Abstract
Xylanases are hydrolytic enzymes that have tremendous applications in different sectors of life, but the high cost of their production has limited their use. One solution to reduce costs and enhance xylanase production is the use of agro-wastes as a substrate in fungal cultures. In this study, olive mill pomace (OMP) and barley bran (BB) were used as carbon sources and possible inducers of xylanase production by three species of Trichoderma (atroviride, harzianum, and longibrachiatum), one major xylanase producer. The experiments were conducted under a solid-state fermentation system (SSF) in flask cultures and a packed-bed bioreactor. Cultures of OMP and BB were optimized by examining different ratios of OMP and BB, varied particle sizes, and inoculum size for the three species of Trichoderma. The ratio of 8:2 OMP and BB yielded the highest xylanase activity, with a particle size of 1 mm at 29 °C and an inoculum size of 1 × 107 spores/mL. Studying the time profile of the process revealed that xylanase activity was highest after seven days of incubation in flask SSF cultures (1.779 U/mL) and after three days in a packed-bed bioreactor (1.828 U/mL). The maximum percentage of OMP degradation recorded was about 15% in the cultures of T. harzianum flask SSF cultures, compared to about 11% in T. longibrachiatum bioreactor cultures. Ammonium sulfate precipitation and dialysis experiments showed that Xylane enzyme activity ranged from 0.274 U/mL in T. harzianum to 0.837 U/mL in T. atroviride when crude extract was used, with the highest activity (0.628 U/mL) at 60% saturation. Xylose was the main sugar released in all purified fractions, with the G-50 and G-75 fractions showing the maximum units of xylanase.
1. Introduction
There is a growing interest in screening natural sources to find bioactive compounds with nutraceutical and industrial benefits [1]. Increasing synthetic costs and environmental concerns rationalize the search for natural, high-value products to produce different commodities [2]. Therefore, attention has been drawn to plant biomass and agricultural wastes (agro-industrial residues) because they are cheap and abundant around the world, and major research efforts are underway to use them from different perspectives [3]. The agro-residues are primary reservoirs of lignocellulose, rendering them particularly appealing for many products essential to human society [4].
Xylan, a major hemicellulosic constituent of lignocellulosic materials, is markedly valued from industrial and biomedical perspectives and for the production of value-added products [5,6]. It is the most abundant hemicellulose in plant cell walls, accounting for more than 30% of the plant biomass [4,7,8]. Appreciable proportions of xylan exist in hardwoods and softwoods, as well as herbaceous plants, and form up to 35% of the lignocellulosic materials [9].
Xylan has a backbone of β-1,4-D-xylose residues with different α-glycosidically linked substitutions in side chains, primarily including L-arabinofuranose, D-galactose, and D-glucuronic or 4-O-methyl-d-glucuronic acid [10,11]. Substituents such as acetyl, arabinosyl, glucuronysyl, coumaroyl, and ferulic acid esters can also be found. The proportion of these substituents and the degree of branching are variable, depending on the plant source [12]. Complete degradation of xylan is achieved by a set of hydrolytic enzymes collectively called xylanases or xylanolytic enzymes [13]. These enzymes have been the target of many research endeavors; yet they are expensive and required in large amounts to be recruited in food, industrial, and medical applications. For this reason, attention has been paid to the natural producers of xylanases and agro-industrial wastes to develop nutrient-rich media for xylanase-producing organisms.
Xylanases find extensive applications across various industries, including food and feed, paper and pulp, textiles, pharmaceuticals, and lignocellulosic biorefinery [9]. Xylanases are ubiquitous and produced by a variety of organisms, including bacteria, protozoa, algae, fungi, snails, crustaceans, and insects [9,10,14]. Among these, multicellular fungi have been highly acknowledged as tremendous producers of xylanases [9,15]. Their filamentous structure provides large surface areas for absorptive nutrition and enhanced production of xylanases [2,16]. Species of the genus Trichoderma such as T. reesi, T. viride, and T. harzianum have held a key position in research studies aiming at xylanase production and purification [9,17].
Olive mill pomace (OMP) is one of the most cost-effective and nutrient-rich substrates used for cultivating fungi. Large quantities of OMP are produced in the Mediterranean region. The extraction of olive oil yields ca. 15,000 tons of olive oil and 80,000 tons of OMP a year [18]. In recent years, Jordan has been the eighth largest producer of olive oil worldwide, with 24,000 tons of oil and 60,000 tons of OMP per year [19]. Traditionally, OMP is used as firewood, but this causes air pollution. Consequently, the authorities placed restrictions on olive oil extraction factories in terms of waste accumulation and re-use. One solution is to make use of OMP as a growth medium for many microorganisms in attempts to produce commercial and high-value products (i.e., enzymes, bio-control agents, fertilizers, and biofuel), and interest in this is increasing [20]. However, few studies exploited OMP to produce enzymes and other value-added products.
The present study aims to examine the capacity of selected Trichoderma species isolated from plant sources for xylanase production using solid-state fermentation (SSF) and varied combinations of OMP and barely bran (BB) substrates, to determine optimal growth conditions for xylanase production, and to extract and partially purify xylanase. Despite the many studies on xylanase production from Trichoderma spp. around the world, this study is, to our knowledge, the first on Jordanian isolates of Trichoderma. The process of SSF has been accredited for the production of value-added microbial enzymes using agro-industrial origins. It is highly productive, with higher product stability and lower processing costs compared to the submerged fermentation process [21].
2. Materials and Methods
2.1. Fungal Species Isolates and Inoculum Preparation
Isolates of three distinct Trichoderma species (T. atroviride [accession MT626716], T. harzianum [accession MT626717], and T. longibrachiatum [accession MT626720]) were examined for xylanase production. The isolates were sub-cultured on potato dextrose agar (PDA) growth medium, incubated for 5 days at 28 °C, and used for inoculum preparation.
The inoculum of each isolate was prepared by adding 1 mL of sterile distilled water to a fresh culture of PDA plate, swept with a loop, and filtered to produce a slurry. After that, the suspension was transferred to 99 mL of sterile distilled water. The spores were counted using a hemocytometer to obtain a spore concentration of (107 spores/mL), and the suspension was stored in the refrigerator at 4 °C until use [22].
2.2. Preparation of OMP
Freshly harvested OMP was collected from olive mills located in Zarqa city (Jordan) during the period of olive oil extraction (October–December 2019). The collected pomace was dried in the greenhouse and used as the main substrate in culturing and fermentation processes.
2.3. Flask Solid State Fermentation for Xylanase Production
This part of the study was carried out following [8,23]. For each Trichoderma sp., a flask containing a 10 g mixture of OMP and barley bran (BB) was autoclaved at 121 °C for 15 min. Then, the mixture was moistened with 5 mL of sterile distilled water and inoculated with a spore suspension of each Trichoderma species (107 spores/mL). All flasks were incubated for seven days at 29 °C until further use in further biochemical analyses.
