Identification and Biological Characteristics of Mortierella alpina Associated with Chinese Flowering Cherry (Cerasus serrulata) Leaf Blight in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Disease Investigation and Isolate Collection
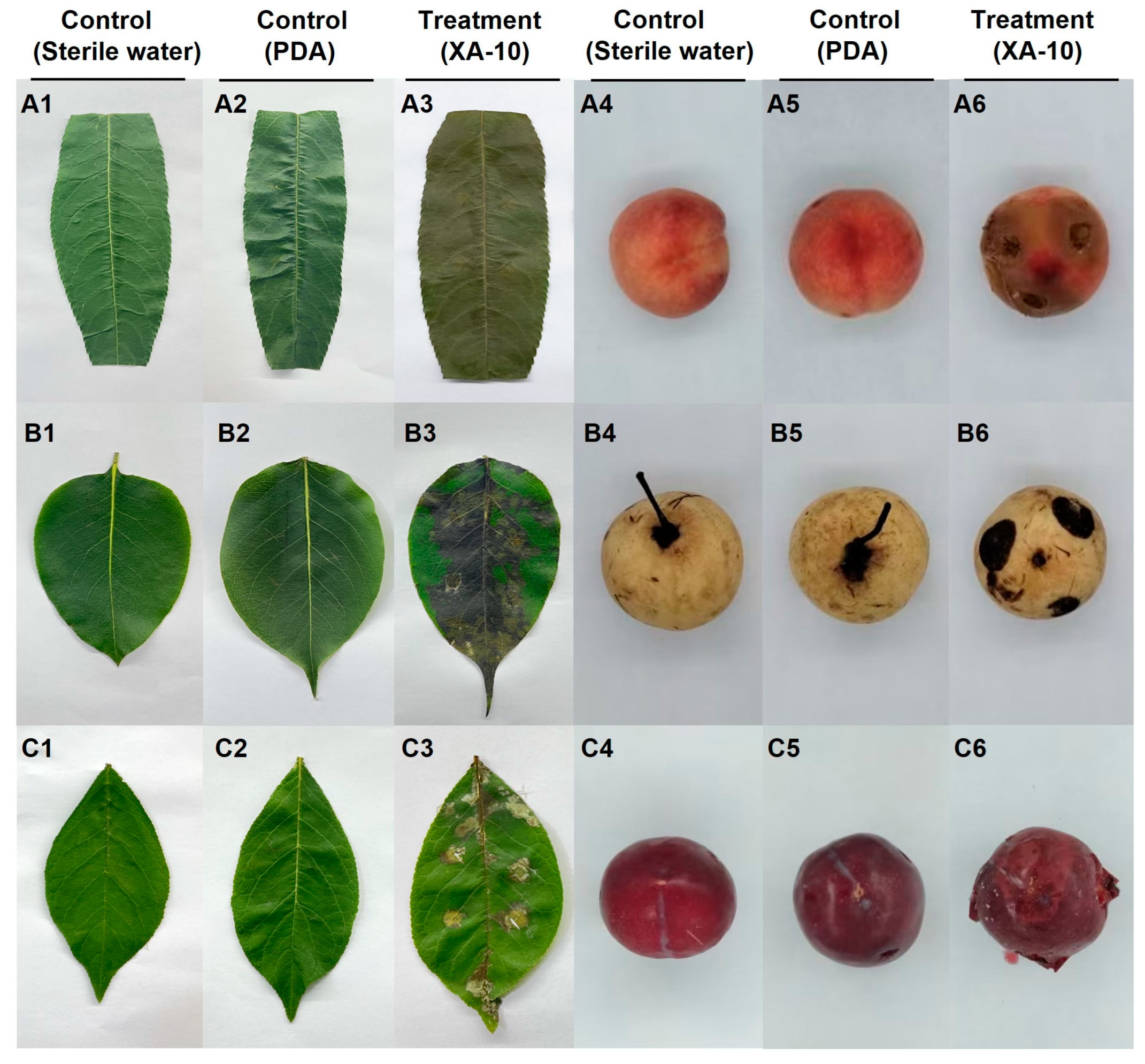
2.2. Pathogenicity Tests on Host Leaves
2.3. Molecular Identification of Fungal Isolates
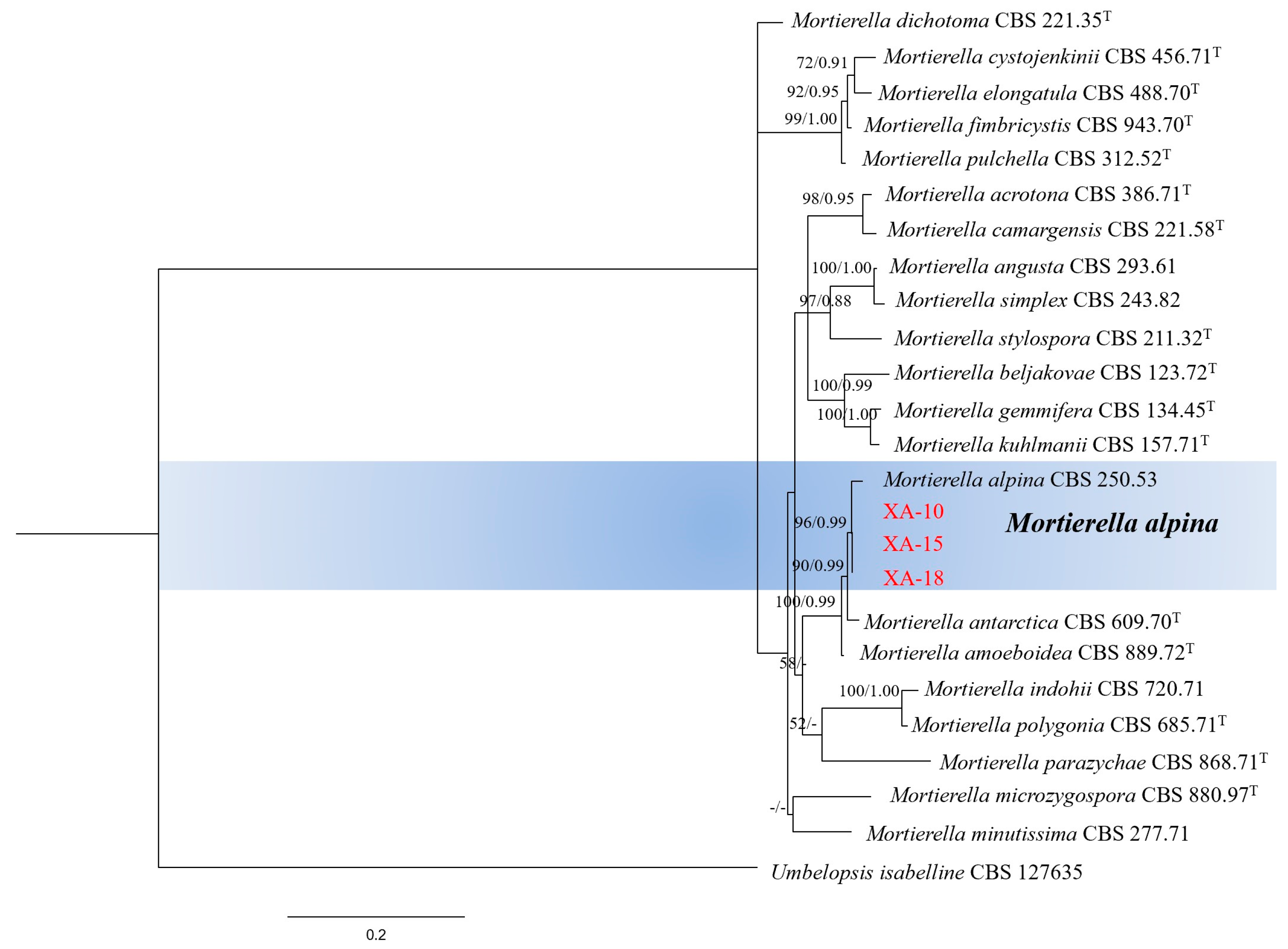
2.4. Phylogenetic Analysis
2.5. Morphological Study
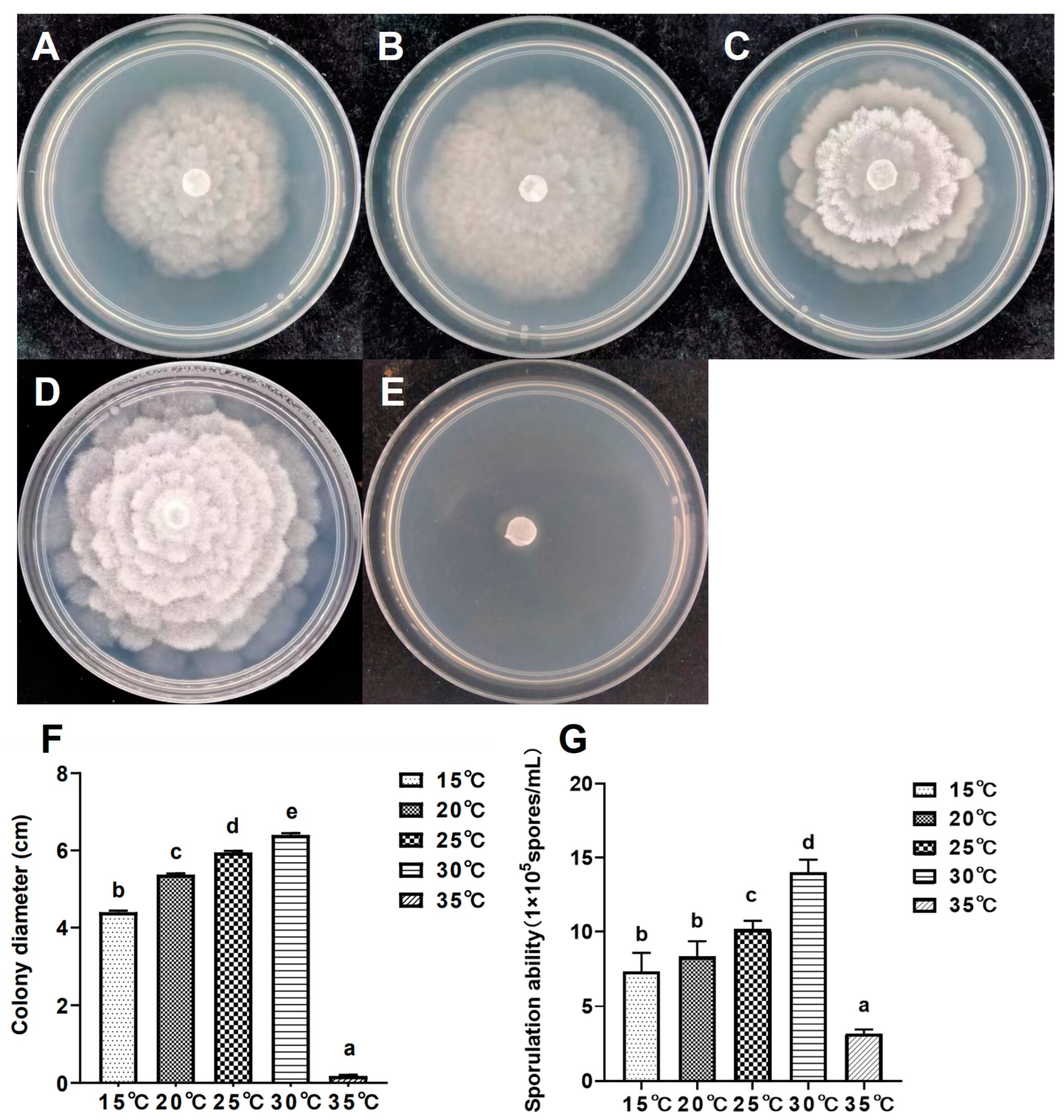
2.6. Growth Characteristics at Different Temperatures
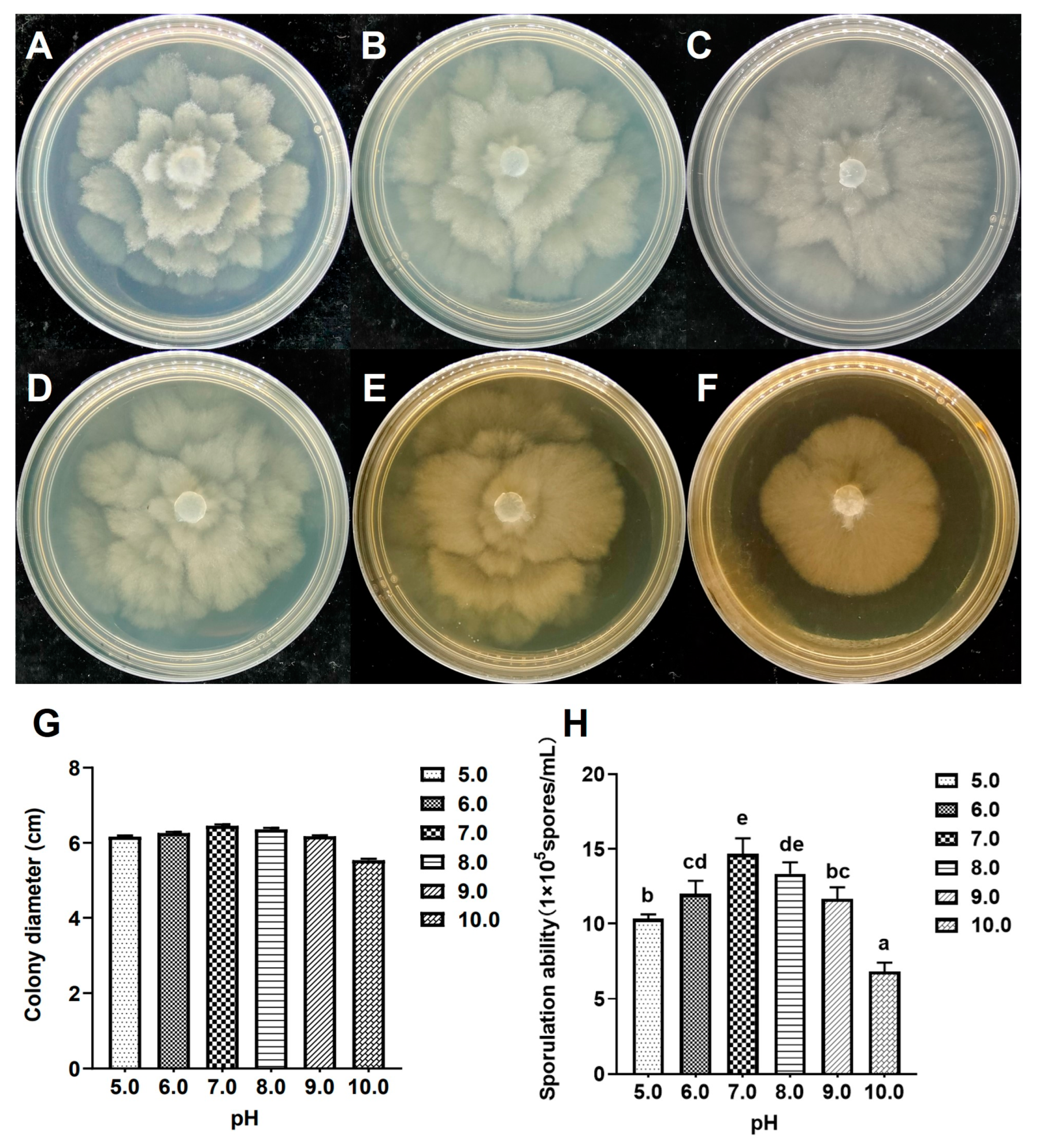
2.7. Growth Characteristics at Different pH Values
2.8. Pathogenicity Tests on Leaves and Fruits of the Other Three Species of Rosaceae
2.9. Statistical Analysis
3. Results
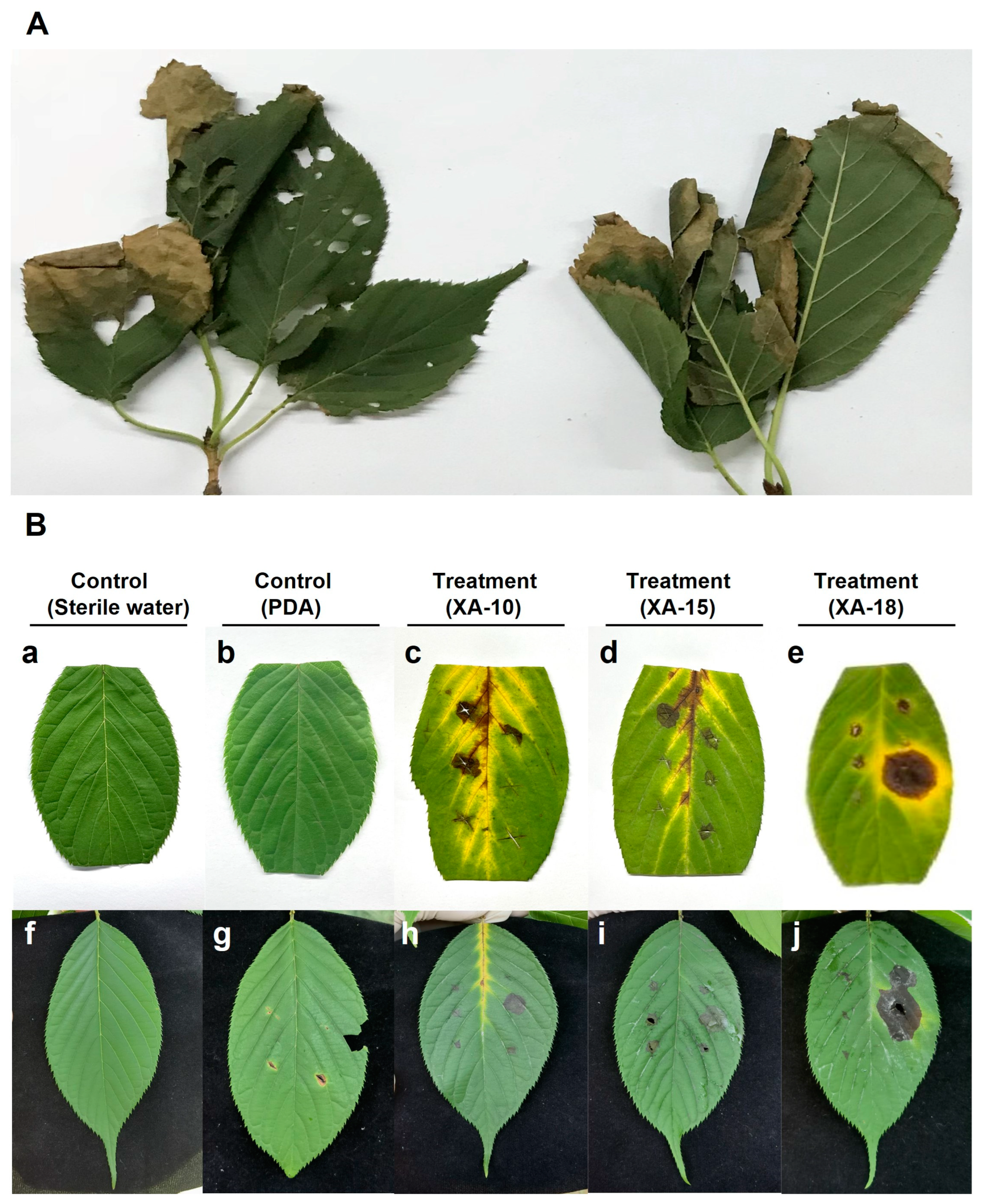
3.1. Natural Symptoms and Pathogen Isolation
3.2. Pathogenicity Tests on Host Leaves
3.3. Molecular Identification of Fungal Isolates
3.4. Morphology of Pathogenic Fungi
3.5. Growth Characteristics at Different Temperatures
3.6. Growth Characteristics at Different pH Values
3.7. Pathogenicity Tests on Leaves and Fruits of the Other Three Species of Rosaceae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirasawa, K.; Esumi, T.; Itai, A.; Isobe, S. Cherry blossom forecast based on transcriptome of floral organs approaching blooming in the flowering cherry (Cerasus × yedoensis) cultivar ‘Somei-Yoshino’. Front. Plant Sci. 2022, 13, 802203. [Google Scholar] [CrossRef] [PubMed]
- Shibato, J.; Takenoya, F.; Hirabayashi, T.; Kimura, A.; Iwasaki, Y.; Toyoda, Y.; Hori, M.; Tamogami, S.; Rakwal, R.; Shioda, S. Towards identification of bioactive compounds in cold vacuum extracted double cherry blossom (Gosen-Sakura) leaves. Plant Signal Behav. 2019, 14, e1644594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Guan, L.; Zhong, Z.Y.; Chang, M.; Zhang, D.K.; Li, H.; Lai, W. The anti-inflammatory effect of cherry blossom extract (Prunus yedoensis) used in soothing skincare product. Int. J. Coxmetic Sci. 2014, 36, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Nakamura, S.; Morioka, M.; Tanaka, J.; Matsuda, H.; Yoshikawa, M. Effect of cinnamoyl and flavonol glucosides derived from cherry blossom flowers on the production of advanced glycation end products (AGEs) and AGE-induced fibroblast apoptosis. Phytother. Res. 2011, 25, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hwang, E.; Ngo Hien, T.T.; Lin, P.; Gao, W.; Liu, Y.; Yi, T.H. Antiphotoaging effect of Prunus yeonesis blossom extract via inhibition of MAPK/AP-1 and regulation of the TGF-βI/Smad and Nrf2/ARE signaling pathways. Photochem. Photobiol. 