Mid-Infrared Spectroscopic Study of Cultivating Medicinal Fungi Ganoderma: Composition, Development, and Strain Variability of Basidiocarps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Cultivation
2.2. Analysis of the Basidiocarp Composiiton
2.2.1. Organic Elements and Moisture
2.2.2. Polysaccharides
- Total acidic hydrolysis: samples dissolved in ice-cold concentrated hydrochloric acid underwent hydrolysis with the same acid diluted to 1.3 mol L−1 at 100 °C for 2 h; then a mixture of exo-(1 → 3)-β-glucanase and β-glucosidase was applied to hydrolyze β-glucan residues.
- Enzymatic hydrolysis of α-d-glucans: samples dissolved in 1.7 mol L−1 sodium hydroxide were hydrolyzed by amyloglucosidase in 1.2 mol L−1 sodium acetate buffer (pH 3.8).
- Leading electrolyte (pre-separation capillary, ITP step): 10 mM sodium acetate, 10 mM acetic acid, and 0.05% hydroxyethyl cellulose (pH 4.7);
- Background electrolyte (analytical capillary, ZE step): 20 mM β-alanine, 40 mM acetic acid, and 0.1% hydroxyethyl cellulose (pH 3.9);
- Terminating electrolyte: 10 mM ε-amino-n-caproic-acid and 5 mM acetic acid (pH 4.8).
2.2.3. Proteins
2.3. Preparation of Basidiocarp Slices
2.4. Recording of ATR-FTIR Spectra
3. Results and Discussion
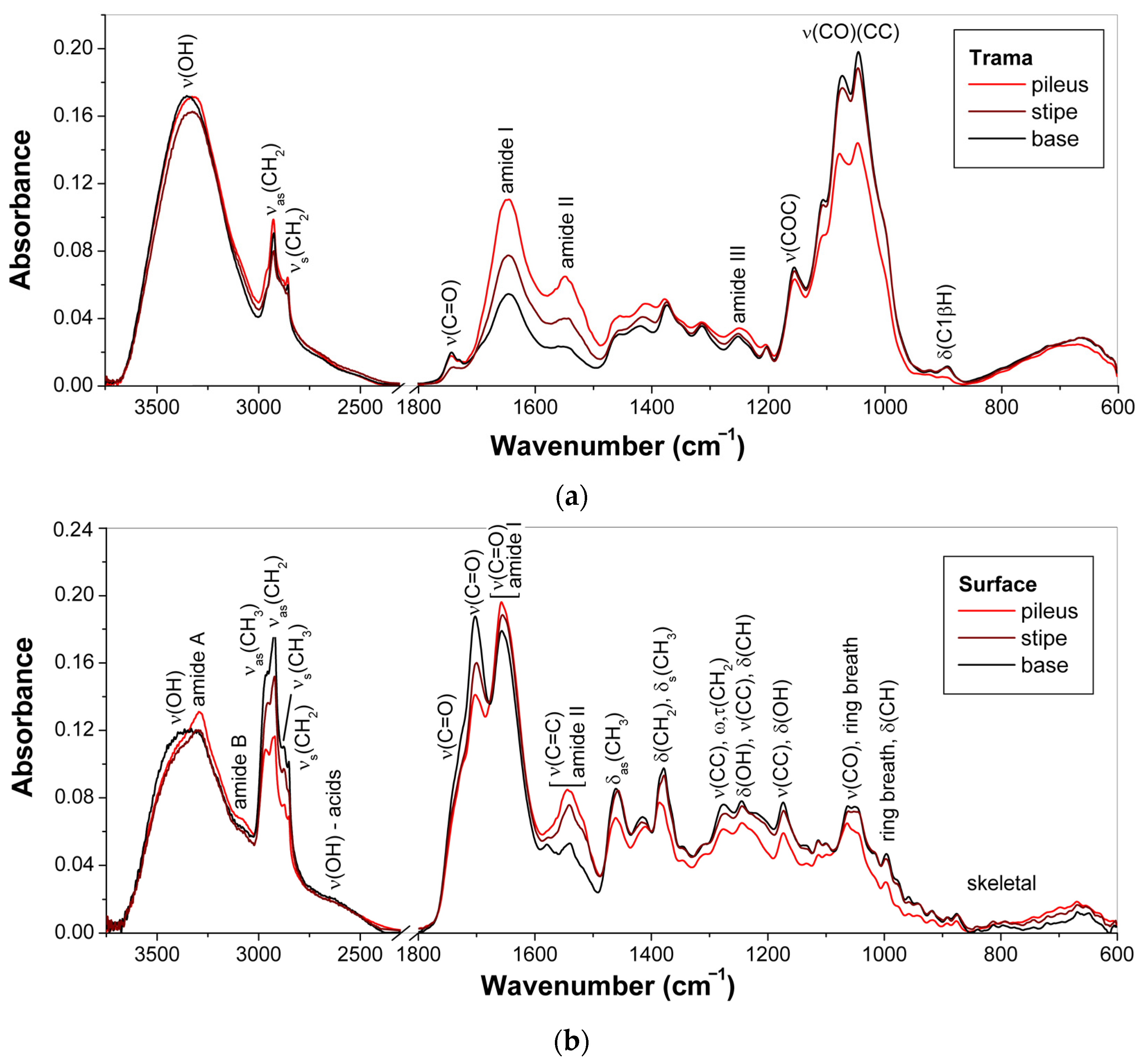
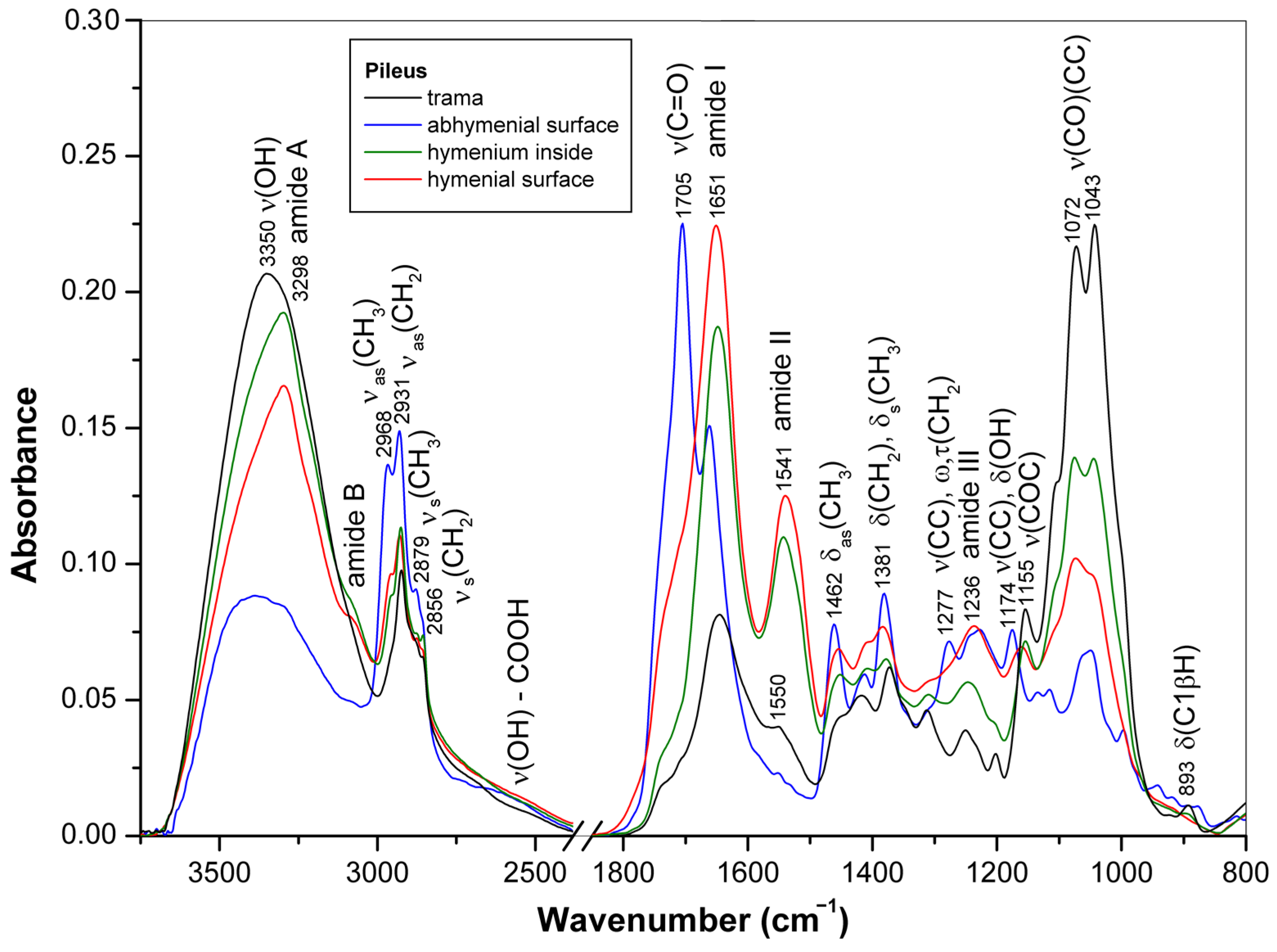
