (1)
2.4.2. Prototenellin D (2)
2.4.3. Pretenellin B (3)
2.4.4. Tenellin (4)
2.4.5. 15-Hydroxytenellin (5)
2.4.6. Pyridovericin (6)
2.5. Biological Assays
3. Results
3.1. Isolation and Structure Elucidation of Secondary Metabolites
| pos. | δH a (multi, J [Hz]) | δC, b Type |
|---|---|---|
| 2 | 161.2, CO | |
| 3 | 98.2, C | |
| 4 | 170.5, CO | |
| 5 | 123.6, C | |
| 6 | 8.18 (s, 1H) | 138.7, CH |
| 7 | 13.94 (br) | n.d. c |
| 8 | 115.3, C | |
| 9,13 | 7.42 (d, 8.3, 2H) | 131.2, CH |
| 10,12 | 6.92 (d, 8.3, 2H) | 115.9, CH |
| 11 | 158.4, C |
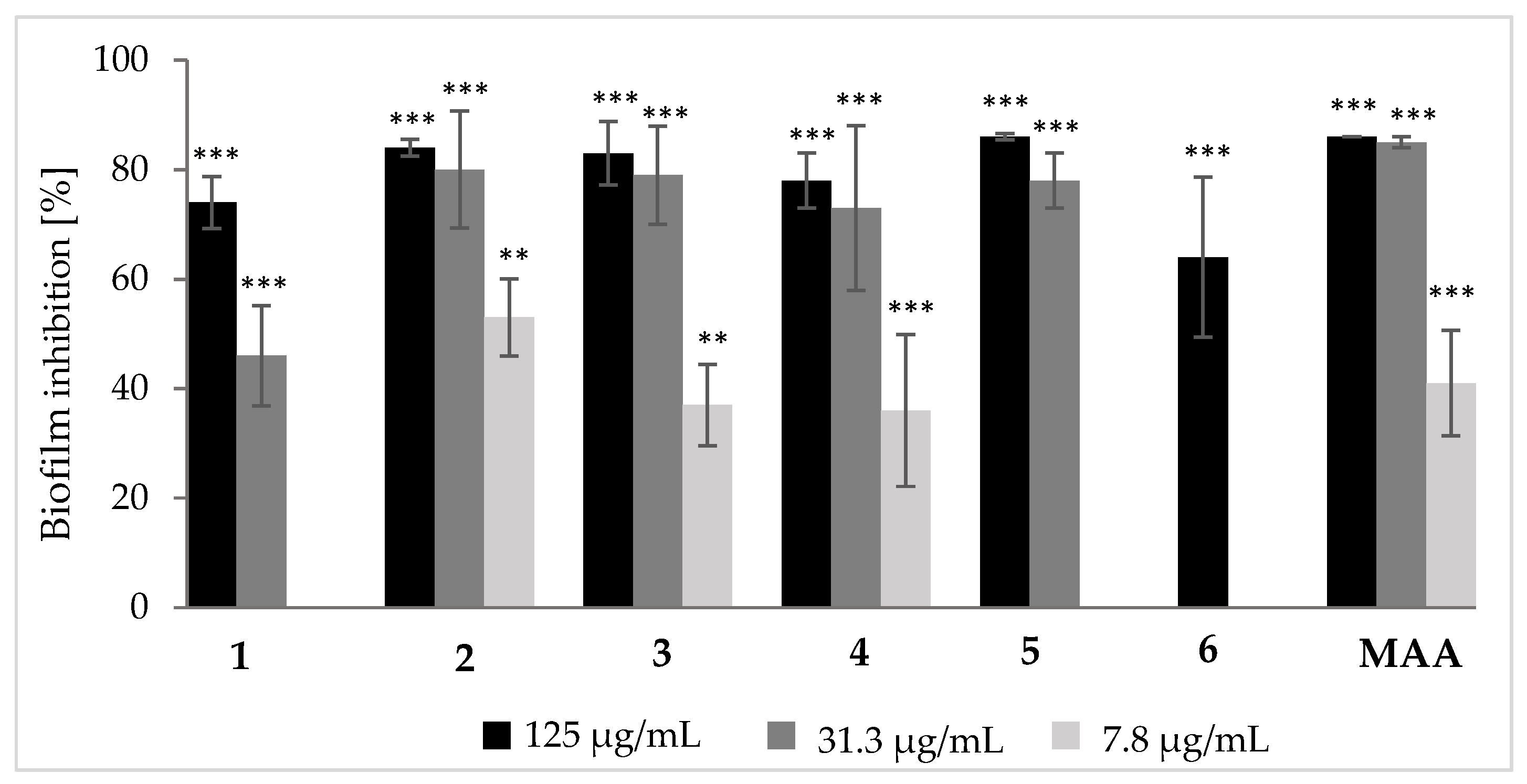
3.2. Biological Effects of Tenellin-Derived Metabolites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mapook, A.; Hyde, K.D.; Hassan, K.; Kemkuignou, B.M.; Čmoková, A.; Surup, F.; Kuhnert, E.; Paomephan, P.; Cheng, T.; de Hoog, S.; et al. Ten decadal advances in fungal biology leading towards human well-being. Fungal Divers. 2022, 116, 547–614. [Google Scholar] [CrossRef]
- Niego, G.A.T.; Lambert, C.; Mortimer, P.; Thongklang, N.; Rapior, S.; Grosse, M.; Schrey, H.; Charria-Girón, E.; Hyde, K.D.; Stadler, M. The contribution of fungi to the global economy. Fungal Divers. 2023, 121, 95–137. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Khonsanit, A.; Thanakitpipattana, D.; Tasanathai, K.; Noisripoom, W.; Lamlertthon, S.; Himaman, W.; Houbraken, J.; Samson, R.A.; Luangsa-ard, J. Revisiting Metarhizium and the description of new species from Thailand. Stud. Mycol. 2020, 95, 171–251. [Google Scholar] [CrossRef] [PubMed]
- Kobmoo, N.; Arnamnart, N.; Pootakham, W.; Sonthirod, C.; Khonsanit, A.; Kuephadungphan, W.; Suntivich, R.; Mosunova, O.V.; Giraud, T.; Luangsa-ard, J.J. The integrative taxonomy of Beauveria asiatica and B. bassiana species complexes with whole-genome sequencing, morphometric and chemical analyses. Persoonia 2021, 47, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Li, X.M.; Sun, Z.C.; Meng, L.H.; Wang, B.G. Secondary metabolites with agricultural antagonistic potentials from Beauveria felina, a marine-derived entomopathogenic fungus. J. Agric. Food Chem. 2020, 68, 14824–14831. [Google Scholar] [CrossRef]
- Buchanan, G.O.; Williams, L.A.; Reese, P.B. Biotransformation of cadinane sesquiterpenes by Beauveria bassiana ATCC 7159. Phytochemistry 2000, 54, 39–45. [Google Scholar] [CrossRef]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef] [PubMed]
- Halo, L.M.; Heneghan, M.N.; Yakasai, A.A.; Song, Z.; Williams, K.; Bailey, A.M.; Cox, R.J.; Lazarus, C.M.; Simpson, T.J. Late stage oxidations during the biosynthesis of the 2-pyridone tenellin in the entomopathogenic fungus Beauveria bassiana. J. Am. Chem. Soc. 2008, 130, 17988–17996. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Takahashi, S.; Kakinuma, N.; Uchida, K.; Hashimoto, R.; Yanagisawa, T.; Nakagawa, A. Pyridovericin and pyridomacrolidin: Novel metabolites from entomopathogenic fungi Beauveria bassiana. J. Antibiot. 1998, 51, 596–598. [Google Scholar] [CrossRef]