2.4. Xylanase Assay
The extraction and assaying of xylanase were carried out according to Al Sheikh [24], Assamoi et al. [25], and Mardawati et al. [8]. The contents of each prepared flask were collected in new flasks, and 50 mM sodium citrate solution (pH 5) was added. Then, the flasks were rotated at 150 rpm at room temperature for 30 min on an orbital shaker (Human Lab, Seoul, Republic of Korea) and filtered through Whatman No. 1 filter paper. The filtrate was centrifuged at 6000 rpm (Wagtek, Thatcham, UK) for 15 min at 4 °C, and the supernatant was used for the xylanase assay. Xylanase activity was measured by adding 0.2 mL of the sample supernatant to 0.5 mL of 1% oat-spelt xylan solution in 0.3 mL of 50 mM citrate buffer (pH 5) at 45 °C for 30 min. Reducing sugars released by xylanase were determined using 3 mL of 3.5-dinitrosalicylic acid (DNS) at 100 °C for 5 min according to the DNS method [15,26,27]. The amount of reducing sugars released in the reactions was measured spectrophotometrically at 540 nm. Xylanase activity was calculated based on a standard curve of serial concentrations of xylose. One unit (U) of xylanase activity was defined as the amount of enzyme releasing one µmole of reducing sugar (xylose equivalent) per min under the assay conditions [8,24].
2.5. Assessment of Reducing Sugars
Reducing sugar released by each culture was assessed using the Nelson–Somogyi methodology [28]. One milliliter of Somogyi reagent was added to 1 mL of the sample supernatant. The mixture was vortexed and boiled for 15 min at 100 °C. Next, 1 mL of Nelson reagent was added, and the absorbance was measured at 520 nm. Concentrations of reducing sugars were determined based on a standard curve of xylose concentrations and following the methodology of [29].
2.6. Total Carbohydrates
The total carbohydrates of each culture were analyzed by the phenol–sulfuric acid method and detected photometrically [30]. Fifty microliters of 80% phenol solution was added to 50 µL of sample supernatant, then 2 mL of sulfuric acid were added. The absorbance was read at 490 nm after 10 min of reaction progress. Concentrations of the total carbohydrates for each culture were estimated using a standard curve.
2.7. Optimization of Xylanase Production by Trichoderma sp.
Different ratios of OMP and BB (9.5:0.5, 9:1, and 8:2) and of different supplement particle sizes of OMP and BB (5 mm, 2 mm, and 1 mm) were prepared to determine the best combination that enhances growth of Trichoderma species and xylanase production. Test trials were conducted in flasks containing trace elements of a nitrogen source (ammonium sulfate or peptone) and a carbon source (xylose or xylan) at a concentration of 1% of the culture medium [31]. A culture containing only OMP was included as a control. The mixture of 8:2 OMP and BB (8 and 2 g/10 mL of distilled water) with particle sizes of 1 mm supplied with xylan (1% of medium) and peptone (1% of medium) yielded the highest growth and so was chosen as the culture medium in the following packed-bed column SSF.
2.8. Packed-Bed Column Solid State Fermentation of OMP and BB for Xylanase Production
This experiment was conducted following the method of [32]. Cultures yielding optimal growth from the flask fermentation were transferred into a sterile packed-bed bioreactor composed of a double-jacketed glass column (50 × 5 cm) connected to an air filter pump via silica tubes supplied through a 0.22 µm pore size filter at the bottom with a 20–25 air bubble flow rate that is controlled by a flow meter. The bioreactor was packed with multilayers of sterile stainless-steel mesh, and the culture medium was moistened with sterile distilled water and mixed with a spore suspension of Trichoderma species (107 spores/mL). The column was then incubated at 29 °C for 3 days and supplied with air at 1 vvm. Finally, the fermented substrate was used for the different biochemical analyses employed in the abovementioned flask SSF. This experiment was repeated three times.
2.9. Percentage of Degradation
To determine the percentage of the substrate consumed by Trichoderma sp., the fermented culture medium was weighed (initial weight) and then dried at 50 °C for 24 h and weighed (final weight). The degradation percentage was calculated using the following equation:
(Initial weight − Final weight)/Initial weight × 100%
2.10. Times Profile of the Process
To determine the optimal incubation time for the cultures of the three Trichoderma isolates, two flask SSF replicates of each species were mixed with its spore suspension (107 spores/mL) and incubated at 29 °C for five days. Afterwards, they were filtered daily, and the filtrate was used for biochemical assays of xylanase activity, total carbohydrates, reducing sugars, and percentages of degradation.
2.11. Partial Purification of Xylanase
2.11.1. Ammonium Sulfate Precipitation and Dialysis
Crude xylanase extract was subjected to a graded ammonium sulfate saturation (20 to 70%) with continuous stirring on ice. Samples were then centrifuged at 5000 rpm for 15 min. For each percentage, the pellet formed was dissolved in a minimum amount of 50 Mm citrate buffer (pH 5), and enzyme activity and protein concentration were measured. The sample with the highest xylanase activity was further processed using dialysis bags in citrate buffer for 24 h at 4 °C. Afterwards, xylanase activities and protein concentrations were re-measured [33,34].
2.11.2. Size-Exclusion Chromatography
After ammonium sulfate precipitation and desalting of proteins, the highest protein concentration of dialyzed samples was subjected to a column (diameter: 1.5 cm, length: 30 cm, Bio-Rad, Hercules, CA, USA) containing Sephadex G-75 as the stationary phase, and in another trial, the column contained Sephadex G-50. The column was eluted with citrate buffer, pH 5. The eluted solution was collected in Cuvette tubes as fractions, each of 1 mL in size. Each fraction was used to measure dissolved protein concentration at 280 nm, enzyme activity in U/mL, and specific activity in U/mg protein as previously described [35,36].
2.12. Statistical Analysis
Standard curves of xylanase enzyme activity, reducing sugar, total carbohydrates, and protein concentration were drawn using Microsoft Excel 2010. One-way analysis of variance (ANOVA) was used for different testing among the experimental groups. Data were calculated as the mean ± standard error of the mean (SE) for duplicate independent experiments, and p ≤ 0.05 was considered a significant difference.
3. Results
3.1. Flask Solid State Fermentation (SSF) for Xylanase Production by Trichoderma sp.
Three Trichoderma species (T. atroviride MT626716, T. harzianum MT626717, and T. longibrachiatum MT626720) were tested for xylanase production in flask SSF. Their optimum growth temperature was examined using PDA agar plates. The optimum temperature for their growth was 29–30 °C, where maximum biomass concentration was observed. The three species entered the stationary phase, as indicated by spore formation, after five days of incubation.