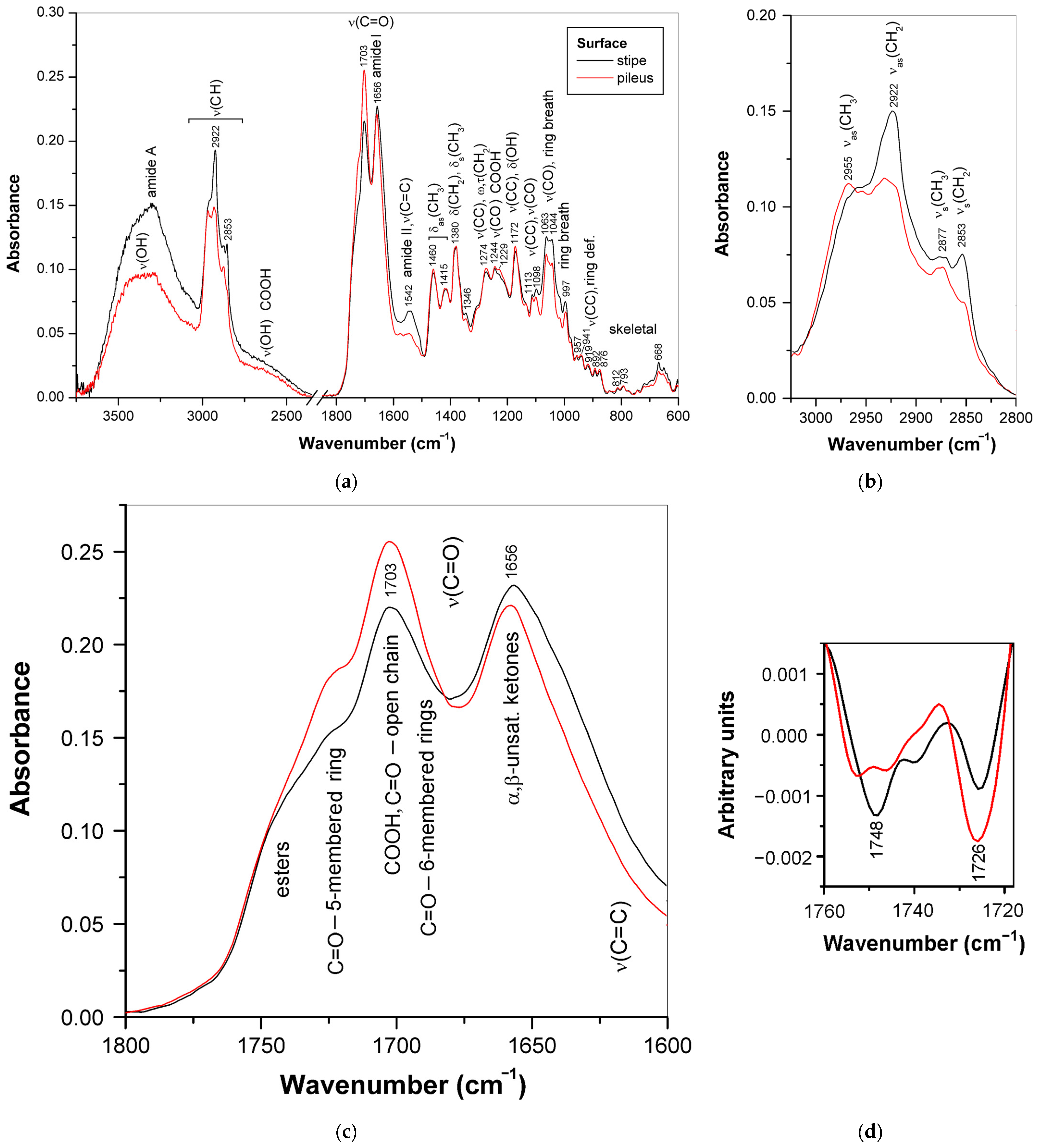
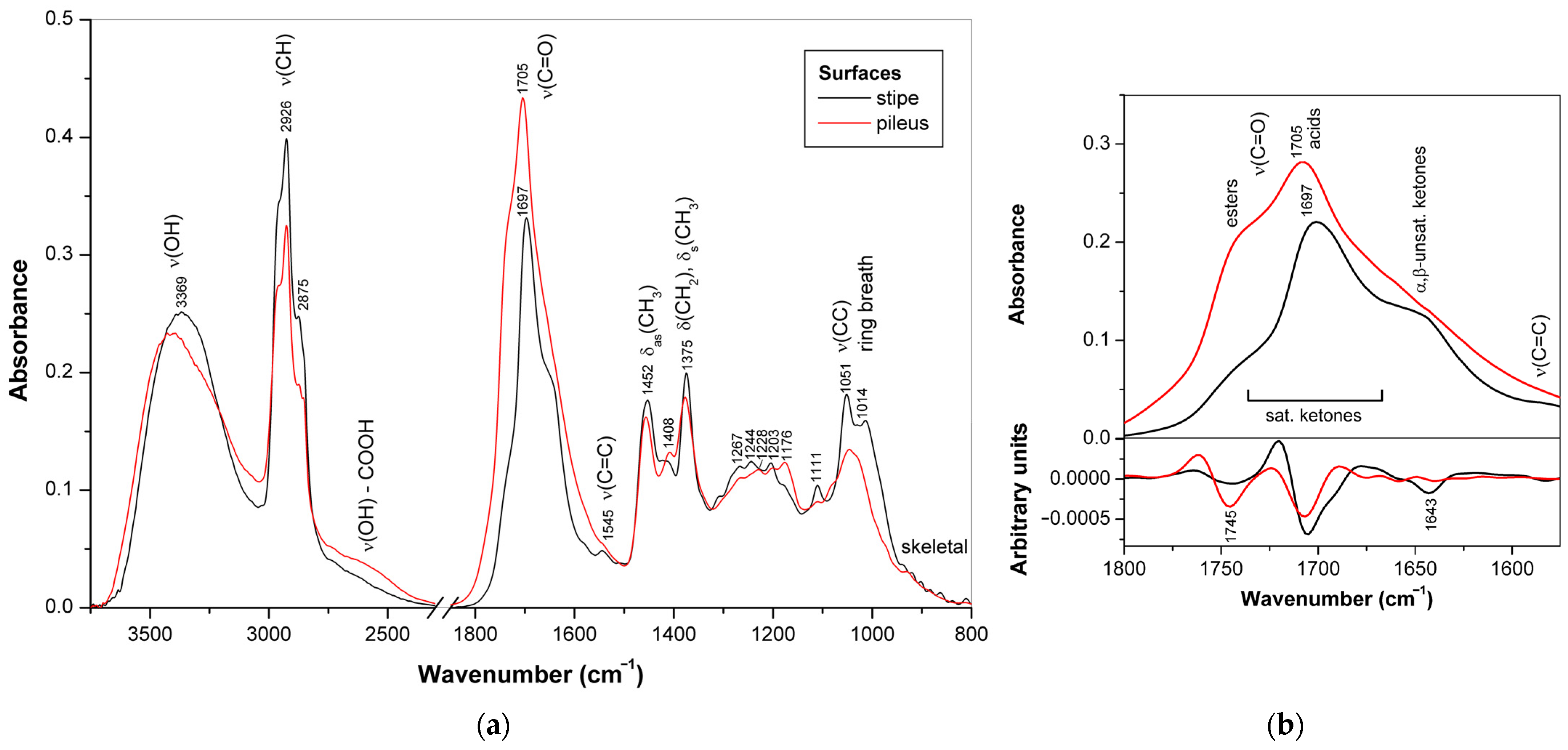
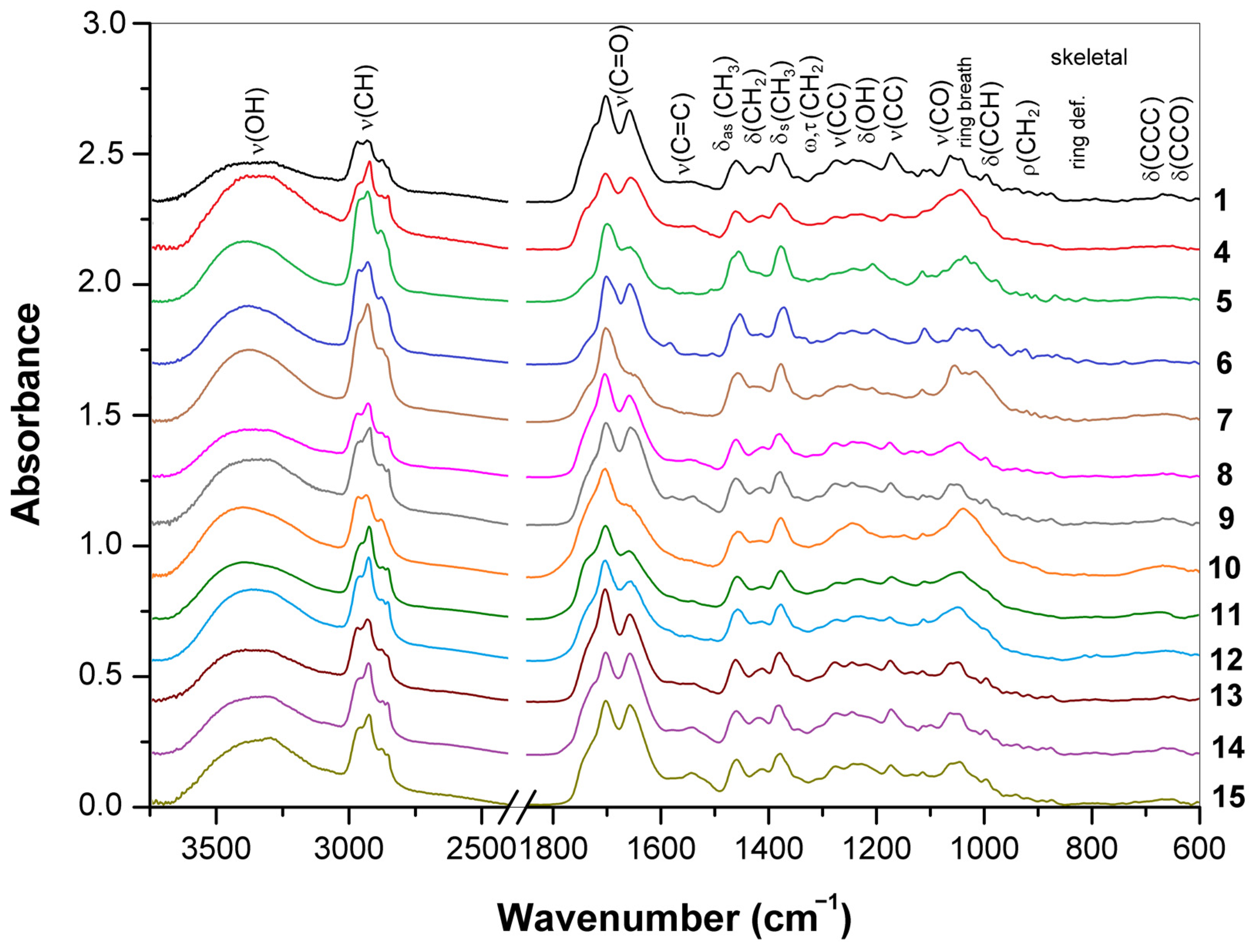
3.1. ATR-FTIR Spectra of Young Basidiocarps
3.1.1. Composition of Trama
3.1.2. Composition of Surface Layer
3.2. ATR-FTIR Spectra of Mature Basidiocarps
3.3. Triterpenoids in Surface Layer of Ganoderma Basidiocarps
3.3.1. Triterpenoid Distribution in Ganoderma Basidiocarps
3.3.2. Differences in Triterpenoid Composition for the Ganoderma Strains and Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sułkowska-Ziaja, K.; Balik, M.; Szczepkowski, A.; Trepa, M.; Zengin, G.; Kała, K.; Muszyńska, B. A Review of Chemical Composition and Bioactivity Studies of the Most Promising Species of Ganoderma spp. Diversity 2023, 15, 882. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.Q.; Zhang, J.; Li, Z.M.; Liu, H.G.; Wang, Y.Z. Traditional uses, chemical components and pharmacological activities of the genus Ganoderma P. Karst.: A review. RSC Adv. 2020, 10, 42084–42097. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef] [PubMed]
- El Sheikha, A.F. Nutritional profile and health benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as functional foods: Current scenario and future perspectives. Foods 2022, 11, 1030. [Google Scholar] [CrossRef]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Ann. Rev. 2007, 13, 265–301. [Google Scholar] [CrossRef]