- Becker, K.; Wessel, A.-C.; Luangsa-ard, J.J.; Stadler, M. Viridistratins A-C, antimicrobial and cytotoxic benzo[j]fluoranthenes from stomata of Annulohypoxylon viridistatum (Hypoxylaceae, Ascomycota). Biomolecules 2020, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Charria-Girón, E.; Stchigel, A.M.; Čmoková, A.; Kolařík, M.; Surup, F.; Marin-Felix, Y. Amesia hispanica sp. nov., producer of the antifungal class of antibiotics dactylfungins. J. Fungi 2023, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Soliga, K.J.; Bär, S.I.; Oberhuber, N.; Zeng, H.; Schrey, H.; Schobert, R. Synthesis and bioactivity of ancorinoside B, a marine diglycosyl tetramic acid. Mar. Drugs 2021, 19, 583. [Google Scholar] [CrossRef] [PubMed]
- Chepkirui, C.; Yuyama, K.T.; Wanga, L.A.; Decock, C.; Matasyoh, J.C.; Abraham, W.-R.; Stadler, M. Microporenic acids A-G, biofilm inhibitors, and antimicrobial agents from the basidiomycete Microporus Species. J. Nat. Prod. 2018, 81, 778–784. [Google Scholar] [CrossRef]
- Gerth, K.; Bedorf, N.; Höfle, G.; Irschik, H.; Reichenbach, H. Epothilons A and B: Antifungal and cytotoxic compounds from Sorangium cellulosum (Myxobacteria): Production, physico-chemical and biological properties. J. Antibiot. 1996, 49, 560–563. [Google Scholar] [CrossRef]
- Goodin, S.; Kane, M.P.; Rubin, E.H. Epothilones: Mechanism of action and biologic activity. J. Clin. Oncol. 2004, 22, 2015–2025. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, D.; Wu, D.; He, W.; Zuo, M.; Zhu, W.; Xu, Y.; Wang, L. Two New 4-Hydroxy-2-pyridone Alkaloids with antimicrobial and cytotoxic activities from Arthrinium sp. GZWMJZ-606 endophytic with Houttuynia cordata Thunb. Molecules 2023, 28, 2192. [Google Scholar] [CrossRef]
- Bergmann, S.; Schümann, J.; Scherlach, K.; Lange, C.; Brakhage, A.A. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007, 3, 213–217. [Google Scholar] [CrossRef]
- Rizwan-ul-Haq, M.; Hu, Q.B.; Hu, M.Y.; Lin, Q.S.; Zhang, W.L. Biological impact of harmaline, ricinine and their combined effects with Bacillus thuringiensis on Spodoptera exigua (Lepidoptera: Noctuidae). J. Pest Sci. 2009, 82, 327–334. [Google Scholar] [CrossRef]
- Schmidt, K.; Riese, U.; Li, Z.; Hamburger, M. Novel tetramic acids and pyridone alkaloids, militarinones B, C, and D, from the insect pathogenic fungus Paecilomyces militaris. J. Nat. Prod. 2003, 66, 378–383. [Google Scholar] [CrossRef]
- Kornsakulkarn, J.; Pruksatrakul, T.; Surawatanawong, P.; Thangsrikeattigun, C.; Komwijit, S.; Boonyuen, N.; Thongpanchang, C. Antimicrobial, antimalarial, and cytotoxic substances from the insect pathogenic fungus Beauveria asiatica BCC 16812. Phytochem. Lett. 2021, 43, 8–15. [Google Scholar] [CrossRef]