To perform SSF for enzyme production, the three Trichoderma spp. were grown on OMP with or without BB under static conditions without any supplements. It was noticed that fungal mycelia penetrated through the substrate particles of the culture after three days of incubation, and sporulation started to appear afterward. Xylanase activity, reducing sugar, and total carbohydrate concentrations were measured for seven-day-old Trichoderma spp. cultures (Figure 1). The data illustrate that supplementing OMP with BB enhanced enzyme production and fungal growth as well. The three fungal species showed almost the same behavior in producing the xylanase enzyme (Figure 1).
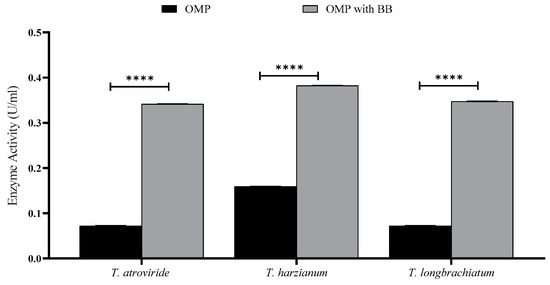
Figure 1.
Xylanase activity of T. atroviride, T. harzianum, and T. longibrachiatum cultured on OMP with and without BB after seven days of fermentation. Bars represent the means (±SE) of two replicates for each culture. Asterisks indicate statistically significant differences at p ≤ 0.05. **** p < 0.0001.
T. atroviride was able to liberate 43.725 μg/mL of reducing sugars compared to 22.835 mg/mL in the culture of T. longibrachiatum. On the other hand, the maximum total carbohydrate concentration obtained was from the cultures of T. longibrachiatum (1.92 μg/mL). Based on these results, combination cultures of OMP and BB were used for further studies.
3.2. Optimization of SSF for Xylanase Production
The effect of OMP and BB mixing ratios and sizes, in addition to the supplementation of cultures with trace elements, was studied. The results revealed a significant difference in the activity of xylanase and concentrations of reducing sugars and total carbohydrates in the three cultures of Trichoderma sp. compared to that of the control (p < 0.001; Figure 2 and Figure 3).
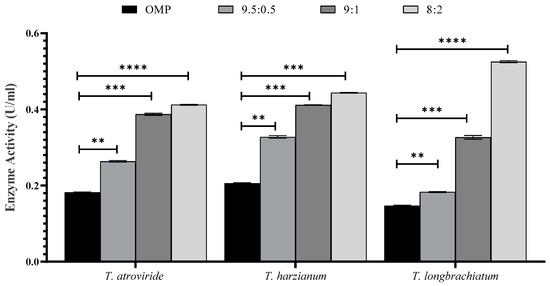
Figure 2.
The effect of the culture ratio between OMP and BB on xylanase activity in the three studied Trichoderma species after seven days of fermentation. Bars represent the means (±SE) of two replicates for each culture. Asterisks indicate statistically significant differences at p ≤ 0.05. ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
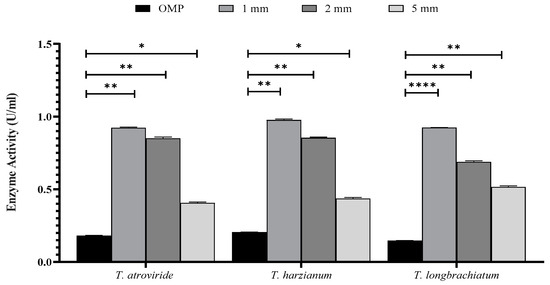
Figure 3.
The effect of particle size of OMP and BB on xylanase activity in the three studied Trichoderma species after seven days of fermentation. Bars represent the means (±SE) of two replicates for each culture. Asterisks indicate statistically significant differences at p ≤ 0.05. * p < 0.05, ** p < 0.01, and **** p < 0.0001.
The activity of xylanase was significantly higher in all ratios and sizes tested compared to that of the control. It was noticed that there were variations in xylanase activity within each Trichoderma sp. culture (Figure 2 and Figure 3). The ratios of 8:2 and 9.5:0.5 of OMP and BB gave the highest and lowest xylanase activity, respectively (Figure 2). In Figure 3, the particle size of 1 mm was found to be the most effective in xylanase production compared to 2 mm and 5 mm in all Trichoderma species studied. Similarly, the inclusion of trace elements in growth cultures exerted a significant effect on enhancing Trichoderma to produce more active xylanase (Figure 3).
However, the effect was dependent on the type of supplement included in the culture. In all species, supplementation with xylan, peptone, or a combination of both yielded higher xylanase activity compared to all other types of supplements and the controls (Figure 4). Notably, peptone alone was found to be the most effective in xylanase production in all Trichoderma species, irrespective of the presence or absence of xylan and despite the overall variations observed (Figure 4).
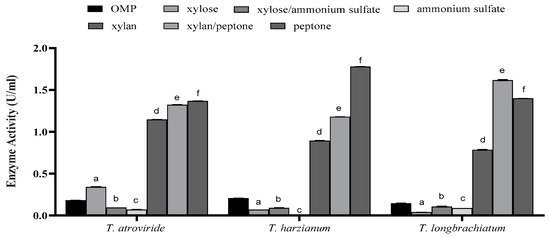
Figure 4.
The effect of supplementation of growth cultures with trace elements on xylanase activity for the three studied Trichoderma species after seven days of fermentation. Bars represent the means (±SE) of two replicates for each culture. Different letters indicate statistically significant differences at p ≤ 0.05.
3.3. Time Profile of the Process
The time profile for the production process was studied in the three tested cultures of Trichoderma sp. at the optimum conditions determined previously. The xylanase activity, reducing sugar, and total carbohydrate concentrations were measured independently every day over the incubation period (Figure 5). Over the incubation period, the production of the xylanase enzyme for the three tested species of Trichoderma reached its maximum on day three. Therefore, three-day-old cultures were chosen for further studies.
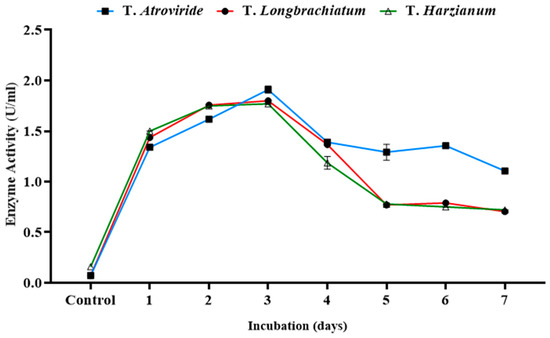
Figure 5.
Xylanase activity over a seven-day incubation period in three studied Trichoderma species incubated at 29 °C.
3.4. Packed-Bed Column Solid State Fermentation
The data of the flask SSF process for xylanase enzyme production were translated into a column bioreactor packed with OMP and BB under SSF conditions. After three days of fermentation, all measures were significantly higher compared to those achieved from flask SSF cultures (Figure 6 and Figure 7). For instance, the maximum xylanase activity achieved was 1.998 U/mL for T. harizianum (Figure 7). The maximum total reducing sugars (867.08 mg/mL) and total carbohydrate (1.667 mg/mL) were the highest in T. atroviride compared to the other two species.