2018, 94, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.G.; Chen, J.; Zhu, H.; Li, X.X.; Li, M.; Duan, Y.F.; Chen, L.; Wang, X.R. Phylogeography and the population genetic structure of flowering cherry Cerasus serrulata (Rosaceae) in subtropical and temperate China. Ecol. Evol. 2020, 10, 11262–11276. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L. Study on the Ornamental Evaluation Of Fourteen Cerasus serrulata Cultivars and the Application in Landscape. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2012. [Google Scholar]
- Zhang, J. Studies on the Cultivar Resources of Cerasus and the Application in Landscape. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2010. [Google Scholar]
- Yi, X.G.; Yu, X.Q.; Chen, J.; Zhang, M.; Liu, S.W.; Zhu, H.; Li, M.; Duan, Y.F.; Chen, L.; Wu, L.; et al. The genome of Chinese flowering cherry (Cerasus serrulata) provides new insights into Cerasus species. Hortic. Res. 2020, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.G.; Chen, J.; You, L.X.; Cong, R.; Wang, H.C.; Duan, Y.F.; Wang, X.R. Genetic diversity of Cerasus serrulata populations assessed by SSR markers. J. Nanjing Forestry Univ. 2018, 42, 25–31. [Google Scholar]
- Yi, X.G. The Variation and Phylogeography of Cerasus serrulata Mill. Populations. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2018. [Google Scholar]
- Li, M.; Yin, X.G.; Wang, H.C.; Shang, T.; Gu, Y.; Wang, X.R. Studies on the relationship between Cerasus serrulata distribution region and the environmental factors. J. Nanjing Forestry Univ. 2014, 38, 74–80. [Google Scholar]
- Wang, X.R. An Illustrated Monograph of Cherry Cultivars in China; Science Press: Beijing, China, 2014; pp. 24–28. [Google Scholar]
- Liao, Z.J.; Zeng, H.L.; Gao, C.; Zhou, S.Y.; Liu, G.S.; Li, R.G. Identification of the pathogen causing brown spot in Cerasus in Jiangxi and its sensitivity to fungicides. J. Nanjing For. Univ. 2022, 48, 449–453. [Google Scholar]