- Li, L.D.; Mao, P.W.; Shao, K.D.; Bai, X.H.; Zhou, X.W. Ganoderma proteins and their potential applications in cosmetics. Appl. Microbial. Biotechnol. 2019, 103, 9239–9250. [Google Scholar] [CrossRef]
- Guo, Z.J.; Liu, Y.; Yang, J.Y.; Jin, M.Y.; Mao, P.W.; Zhou, X.W. Evaluating the Application Potential of a Recombinant Ganoderma Protein as Bioactive Ingredients in Cosmetics. Molecules 2023, 28, 3272. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, T.; Li, L.; Bao, Z.; Tu, W.; Xiang, P.; Wu, Q.; Li, P.; Cao, M.; Huang, W. Comparative mitogenomic analysis reveals intraspecific, interspecific variations and genetic diversity of medical fungus Ganoderma. J. Fungi 2022, 8, 781. [Google Scholar] [CrossRef]
- Pristaš, P.; Beck, T.; Nosalová, L.; Gaperová, S.; Gáper, J. How Different Molecular Markers Estimate the Diversity of European Species of the Ganoderma Genus. J. Fungi 2023, 9, 1023. [Google Scholar] [CrossRef]
- Veljović, S.; Veljović, M.; Nikićević, N.; Despotović, S.; Radulović, S.; Nikšić, M.; Filipović, L. Chemical composition, antiproliferative and antioxidant activity of differently processed Ganoderma lucidum ethanol extracts. J. Food Sci. Technol. 2017, 54, 1312–1320. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Srivastava, M.; Pal, M.; Sharma, Y.K.; Bhattacharya, S.; Tulsawani, R.; Sugadev, R. Screening of Indian lingzhi or reishi medicinal mushroom, Ganoderma lucidum (agaricomycetes): A upc 2-sqd-ms approach. Int. J. Med. Mushrooms 2016, 18, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.T.; Wang, S.H.; Chen, C.H.; Yang, W.B. Structure determination of β-glucans from Ganoderma lucidum with matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. Molecules 2008, 13, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Náplavová, K.; Beck, T.; Pristaš, P.; Gáperová, S.; Šebesta, M.; Piknová, M.; Gáper, J. Molecular data reveal unrecognized diversity in the European Ganoderma resinaceum. Forests 2020, 11, 850. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Zhang, X.; Xu, J.; Wang, H.; Zhao, L.; Wang, Y. Screening immunoactive compounds of Ganoderma lucidum spores by mass spectrometry molecular networking combined with in vivo zebrafish assays. Front. Pharmacol. 2020, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Bleha, R.; Třešnáková, L.; Sushytskyi, L.; Capek, P.; Čopíková, J.; Klouček, P.; Jablonský, I.; Synytsya, A. Polysaccharides from basidiocarps of the polypore fungus Ganoderma resinaceum: Isolation and structure. Polymers 