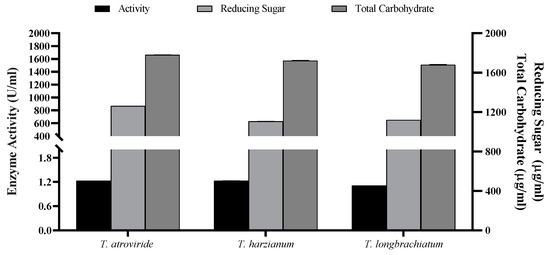
Figure 6.
Xylanase activity and concentrations of reducing sugars and total carbohydrate of the three Trichoderma cultured species using packed-bed bioreactor.
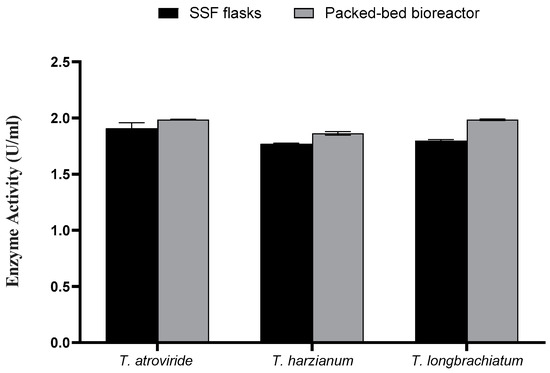
Figure 7.
Xylanase activity using SSF and packed-bed bioreactor.
3.5. Percentage of OMP Degradation
The percentage of substrate degradation varied between SSF in flasks and in the packed-bed bioreactor, with the former being generally greater than the latter except for T. longibrachiatum. Using the SSF, the percentage varied in the following order: T. harzianum > T. longibrachiatum > T. atroviride (Figure 8). The maximum percentage of degradation recorded was about 15% in the cultures of T. harzianum flask SSF cultures, compared to about 11% in T. longibrachiatum bioreactor cultures.
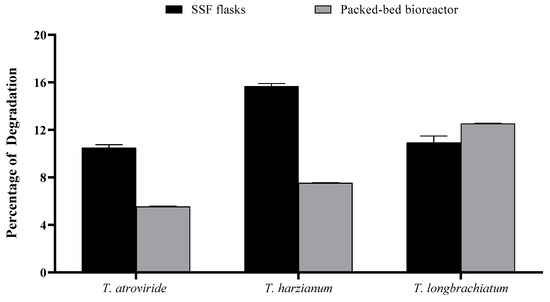
Figure 8.
Consumed and percentage of culture media after seven days of incubation using SSF and packed-bed bioreactor by the three Trichoderma species studied. Bars represent the means (±SE) of four replicates per species.
3.6. Partial Purification of Xylanase
The crude extract of each Trichoderma species that gave the highest xylanase activity using a packed-bed bioreactor was partially purified through a series of ammonium sulfate precipitation reactions and dialysis. The highest dissolved protein concentration was obtained using a 60% ammonium sulfate saturation, and this percentage was chosen for further purification of xylanase using size-exclusion chromatography.
The experiment of size-exclusion chromatography yielded 25 fractions per Trichoderma species tested (Figure 9). Dissolved proteins and xylanase activity were highest in fractions 9 to 12 for T. atroviride, 4 to 7 for T. harzianum, and 9 to 14 for T. longibrachiatum (Figure 9).
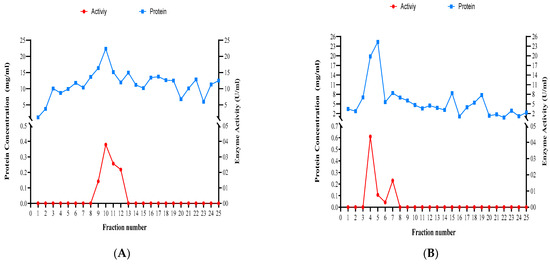
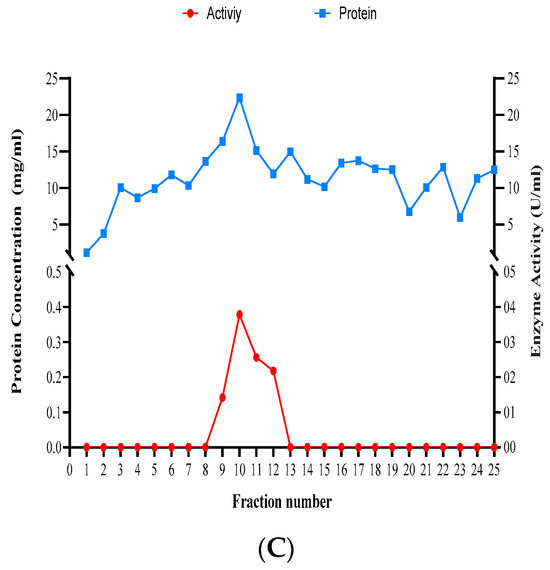
Figure 9.
Concentrations of dissolved proteins and xylanase activity in the fractions obtained using size-exclusion chromatography for the three studied Trichoderma species. (A) T. atroviride, (B) T. harzianum, and (C) T. longibrachiatum.
Xylanase enzymatic activity ranged from 0.274 U/mL in T. harzianum to 0.837 U/mL in T. atroviride when crude extract was used. In general, xylanase activity was the highest in T. atroviride compared to the other two species across all the ammonium sulfate precipitation concentrations (20–70%). In addition, the specific activity of xylanase was measured for the three tested species of Trichoderma, and data ranging from 0.001 to 0.064 U/mg for T. atroviride, 0.004 to 0.031 mg/U for T. harzianum, and from 0.003 to 0.057 for T. longibrachiatum were obtained (Appendix A).
4. Discussion
In this study, a combination of olive mill pomace (OMP) and barley bran (BB) was studied as substrates for the production of the xylanase enzyme by Trichoderma sp. Different lignocellulosic materials were used as substrates to produce xylanase, and several reports illustrated the ability of Trichoderma species to use lignocellulosic materials as nutrient sources [37]. The agro-industrial residues of OMP have been well recognized in the literature as a rich source of organic matter, comprising a large amount of cellulose, hemicellulose, lignin, lipids, carbohydrates, and phenols [38]. This complex structure makes it suitable for the growth and metabolism of many microorganisms, specifically Trichoderma [39]. However, there are limited studies in the literature on using a combination of OMP and BB as an enzyme inducer to produce xylanase.
The activity of xylanase was studied with and without BB throughout the incubation period. The highest activity of xylanase was recorded with BB, and the incubation period was seven days. This indicates that the BB used in this study supports the growth of Trichoderma spp. and enhances their capacity to produce xylanase, an inducible enzyme. This is consistent with the findings of Soliman [40], who underscored BB as an excellent nutritive material that stimulates Trichoderma to produce xylanases because its hemicellulosic content is composed largely of xylan [40]. The substrates OMP and BB are thus vital for enhancing gene expression and protein synthesis, and, in consequence, the activity of cells to produce xylanase is augmented. It is well accepted that when cells are in the stationary phase of growth, their priority to express a specific gene varies depending on their needs and the signals they receive from their environment.