- Chen, J.X.; Wu, Z.Q.; Wu, F.J.L.; Ma, H.C.; Han, Q.L.; Wu, J.R. First report of ‘Candidatus Phytoplasma asteris’ associated with witches’-broom and plexus bud disease of Cerasus serrula in China. Plant Dis. 2023, 107, 3274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Liu, C.Y.; Xu, S.F. Occurrence and Control of Landscape Plant Diseases; China Agricultural University Press: Beijing, China, 2014; pp. 156–157. [Google Scholar]
- Wang, X.W.; Ma, X.Y.; Zhou, Q.; Long, J.B.; Tian, H.Q.; Liu, D.M.; Yu, C. First report of root rot in Cerasus subhirtella caused by Fusarium solani and Fusarium oxysporum in China. Plant Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, G.F.; Turatsinze, A.N.; Xie, X.F.; Sha, Y.X.; Wang, R.Y. First report of Fusarium tricinctum causing root rot on Chinese dwarf cherry (Cerasus humilis) in China. Plant Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.; Rollins, L.; Bourret, T.; Chastagner, G. First report of leaf blight caused by Phytophthora ramorum on cherry laurel (Prunus laurocerasus) in Washington State, U.S.A. Plant Dis. 2021, 105, 712. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Ann, P.J. First report of Phytophthora cambivora causing leaf and stem blight and root rot on Taiwan cherry (Prunus campanulata) in Taiwan. Plant Dis. 2012, 96, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.D.; Zhou, C.H.; Ai, J.Y.; Zhang, Q.J. Pathogen identification of sweet cherry leaf blight in Liaoning Province. China Fruits 2022, 9, 12–19. [Google Scholar]
- Fang, Z.D. Research Methods for Plant Pathology, 3rd ed.; China Agriculture Press: Beijing, China, 1998; pp. 156–168. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, F.J.R.M.; Lee, S.H.; Taylor, L.; Shawe-Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Lee, S.B.; Taylor, J.W. Isolation of DNA from fungal mycelia and single spores. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 282–287. [Google Scholar]
- Liu, S.B.; Liu, X.Y.; Zhang, Z.X.; Xia, J.W.; Zhang, X.G.; Meng, Z. Three New Species of Microdochium (Sordariomycetes, Amphisphaeriales) on Miscanthus sinensis and Phragmites australis from Hainan, China. J. Fungi 2022, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.N.; Addrah, M.E.; Zhang, Y.Y.; Jia, R.F.; Kang, L.R.; Zhao, J.; Zhang, J. Identification and comparison of biological characteristics and pathogenicity of different mating types of V. dahliae isolated from potato and sunflower. Sci. Rep. 2022, 12, 12840. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Wagner, L.; Stielow, B.; Hoffmann, K.; Petkovits, T.; Papp, T.; Vágvölgyi, C.; de Hoog, G.S.; Verkley, G.; Voigt, K. A comprehensive molecular phylogeny of the Mortierellales (Mortierellomycotina) based on nuclear ribosomal DNA. Persoonia 2013, 30, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Petkovits, T.; Nagy, L.G.; Hoffmann, K.; Wagner, L.; Nyilasi, I.; Griebel, T.; Schnabelrauch, D.; Vogel, H.; Voigt, K.; Vagvoelgyi, C.; et al. Data partitions, bayesian analysis and phylogeny of the Zygomycetous fungal family Mortierellaceae, inferred from nuclear ribosomal DNA sequences. PLoS ONE 2011, 6, e27507. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Park, S.W.; Pangging, M.; Lee, H.B. Molecular and morphological confirmation of three undescribed species of Mortierella from Korea. Mycobology 2019, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.B.; Su, J.Y.; Fang, T.; Pan, A.F.; He, X.Y.; Fan, G.L.; Wang, Z.H.; Hu, H.L. Pathogen of liquidambar formosana leaf spot. Mycosystema 2022, 41, 420–434. [Google Scholar]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Wei, J.C. Identification Manua; Shanghai Scientific & Technical Publishers: Shanghai, China, 1979; pp. 1–780. [Google Scholar]
- Li, C.L.; Bartholomew, B. Flora of China; Science Press: Beijing, China, 2003; pp. 404–420. [Google Scholar]
- Wang, Y. Mechanism Analysis of Inoculation with Mortierella alplina on Defense against Root of Cultivated Ginseng. Master’s Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar]
- Liu, Z. Studies on the Taxonomy and Molecular Phylogeny of Mortierella and Allied Genera in China. Master’s Thesis, Beijing Forestry University, Beijing, China, 2020. [Google Scholar]
- Wani, Z.A.; Kumar, A.; Sultan, P.; Bindu, K.; Riyaz-Ul-Hassan, S.; Ashraf, N. Mortierella alpina CS10E4, an oleaginous fungal endophyte of Crocus sativus L. enhances apocarotenoid biosynthesis and stress tolerance in the host plant. Sci. Rep. 2017, 7, 8598. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.W.; Suo, M.; Qiu, Z.J.; Wu, H.; Zhao, M.; Yang, H.Y. Regulating root fungal community using Mortierella alpina for Fusarium oxysporum resistance in Panax ginseng. Front. Microbiol. 2022, 13, 850917. [Google Scholar] [CrossRef]