2022, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Peng, X.; Adegoke, A.T.; Chen, J.; Su, H.; Hu, G.; Wei, G.; Qiu, M. NMR-based structural classification, identification, and quantification of triterpenoids from edible mushroom Ganoderma resinaceum. J. Agric. Food Chem. 2020, 68, 2816–2825. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Huang, Y.J.; Su, H.G.; Gao, Y.; Lu, S.Y.; Peng, X.R.; Li, X.N.; Zhou, L.; Qiu, M.H. Structurally diverse lanostane triterpenoids from medicinal and edible mushroom Ganoderma resinaceum Boud. Bioorg. Chem. 2020, 100, 103871. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, H.P.; Li, X.; Liu, J.K. Ganoaustralins A and B, Unusual Aromatic Triterpenes from the Mushroom Ganoderma australe. Pharmaceuticals 2022, 15, 1520. [Google Scholar] [CrossRef]
- Zhou, L.; Akbar, S.; Wang, M.X.; Chen, H.P.; Liu, J.K. Tetra-, penta-, and hexa-nor-lanostane triterpenes from the medicinal fungus Ganoderma australe. Nat. Prod. Bioprospect. 2022, 12, 32. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; Daba, G.M.; Sheir, D.; El-Dein, A.N.; Fayad, W.; Elmahdy, E.M.; Shaheen, M.N.; Thomas, P.W.; Wen, T.C. GC-MS analysis and in-vitro hypocholesterolemic, anti-rotavirus, anti-human colon carcinoma activities of the crude extract of a Japanese Ganoderma spp. Egypt. Pharm. J. 2019, 18, 102–110. [Google Scholar] [CrossRef]