Xylanase activity increased after the inclusion of OMP and BB at a ratio of 8:2 and a particle size of 1 mm. It was necessary to reduce the substrate particle size, which may provide a favorable surface area for fungal growth, uptake of gases, accessibility, and hence improved production of xylanase. Lakshmi [41] affirmed that a smaller substrate particle size provides more efficient nutrient uptake, better support for microbial attachment, and better transport of substrate components compared to larger particle sizes.
To further enhance fungal growth and enzyme production, 1% xylan and peptone were added to the cultures as additional carbon and nitrogen sources, respectively. This supplement increases xylanase activity. Xylan was added as a simple inducer of gene expression and xylanase synthesis, whereas peptone was utilized by Trichoderma as a protein source. Once a high fungal biomass is established, Trichoderma can achieve complete hydrolysis of xylan. Therefore, the addition of 1% of these constituents to the fermentation media helps the microorganism attain its maximum xylanase production capability in a shorter period of time owing to the easily utilized carbon and nitrogen sources in the media. Our results support those of Gauterio et al. [42], who maximized xylanase production by Aureobasidium pullulans using a by-product of rice grain supplied with xylan, peptone, and other nutritional sources. Moreover, a considerable increase in xylanase activity by T. harzianum 1073 D3 was reported when 1% xylose was included, surpassing the effects observed using alternative sugars such as glucose, galactose, fructose, lactose, and sucrose [43]. In a study by El-Gendi et al. [44] on xylanase production by Bacillus subtilis using varying peptone concentrations, a direct correlation between peptone concentration and xylanase production and strain growth was reported. The highest growth and productivity were found at a peptone concentration of 1.4%. Taking these findings as a baseline, peptone emerged as a primary organic nitrogen source required to enhance xylanase production and activity by bacterial and fungal species [43].
In this study, examining the temporal profile of the production process reveals that xylanase activity peaked after three days of incubation, followed by a sudden drop. This indicates that the fungal ability to generate the enzyme was strikingly restricted to the early phases of growth, allowing them to access available substrates necessary for their survival and reproduction. Subsequently, xylanase generation decreased during the transition between log and stationary phases of their normal growth cycles. From a commercial perspective, repeating solid-state fermentation (SSF) beyond a three-day incubation period would not yield significant results for xylanase production. The decline in nutrient availability and the build-up of metabolic by-products adversely impact fungal growth, pushing them into the stationary phase, where spore formation is induced. A similar trend of xylanase activity by Trichoderma using SSF was previously reported by Pandey [45].
To study xylanase enzyme properties, it was necessary to produce the enzyme in a larger quantity. For that reason, a packed-bed bioreactor was employed. Xylanase enzyme production was enhanced in the packed-bed bioreactor compared to the SSF flask due to the efficient aeration pattern in the packed-bed bioreactor, which provides air circulation in a closed system [45]. Also, a packed-bed bioreactor maintains adequate and homogeneous levels of temperature and moisture, allowing efficient transfer of nutrients and metabolites [46]. In a recent study by Massadeh et al. [47], the entrapment/immobilized fermentation method has been proposed as an alternative to classical free-cell fermentation owing to its prolonged periods of growth, enhanced metabolic activities, and repeated use of cells. Cell entrapment is a common methodology for whole-cell immobilization carried out in a polymer matrix, carrageenan and alginate, and synthetic fibers. As a result, fungal growth was greatly improved, xylanase activity increased, and the maximum percentage of substrate degradation was accomplished.
Partial purification of the xylanase enzyme was performed after protein precipitation with ammonium sulfate. The highest xylanase activity was achieved at 60% saturation of ammonium sulfate. The crude enzyme precipitate was dialyzed against solid sodium citrate, which caused a reduction in enzyme activity compared to the crude enzyme extract, which might be due to the high polarity of the protein that forced them to remain in their physical medium. This indicates that it is possible to separate proteins from a mixture on the basis of their relative hydrophilicity by gradually increasing the concentration of ammonium sulphate [42]. Gauterio et al. [42] reported a reduction in xylanase and cellulase activities when employing ammonium sulfate precipitation and dialysis against different buffer solutions.
The dialyzed samples with the highest activity were subjected to gel filtration chromatography using Sephadex G-50 and G-75. The results revealed that xylanase was partially purified, and its activity increased. This improvement could be due to the removal of other compounds affecting xylanase activity, as Sephadex G-50 and G-75 allowed the adhesion of proteins of specific sizes. Our results are in agreement with Silva et al. [48], who purified xylanase from Trichoderma sp. using Sephadex G-75 and claimed that the xylanase enzyme was purified [48].
Fractions showing the highest activity from the gel filtration chromatography were collected, and a sample of each fraction was spotted on silica gel plates to perform TLC. It was shown that xylose and glucose were the main sugars present in the enzyme extracts when compared to the standard employed (R. Alkfoof, K., Alananbeh, M. Massadeh and R. Muhaidat, unpublished results). Two distinct materials, namely OMP and BB, were employed as substrates for Trichoderma culture. The induction process triggered the enzymatic activity, leading to enzyme-mediated transformations. Upon conducting thin layer chromatography (TLC) post-enzyme purification, it was observed that OMP and BB exhibited high heterogeneity and intricate structural complexity. Their native structures posed challenges in comprehension. The enzymatic action on these materials resulted in not only xylose but also the formation of disaccharides and oligosaccharides. Given the endoxylanase nature of the enzyme, it acts on internal bonds within these structurally complex substrates. Unfortunately, due to the limited availability of disaccharides and oligosaccharides in the laboratory, a comprehensive experiment elucidating the complete spectrum of these materials was not conducted. Nevertheless, our analysis confirmed the presence of xylose in the reaction mixture. Our results agree with those of Silva et al. [48], who claimed that complete degradation of xylan releases xylose as the final product.
The xylanase enzyme has tremendous applications in industry, and it can be used in the synthesis of biofuels, foodstuffs, textiles, organic acids, and many value-added products. The agro-residues of OMP are cheap and available all through the year, and hence it is recommended to study its supplementation with different cost-effective elements to support the growth and metabolism of Trichoderma. To elucidate the fungal behavior in producing an active xylanase enzyme, it is recommended to investigate the type of xylanase produced by using SDS-PAGE, native PAGE electrophoresis, and HPLC for detecting the reaction products. In doing so, additional species of Trichoderma should be studied.