- Yadav, D.R.; Kim, S.W.; Babu, A.G.; Adhikari, M.; Kim, C.; Lee, H.B.; Lee, Y.S. First report of Mortierella alpina (Mortierellaceae, Zygomycota) isolated from crop field soil in Korea. Mycobiology 2014, 42, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Streekstra, H. On the safety of Mortierella alpina for the production of food ingredients, such as arachidonic acid. J. Biotechnol. 1997, 56, 153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z. Studies on the Diversity of Soil Fungi in the Alpine Grassland of Eastern Qilian Mountains. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2010. [Google Scholar]
- Melo, I.; Santos, S.; Rosa, L.; Parma, M.; Silva, L.; Queiroz, S.; Pellizari, V. Isolation and biological activities of an endophytic Mortierella alpina strain from the Antarctic moss Schistidium antarctici. Extremophiles 2014, 18, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, V.W. Physiology of Fungi; John Wiley & Sons: New York, NY, USA, 1958; pp. 19–23. [Google Scholar]
- de León, Y.M.P.; Muñoz-Castellanos, L.N.; Ruiz-Cisneros, M.F.; Pérez-Corral, D.A.; Ornelas-Paz, J.J.; AcostaMuñiz, C.H.; Berlanga Reyes, D.I.; Rios Velasco, C. Morphological and molecular identification of Mortierella species associated to rhizosphere of apple trees with symptoms of root diseases. Rev. Mex. Fitopatol. 2017, 36, 184–195. [Google Scholar]
- Johnson, J.M.; Ludwig, A.; Furch, A.C.U.; Mithofer, A.; Scholz, S.; Reichelt, M.; Oelmtiller, R. The beneficial root-colonizing fungus Mortierella hyaline promotes the aerial growth of Arabidopsis and activates calcium-dependent responses that restrict Alternaria brassicae-induced disease development in roots. Mol. Plant Microbe Interact. 2019, 32, 351–363. [Google Scholar] [CrossRef]
- Li Destri Nicosia, M.G.; Mosca, S.; Mercurio, R.; Schena, L. Dieback of Pinus nigra seedlings caused by a strain of Trichoderma viride. Plant Dis. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Vélez, M.L.; Marfetán, J.A.; Salgado Salomón, M.E.; Taccari, L.E. Mortierella species from declining Araucaria araucana trees in Patagonia, Argentina. Forest Pathol. 2020, 50, e12591. [Google Scholar] [CrossRef]
- Hernández Pérez, A.; Cerna Chávez, E.; Delgado Ortiz, J.C.; Beltrán Beache, M.; Hernández Bautista, O.; Tapia Vargas, L.M.; Ochoa Fuentes, Y.M. First report of Mortierella elongata as a pathogen of avocado crop in Michoacán, Mexico. Sci. Fungorum. 2018, 48, 95–98. [Google Scholar] [CrossRef]