- Wu, L.; Liang, W.; Chen, W.; Li, S.; Cui, Y.; Qi, Q.; Zhang, L. Screening and analysis of the marker components in Ganoderma lucidum by HPLC and HPLC-MSn with the aid of chemometrics. Molecules 2017, 22, 584. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Yue, G.G.L.; Li, P.; Wong, E.C.W.; Lee, J.K.M.; Kennelly, E.J.; Bik-San Lau, C. Screening and analysis of potential anti-tumor components from the stipe of Ganoderma sinense using high-performance liquid chromatography/time-of-flight mass spectrometry with multivariate statistical tool. J. Chromatogr. A 2017, 1487, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Biswal, R.P.; Dandamudi, R.B.; Patnana, D.P.; Pandey, M.; Vutukuri, V.R.K. Metabolic fingerprinting of Ganoderma spp. using UHPLC-ESI-QTOF-MS and its chemometric analysis. Phytochemistry 2022, 199, 113169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, J.; Li, Q.; Gan, B.; Peng, W.; Zhang, X.; Tan, W.; Jiang, L.; Li, X. Manganese affects the growth and metabolism of Ganoderma lucidum based on LC-MS analysis. PeerJ 2019, 7, e6846. [Google Scholar] [CrossRef] [PubMed]
- Wubshet, S.G.; Johansen, K.T.; Nyberg, N.T.; Jaroszewski, J.W. Direct 13C NMR detection in HPLC hyphenation mode: Analysis of Ganoderma lucidum terpenoids. J. Nat. Prod. 2012, 75, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Kosa, G.; Shapaval, V.; Kohler, A.; Zimmermann, B. FTIR spectroscopy as a unified method for simultaneous analysis of intra-and extracellular metabolites in high-throughput screening of microbial bioprocesses. Microb. Cell Fact. 2017, 16, 195. [Google Scholar] [CrossRef] [PubMed]
- Rudakiya, D.M.; Gupte, A. Assessment of white rot fungus mediated hardwood degradation by FTIR spectroscopy and multivariate analysis. J. Microbial. Methods 2019, 157, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Baeva, E.; Bleha, R.; Sedliaková, M.; Sushytskyi, L.; Švec, I.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Evaluation of the cultivated mushroom Pleurotus ostreatus Basidiocarps using vibration spectroscopy and chemometrics. Appl. Sci. 2020, 10, 8156. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Qi, Z.; Liu, X.; Li, W.; Wang, S. A study of Ganoderma lucidum spores by FTIR microspectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 91, 285–289. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Sheng, D.; Huang, D.; Li, W.; Wang, X. Distinction of broken cellular wall Ganoderma lucidum spores and G. lucidum spores using FTIR microspectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 667–672. [Google Scholar] [CrossRef]