- Colarusso, S.; Narjes, F. Preparation of Pyridine N-oxides as Antiviral Agents. WO2004110442 A1, 23 December 2004. [Google Scholar]
| IC50 (µM) | Positive Control | ||||||
|---|---|---|---|---|---|---|---|
| Test Cell Line | 1 | 2 | 3 | 4 | 5 | 6 | Epothilone B (nM) |
| L929 (fibroblast) | n.a. | 70.1 | 48.2 | 0.79 | 6.8 | 5.7 | 0.65 |
| KB3.1 (ovary) | n.a. | 64.7 | 20.2 | 0.79 | 6.0 | 4.9 | 0.17 |
| A549 (lung) | n.d. | n.d. | n.d. | 0.24 | 2.6 | 24.1 | 0.05 |
| MCF-7 (breast) | n.d. | n.d. | n.d. | 2.0 | 8.1 | 7.3 | 0.07 |
| Test Microorganism | MIC (µg/mL) | Positive Control (µg/mL) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Staphylococcus aureus | n.i. | n.i. | 66.6 | 16.6 | 66.6 | 66.6 | 0.21 G |
| Escherichia coli | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.42 G |
| Bacillus subtilis | n.i. | n.i. | 66.6 | 8.3 | 66.6 | 66.6 | 16.6 O |
| Pseudomonas aeruginosa | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.21 G |
| Wickerhamomyces anomalus | n.d. | n.i. | n.d. | n.i. | n.i. | n.d. | 16.6 N |
| Candida albicans | n.i. | n.i. | n.i. | 66.6 | n.i. | n.i. | 8.3 N |
| Acinetobacter baumannii | n.d. | n.i. | n.d. | n.i. | n.i. | n.d. | 0.52 C |
| Chromobacterium violaceum | n.d. | n.i. | n.i. | n.i. | n.i. | n.d. | 1.70 G |
| Schizosaccharomyces pombe | n.d. | n.i. | n.d. | n.i. | n.i. | n.d. | 4.20 N |
| Mucor hiemalis | n.i. | n.i. | n.i. | 66.6 | n.i. | n.i. | 8.30 N |
| Rhodotorula glutinis | n.d. | n.i. | n.d. | n.i. | n.i. | n.d. | 4.20 N |
| Mycobacterium smegmatis | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 1.70 K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toshe, R.; Charria-Girón, E.; Khonsanit, A.; Luangsa-ard, J.J.; Khalid, S.J.; Schrey, H.; Ebada, S.S.; Stadler, M. Bioprospection of Tenellins Produced by the Entomopathogenic Fungus Beauveria neobassiana. J. Fungi 2024, 10, 69. https://doi.org/10.3390/jof10010069
Toshe R, Charria-Girón E, Khonsanit A, Luangsa-ard JJ, Khalid SJ, Schrey H, Ebada SS, Stadler M. Bioprospection of Tenellins Produced by the Entomopathogenic Fungus Beauveria neobassiana. Journal of Fungi. 2024; 10(1):69. https://doi.org/10.3390/jof10010069
Chicago/Turabian StyleToshe, Rita, Esteban Charria-Girón, Artit Khonsanit, Janet Jennifer Luangsa-ard, Syeda Javariya Khalid, Hedda Schrey, Sherif S. Ebada, and Marc Stadler. 2024. "Bioprospection of Tenellins Produced by the Entomopathogenic Fungus Beauveria neobassiana" Journal of Fungi 10, no. 1: 69. https://doi.org/10.3390/jof10010069
APA StyleToshe, R., Charria-Girón, E., Khonsanit, A., Luangsa-ard, J. J., Khalid, S. J., Schrey, H., Ebada, S. S., & Stadler, M. (2024). Bioprospection of Tenellins Produced by the Entomopathogenic Fungus Beauveria neobassiana. Journal of Fungi, 10(1), 69. https://doi.org/10.3390/jof10010069