- Miao, C.P.; Mi, Q.L.; Qiao, X.G.; Zheng, Y.K.; Chen, Y.W.; Xu, L.H.; Guan, H.L.; Zhao, L.X. Rhizospheric fungi of Panax notoginseng: Diversity and antagonism to host phytopathogens. J. Ginseng Res. 2016, 40, 127–134. [Google Scholar] [CrossRef]
- Curlevski, N.J.A.; Xu, Z.H.; Anderson, I.C.; Cairney, J.W.G. Diversity of soil and rhizosphere fungi under Araucaria bidwillii (Bunya pine) at an Australian tropical montane rainforest site. Fungal Divers. 2010, 40, 12–22. [Google Scholar] [CrossRef]
- Huang, F.F. Effect of Pesticides on Mortierella in Rhizospheric soil of panax notoginseng and Their Colonization Ability in the Field. Master’s Thesis, Yunnan University, Kunming, China, 2017. [Google Scholar]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Sang, Y.; Jin, L.; Zhu, R.; Yu, X.Y.; Hu, S.; Wang, B.T.; Ruan, H.H.; Jin, F.J.; Lee, H.G. Phosphorus-solubilizing capacity of Mortierella species isolated from rhizosphere soil of a poplar plantation. Microorganisms 2022, 10, 2361. [Google Scholar] [CrossRef] [PubMed]
- Vandepol, N.; Liber, J.; Desirò, A.; Na, H.; Kennedy, M.; Barry, K.; Grigoriev, I.V.; Miller, A.N.; O’Donnell, K.; Stajich, J.E. Resolving the Mortierellaceae phylogeny through synthesis of multi-gene phylogenetics and phylogenomics. Fungal Divers. 2020, 104, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Borrell, A.N.; Shi, Y.; Gan, Y.; Bainard, L.D.; Germida, J.J.; Hamel, C. Fungal diversity associated with pulses and its influence on the subsequent wheat crop in the Canadian Prairies. Plant Soil 2017, 414, 13–31. [Google Scholar] [CrossRef]
- Niu, Y.N.; Bainard, L.D.; May, W.E.; Hossain, Z.; Hamel, C.; Gan, Y.T. Intensified pulse rotations buildup pea rhizosphere pathogens in cereal and pulse based cropping systems. Front. Microbiol. 2018, 9, 1909. [Google Scholar] [CrossRef]
- Li, F.; Zhang, S.Q.; Wang, Y.; Li, Y.; Li, P.P.; Chen, L.; Jie, X.L.; Hu, D.S.; Feng, B.; Yue, K.; et al. Rare fungus, Mortierella capitata, promotes crop growth by stimulating primary metabolisms related genes and reshaping rhizosphere bacterial community. Soil Biol. Biochem. 2020, 151, 108017. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Gordon, M.R.; Zhang, J.B.; Zhang, C.Z.; Ning, Q.; Li, W. Mortierella elongata’s roles in organic agriculture and crop growth promotion in a mineral soil. Land Degrad. Dev. 2018, 29, 1642–1651. [Google Scholar] [CrossRef]