5. Conclusions
This study showed the ability of three Trichoderma species to produce xylanase by using OMP and BB as substrates in solid-state fermentation. After optimizing the growth of the three Trichoderma sp., xylanase enzyme production was assessed. It was found that BB supplementation was necessary to induce xylanase production. The optimum ratio of OMP and BB was 8:2, corresponding with the highest activity of xylanase recorded (0.527 U/mL) from the culture of T. longibrachiatum. Furthermore, using a 1 mm particle size of both substrates enhanced the activity of xylanase (0.983 U/mL) from the culture of T. harzianum. Supplementing the culture with xylan and peptone showed a positive impact on fungal growth and xylanase production in all Trichoderma sp. cultures. The maximum xylanase activity (1.944 U/mL) was obtained in the culture of T. atroviride after 3 days in SSF flask culture and in the packed-bed SSF bioreactor (1.998 U/mL). To extract the enzyme and partially purify it, the crude extract was collected from the packed-bed bioreactor culture of the three Trichoderma sp. and subjected to 60% ammonium sulfate precipitation and dialysis against solid sodium citrate. The dialyzed enzyme samples were introduced to size-exclusion chromatography using Sephadex G-75 and G-50. It was noticed that xylanase activity was concentrated, and to confirm its purity, the fractions were subjected to a TLC reaction. The results revealed that the selected fractions collected from the three Trichoderma sp. were highly active, liberating xylose as the main sugar observed.
Author Contributions
Conceptualization, R.A., R.M., M.M. and K.M.A.; methodology, R.A., R.M., M.M. and K.M.A.; investigation, R.A. and R.M.; resources, R.A., R.M., M.M. and K.M.A.; project administration. R.M., M.M. and K.M.A.; data curation, R.A. and R.M.; writing—original draft preparation, R.A., R.M., M.M. and K.M.A.; writing—review and editing, R.M., M.M. and K.M.A.; supervision, R.M., M.M. and K.M.A.; funding acquisition R.M., M.M. and K.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by research grant number 1600204/9 to M.M. from the Deanship of Research of the Hashemite University, and the research lab facilities of the Department of Biological Sciences of Yarmouk University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Xylanase specific activity at different fractions for the three tested Trichoderma spp.

| Fractions | Trichoderma species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. atroviride | T. harzianum | T. longibrachiatum | ||||||||||
| OD280 | Protein mg/mL | Activity U/mL | Specific Enzyme Activity U/mg | OD280 | Protein mg/mL | Activity U/mL | Specific Enzyme Activity U/mg | OD280 | Protein mg/mL | Activity U/mL | Specific Enzyme Activity U/mg | |
| 1 | -0.035 | -1.750 | 0.000 | 0.000 | 0.068 | 3.410 | 0.000 | 0.000 | 0.006 | 0.300 | 0.000 | 0.000 |
| 2 | -0.030 | -1.510 | 0.000 | 0.000 | 0.055 | 2.735 | 0.000 | 0.000 | 0.009 | 0.450 | 0.000 | 0.000 |
| 3 | -0.001 | -0.050 | 0.000 | 0.000 | 0.140 | 7.015 | 0.000 | 0.000 | 0.065 | 3.250 | 0.000 | 0.000 |
| 4 | 0.005 | 0.260 | 0.000 | 0.000 | 0.396 | 19.820 | 0.610 | 0.031 | 0.149 | 7.440 | 0.000 | 0.000 |
| 5 | 0.013 | 0.640 | 0.000 | 0.000 | 0.487 | 24.325 | 0.106 | 0.004 | 0.212 | 10.595 | 0.000 | 0.000 |
| 6 | 0.070 | 3.486 | 0.000 | 0.000 | 0.111 | 5.550 | 0.042 | 0.007 | 0.213 | 10.670 | 0.000 | 0.000 |
| 7 | 0.016 | 0.796 | 0.000 | 0.000 | 0.168 | 8.415 | 0.230 | 0.027 | 0.300 | 15.005 | 0.000 | 0.000 |
| 8 | 0.035 | 1.750 | 0.000 | 0.000 | 0.140 | 6.980 | 0.000 | 0.000 | 0.309 | 15.440 | 0.000 | 0.000 |
| 9 | 0.159 | 7.950 | 0.511 | 0.064 | 0.121 | 6.040 | 0.000 | 0.000 | 0.397 | 19.840 | 0.059 | 0.003 |
| 10 | 0.200 | 10.005 | 0.093 | 0.009 | 0.093 | 4.635 | 0.000 | 0.000 | 0.485 | 24.235 | 0.469 | 0.019 |
| 11 | 0.350 | 17.500 | 0.106 | 0.006 | 0.072 | 3.600 | 0.000 | 0.000 | 0.391 | 19.540 | 0.140 | 0.007 |
| 12 | 0.456 | 22.780 | 0.041 | 0.002 | 0.090 | 4.475 | 0.000 | 0.000 | 0.286 | 14.315 | 0.250 | 0.017 |
| 13 | 0.388 | 19.400 | 0.000 | 0.000 | 0.076 | 3.775 | 0.000 | 0.000 | 0.209 | 10.425 | 0.597 | 0.057 |
| 14 | 0.300 | 15.000 | 0.000 | 0.000 | 0.062 | 3.120 | 0.000 | 0.000 | 0.244 | 12.220 | 0.050 | 0.004 |
| 15 | 0.298 | 14.900 | 0.000 | 0.000 | 0.167 | 8.345 | 0.000 | 0.000 | 0.304 | 15.205 | 0.000 | 0.000 |
| 16 | 0.266 | 13.300 | 0.000 | 0.000 | 0.022 | 1.120 | 0.000 | 0.000 | 0.300 | 15.005 | 0.000 | 0.000 |
| 17 | 0.255 | 12.750 | 0.000 | 0.000 | 0.079 | 3.935 | 0.000 | 0.000 | 0.223 | 11.130 | 0.000 | 0.000 |
| 18 | 0.250 | 12.500 | 0.000 | 0.000 | 0.108 | 5.390 | 0.000 | 0.000 | 0.270 | 13.480 | 0.000 | 0.000 |
| 19 | 0.203 | 10.165 | 0.000 | 0.000 | 0.155 | 7.745 | 0.000 | 0.000 | 0.257 | 12.830 | 0.000 | 0.000 |
| 20 | 0.100 | 5.005 | 0.000 | 0.000 | 0.007 | 0.345 | 0.000 | 0.000 | 0.270 | 13.500 | 0.000 | 0.000 |
| 21 | 0.125 | 6.250 | 0.000 | 0.000 | 0.034 | 1.715 | 0.000 | 0.000 | 0.200 | 10.005 | 0.000 | 0.000 |
| 22 | 0.200 | 10.000 | 0.000 | 0.000 | 0.015 | 0.765 | 0.000 | 0.000 | 0.190 | 9.480 | 0.000 | 0.000 |
| 23 | 0.100 | 5.010 | 0.000 | 0.000 | 0.058 | 2.885 | 0.000 | 0.000 | 0.156 | 7.775 | 0.000 | 0.000 |
| 24 | 0.109 | 5.450 | 0.000 | 0.000 | 0.023 | 1.170 | 0.000 | 0.000 | 0.098 | 4.910 | 0.000 | 0.000 |
| 25 | 0.100 | 5.000 | 0.000 | 0.000 | 0.047 | 2.335 | 0.000 | 0.000 | 0.085 | 4.260 | 0.000 | 0.000 |
References
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent advances in health benefits of bioactive compounds from food wastes and by-products: Biochemical aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Knob, A.; Fortkamp, D.; Prolo, T.; Izidoro, S.C.; Almeida, J.M. Agro-residues as an alternative for xylanase production by filamentous fungi. BioResources 2014, 9, 5738–5773. [Google Scholar]