- Ning, Q.; Chen, L.; Li, F.; Zhang, C.Z.; Ma, D.H.; Cai, Z.J.; Zhang, J.B. Effects of Mortierella on nutrient availability and straw decomposition in soil. Acta Pedol. Sin. 2022, 59, 206–217. [Google Scholar]

| Locus a | Primer | Primer Sequence (5′–3′) | PCR Conditions | Reference |
|---|---|---|---|---|
| ITS | ITS5 | GGAAGTAAACGTAACAAGG | 94 °C for 5 min (94 °C for 40 s, 58 °C for 40 s, and 72 °C for 60 s) × 35 cycles, 72 °C for 7 min | [23] |
| ITS4 | TCCTCCGCTTATTGATATGC | |||
| LSU | LR0R | ACCCGCTGAACTTAAGC | 94 °C for 5 min (94 °C for 45 s, 50 °C for 45 s, and 72 °C for 1 min) × 35 cycles, 72 °C for 7 min | [27,28] |
| LR5 | TCCTGAGGGAAACTTCG |
| Original Name | Culture Accession Number(s) | Type Status | Accession No. ITS | Accession No. LSU | Reference |
|---|---|---|---|---|---|
| Mortierella acrotona | CBS 386.71, FSU 9788 | Type of Mortierella acrotona | JX975921 | HQ667405.1 | [29,30] |
| Mortierella alpina | CBS 250.53 | JX975955 | KC018184 | [29] | |
| Mortierella amoeboidea | CBS 889.72 | Type of Mortierella amoeboidea | JX976073 | HQ667422.1 | [29,30] |
| Mortierella angusta | CBS 293.61 | Neotype of Mortierella polycephala var.angusta | JX976061 | HQ667358.1 | [29,30] |
| Mortierella antarctica | CBS 609.70, FSU 9792 | Type of Mortierella antarctica | JX975907 | HQ667503.1 | [29,30] |
| Mortierella beljakovae | CBS 123.72, FSU 9794 | Type of Mortierella beljakovae | JX976126 | HQ667428.1 | [29,30] |
| Mortierella camargensis | CBS 221.58, FSU 9796 | Type of Mortierella camargensis | JX975949 | HQ667408.1 | [29,30,31] |
| Mortierella cystojenkinii | CBS 456.71, FSU 9803 | Type of Mortierella cystojenkinii | JX976030 | HQ667504.1 | [29,30] |
| Mortierella dichotoma | CBS 221.35, FSU 9804 | Syntype of Mortierella dichotoma | JX975842 | HQ667393.1 | [29,30] |
| Mortierella elongatula | CBS 488.70, FSU 9808 | Type of Mortierella elongatula | JX975967 | HQ667425.1 | [29,30] |
| Mortierella fimbricystis | CBS 943.70 | Type of Mortierella fimbricystis | GU559986.1 | JX976172 | [29] |
| Mortierella gemmifera | CBS 134.45, FSU 9815 | Type of Mortierella gemmifera | JX975931 | HQ667371.1 | [29,30] |
| Mortierella indohii | CBS 720.71, FSU 9826 | Isotype of Mortierella indohii | JX975856 | HQ667377.1 | [29,30] |
| Mortierella kuhlmanii | CBS 157.71, FSU 9827 | Type of Mortierella kuhlmanii | JX975846 | HQ667372.1 | [29,30] |
| Mortierella microzygospora | CBS 880.97, FSU 9831 | Type of Mortierella microzygospora | JX976027 | HQ667394.1 | [29,30] |
| Mortierella minutissima | CBS 277.71, FSU 832 | JX975938 | KC018293 | [29] | |
| Mortierella parazychae | CBS 868.71, FSU 9836 | Type of Mortierella parazychae | JX975985 | HQ667362.1 | [29,30] |
| Mortierella polygonia | CBS 685.71, FSU 9839 | Type of Mortierella polygonia | JX975900 | HQ667378.1 | [29,30] |
| Mortierella pulchella | CBS 312.52, FSU 9840 | Authentic strain of Mortierella pulchella | JX976054 | HQ667427.1 | [29,30] |
| Mortierella simplex | CBS 243.82 | JX975870 | JX976156 | [29,30] | |
| Mortierella stylospora | CBS 211.32, FSU 9850 | Type of Mortierella stylospora | JX976086 | HQ667359.1 | [29,30] |
| Umbelopsis isabellina | CBS 127635 | MH864646 | MH876082 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, D.; Xu, Y.; Zhang, C.; Lai, Z.; Song, L.; Su, J.; Yang, R.; Jing, X.; Felix, A.; Abubakar, Y.S.; et al. Identification and Biological Characteristics of Mortierella alpina Associated with Chinese Flowering Cherry (Cerasus serrulata) Leaf Blight in China. J. Fungi 2024, 10, 50. https://doi.org/10.3390/jof10010050
Shao D, Xu Y, Zhang C, Lai Z, Song L, Su J, Yang R, Jing X, Felix A, Abubakar YS, et al. Identification and Biological Characteristics of Mortierella alpina Associated with Chinese Flowering Cherry (Cerasus serrulata) Leaf Blight in China. Journal of Fungi. 2024; 10(1):50. https://doi.org/10.3390/jof10010050
Chicago/Turabian StyleShao, Dengke, Yuying Xu, Chunyuan Zhang, Zecheng Lai, Linlin Song, Jiyu Su, Ruixian Yang, Xinhong Jing, Abah Felix, Yakubu Saddeeq Abubakar, and et al. 2024. "Identification and Biological Characteristics of Mortierella alpina Associated with Chinese Flowering Cherry (Cerasus serrulata) Leaf Blight in China" Journal of Fungi 10, no. 1: 50. https://doi.org/10.3390/jof10010050
APA StyleShao, D., Xu, Y., Zhang, C., Lai, Z., Song, L., Su, J., Yang, R., Jing, X., Felix, A., Abubakar, Y. S., Lu, G., & Ye, W. (2024). Identification and Biological Characteristics of Mortierella alpina Associated with Chinese Flowering Cherry (Cerasus serrulata) Leaf Blight in China. Journal of Fungi, 10(1), 50. https://doi.org/10.3390/jof10010050