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier, H.; Jang, H.; Verniquet, A.; Brosze, J.; et al. A Research challenge vision regarding management of agricultural waste in circular bio-based economy. Crit. Rev. Environ. Sci. Technol. 2018, 48, 614–654. [Google Scholar] [CrossRef]
- Basit, A.; Jiang, W.; Rahim, K. Xylanase and its industrial applications. In Biotechnological Applications of Biomass; Basso, T.P., Basso, T.O., Basso, L.C., Eds.; Intechopen: London, UK, 2020; p. 638. [Google Scholar]
- Ocreto, J.B.; Chen, W.H.; Rollon, A.P.; Ong, H.C.; Pétrissans, A.; Pétrissans, M.; De Luna, M.D.G. Ionic liquid dissolution utilized for biomass conversion into biofuels, value-added chemicals and advanced materials: A comprehensive review. Chem. Eng. J. 2022, 445, 136733. [Google Scholar] [CrossRef]
- Rebouillat, S.; Pla, F. A Review: On smart materials based on some polysaccharides; within the contextual bigger data, insiders, “improvisation,” and said artificial intelligence trends. J. Biomater. Nanobiotech. 2019, 10, 41–77. [Google Scholar] [CrossRef]
- Kumar, A.; Naraian, R. Chapter 6—Differential expression of the microbial β-1,4-Xylanase and β-1,4-Endoglucanase genes. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Gupta, V.K., Jogaiah, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 95–111. [Google Scholar]
- Mardawati, E.; Sinurat, Y.; Yuliana, T. Production of crude xylanase from Trichoderma sp. using Reutealis trisperma exocarp substrate in solid state fermentation. In Proceedings of the International Conference of Sustainability Agriculture and Biosystem, Padang, Indonesia, 12–13 November 2019; IOP Publishing: Bristol, UK, 2020; p. 012024. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Verma, P. A Detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Dutta, P.D.; Neog, B.; Goswami, T. Xylanase enzyme production from Bacillus australimaris P5 for prebleaching of bamboo (Bambusa tulda) pulp. Mater. Chem. Phys. 2020, 243, 122227. [Google Scholar] [CrossRef]
- Fang, H.Y.; Chang, S.M.; Hsieh, M.C.; Fang, T.J. Production, Optimization growth conditions, and properties of the xylanase from Aspergillus carneus M34. J. Mol. Catal. B Enzym. 2007, 49, 36–42. [Google Scholar] [CrossRef]
- Rojas, L.F.; Zapata, P.; Ruiz-Tirado, L. Agro-industrial waste enzymes: Perspectives in circular economy. Curr. Opin. Green Sustain. Chem. 2022, 34, 100585. [Google Scholar] [CrossRef]
- Danso, B.; Ali, S.S.; Xie, R.; Sun, J. Valorization of wheat straw and bioethanol production by a novel xylanase- and cellulase-producing Streptomyces strain isolated from the wood-feeding termite, Microcerotermes Species. Fuel 2022, 310, 122333. [Google Scholar] [CrossRef]
- Bajpai, P. Xylanases. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Lederberg, J., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 600–612. [Google Scholar]
- Azzouz, Z.; Bettache, A.; Boucherba, N.; Amghar, Z.; Benallaoua, S. Optimization of xylanase production by newly isolated strain Trichoderma afroharzianum isolate AZ 12 in solid state fermentation using response surface methodology. Cellul. Chem. Technol. 2020, 54, 451–462. [Google Scholar] [CrossRef]
- Haddadin, M.S.; Haddadin, J.; Arabiyat, O.I.; Hattar, B. Biological conversion of olive pomace into compost by using Trichoderma harzianum and Phanerochaete chrysosporium. Bioresour. Technol. 2009, 100, 4773–4782. [Google Scholar] [CrossRef] [PubMed]
- Polizeli, M.; Rizzatti, A.; Monti, R.; Terenzi, H.; Jorge, J.A.; Amorim, D. Xylanases from fungi: Properties and industrial applications. Appl. Microbiol. Biotechnol. 2005, 67, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Abdelhadi, S.O.; Dosoretz, C.G.; Rytwo, G.; Gerchman, Y.; Azaizeh, H. Production of biochar from olive mill solid waste for heavy metal removal. Bioresour. Technol. 2017, 244, 759–767. [Google Scholar] [CrossRef]
- Khdair, A.I.; Abu-Rumman, G.; Khdair, S.I. Pollution estimation from olive mills wastewater in Jordan. Heliyon 2019, 5, e02386. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, K.; Nayak, R.; Shukla, A.; Parmar, P.; Goswami, D.; Saraf, M. Polyhydroxyalkanoates: An exotic gleam in the gloomy tale of plastics. J. Polym. Environ. 2021, 29, 2013–2032. [Google Scholar] [CrossRef]
- Abdollahi, F.; Jahadi, M.; Ghavami, M. Thermal stability of natural pigments produced by Monascus purpureus in submerged fermentation. Food Sci. Nutr. 2021, 9, 4855–4862. [Google Scholar] [CrossRef]
- Singh, A.; Shahid, M.; Srivastava, M.; Pandey, S.; Sharma, A.; Kumar, V. Optimal physical parameters for growth of Trichoderma species at varying pH, temperature, and agitation. Virol Mycol. 2014, 3, 1–7. [Google Scholar]
- Khanahmadi, M.; Arezi, I.; Amiri, M.-S.; Miranzadeh, M. Bioprocessing of agro-industrial residues for optimization of xylanase production by solid-state fermentation in flask and tray bioreactor. Biocatal. Agric. Biotechnol. 2018, 13, 272–282. [Google Scholar] [CrossRef]
- Al Sheikh, A.H.M. Utilization of Dry Mill Residues (DOR) for the Production of Xylanase Enzyme by Aspergillus terreus in Solid State Fermentation. Master’s Thesis, Hashemite University, Zarqa, Jordan, 2011. [Google Scholar]
- Assamoi, A.A.; Destain, J.; Delvigne, F.; Lognay, G.; Thonart, P. Solid-state fermentation of xylanase from Penicillium canescens 10-10c in a multi-layer-packed bed reactor. Appl. Biochem. Biotechnol. 2007, 145, 87–98. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Teixeira, R.S.; da Silva, A.S.; Ferreira-Leitao, V.S.; da Silva Bon, E.P. Amino acids interference on the quantification of reducing sugars by the 3,5-dinitrosalicylic acid assay mislead carbohydrase activity measurements. Carbohydr. Res. 2012, 363, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ranjitha, P.; Karthy, E.; Mohankumar, A. Purification and characterization of the lipase from marine Vibrio fischeri. Int. J. Biol. 2009, 1, 48. [Google Scholar] [CrossRef]
- Saryono, S.; Novianty, R.; Suraya, N.; Piska, F.; Devi, S.; Pratiwi, N.W.; Ardhi, A. Molecular identification of cellulase-producing thermophilic fungi isolated from Sungai Pinang hot spring, Riau Province, Indonesia. Biodivers. J. 2022, 23. [Google Scholar] [CrossRef]
- Shallom, D.; Shoham, Y. Microbial hemicellulases. Curr. Opin. Microbiol. 2003, 6, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Sharma, N.; Verma, S.K. Optimization of cellulase-free xylanase produced by a potential thermoalkalophilic Paenibacillus sp. N1 isolated from hot springs of Northern Himalayas in India. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1–24. [Google Scholar]
- Oliveira, F.; Salgado, J.M.; Abrunhosa, L.; Pérez-Rodríguez, N.; Domínguez, J.M.; Venâncio, A.; Belo, I. Optimization of lipase production by solid-state fermentation of olive pomace: From flask to laboratory-scale packed-bed bioreactor. Bioprocess Biosyst. Eng. 2017, 40, 1123–1132. [Google Scholar] [CrossRef]
- Dhivahar, J.; Khusro, A.; Ahamad Paray, B.; Rehman, M.U.; Agastian, P. Production and partial purification of extracellular Xylanase from Pseudomonas nitroreducens using frugivorous bat (Pteropus giganteus) faeces as an ideal substrate and its role in poultry feed digestion. J. King Saud Univ. Sci. 2020, 32, 2474–2479. [Google Scholar] [CrossRef]
- Siddiqui, M.A.H.; Biswas, M.; Faruk, M.O.; Roy, M.; Asaduzzaman, A.K.M.; Sharma, S.C.D.; Biswas, T.; Roy, N. Optimization, Isolation and characterization of cellulase–free thermostable xylanase from Paenibacillus sp. Am. J. Life Sci. 2016, 4, 93–98. [Google Scholar] [CrossRef]
- Abusara, W.A. Beta-Glucosidase Enzyme Production in Solid-State Fermentation Using Olive Mill Pomace (OMP) and Yeast Industry Wastewater (YIW); MSc. Hashemite University: Zarqa, Jordan, 2019. [Google Scholar]
- Le Strat, Y.; Mandin, M.; Ruiz, N.; Robiou du Pont, T.; Ragueneau, E.; Barnett, A.; Déléris, P.; Dumay, J. Quantification of xylanolytic and cellulolytic activities of fungal strains isolated from Palmaria palmata to enhance R-phycoerythrin extraction of Palmaria palmata: From seaweed to seaweed. Mar. Drugs 2023, 21, 393. [Google Scholar] [CrossRef]
- Naeimi, S.; Khosravi, V.; Varga, A.; Vágvölgyi, C.; Kredics, L. Screening of organic substrates for solid-state fermentation, viability and bioefficacy of Trichoderma harzianum AS12-2, a biocontrol strain against rice sheath blight disease. J. Agron. 2020, 10, 1258. [Google Scholar] [CrossRef]
- Medouni-Haroune, L.; Zaidi, F.; Medouni-Adrar, S.; Kecha, M. Olive pomace: From an olive mill waste to a resource, an overview of the new treatments. J. Crit. Rev. 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Boutiche, M.; Sahir-Halouane, F.; Meziant, L.; Boulaouad, I.; Saci, F.; Fiala, S.; Bekrar, A.; Mesbah, R.; Abdessemed, A. Characterization and valorization of olive pomace for production of cellulase from Trichoderma reesei RUT C30 in solid-state fermentation. Alger. J. Environ. Sci. Technol. 2020, 6, 1381–1387. [Google Scholar]
- Soliman, H.M.; Sherief, A.; EL-Tanash, A.B. Production of xylanase by Aspergillus niger and Trichoderma viride using some agricultural residues. Int. J. Agric. Res. 2012, 7, 46–57. [Google Scholar] [CrossRef]
- Lakshmi, G.S.; Rao, C.S.; Rao, R.S.; Hobbs, P.J.; Prakasham, R.S. Enhanced production of xylanase by a newly isolated Aspergillus terreus under solid state fermentation using palm industrial waste: A statistical optimization. Biochem. Eng. J. 2009, 48, 51–57. [Google Scholar] [CrossRef]
- Gautério, G.V.; da Silva, L.G.G.; Hübner, T.; da Rosa Ribeiro, T.; Kalil, S.J. Xylooligosaccharides production by crude and partially purified xylanase from Aureobasidium pullulans: Biochemical and thermodynamic properties of the enzymes and their application in xylan hydrolysis. Process Biochem. 2021, 104, 161–170. [Google Scholar] [CrossRef]
- Seyis, I.; Aksoz, N. Xylanase production from Trichoderma harzianum 1073 D3 with alternative carbon and nitrogen sources. Food Technol. Biotechnol. 2005, 43, 37–40. [Google Scholar]
- El-Gendi, H.; Badawy, A.S.; Bakhiet, E.K.; Rawway, M.; Ali, S.G. Valorization of lignocellulosic wastes for sustainable xylanase production from locally isolated Bacillus subtilis exploited for xylooligosaccharides’ production with potential antimicrobial activity. Arch. Microbiol. 2023, 205, 315. [Google Scholar] [CrossRef]
- Pandey, S.; Shahid, M.; Srivastava, M.; Sharma, A.; Singh, A.; Kumar, V.; Srivastava, Y. Isolation and optimized production of xylanase under solid state fermentation condition from Trichoderma sp. Int. J. Adv. Res. 2014, 2, 263–273. [Google Scholar]
- De los Santos-Villalobos, S.; Hernández-Rodríguez, L.E.; Villaseñor-Ortega, F.; Peña-Cabriales, J.J. Production of Trichoderma asperellum T8a spores by a” home-made” solid-state fermentation of mango industrial wastes. BioResources 2012, 7, 4938–4951. [Google Scholar] [CrossRef][Green Version]
- Massadeh, M.I.; Fandi, K.; Al-Abeid, H.; Alsharafat, O.; Abu-Elteen, K. Production of citric acid by Aspergillus niger cultivated in olive mill wastewater using a two-stage packed column bioreactor. Fermentation 2022, 8, 153. [Google Scholar] [CrossRef]
- Silva, L.d.O.; Terrasan, C.R.F.; Carmona, E.C. Purification and characterization of xylanases from Trichoderma inhamatum. Electron. J. Biotechnol. 2015, 18, 307–313. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).