- Ma, J.; Fu, Z.; Ma, P.; Su, Y.; Zhang, Q. Breaking and characteristics of Ganoderma lucidum spores by high speed centrifugal shearing pulverizer. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2007, 22, 617–621. [Google Scholar] [CrossRef]
- Qiu, G.; Lan, J.; Zhang, W.; Wen, L.; Keong, C.Y.; Chen, X. Determination on tree species selection for Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes) cultivation by fourier transform infrared and two-dimensional infrared correlation spectroscopy. Int. J. Med. Mushrooms 2023, 25, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Choong, Y.K.; Sun, S.Q.; Zhou, Q.; Ismail, Z.; Rashid, B.A.A.; Tao, J.X. Determination of storage stability of the crude extracts of Ganoderma lucidum using FTIR and 2D-IR spectroscopy. Vibr. Spectrosc. 2011, 57, 87–96. [Google Scholar] [CrossRef]
- Choong, Y.K.; Chen, X.; Jamal, J.A.; Wang, Q.; Lan, J. Preliminary results of determination of chemical changes on Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Higher Basidiomycetes) carried by Shenzhou I spaceship with FTIR and 2D-IR correlation spectroscopy. Int. J. Med. Mushrooms 2012, 14, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Choong, Y.K.; Sun, S.Q.; Zhou, Q.; Lan, J.; Lee, H.L.; Chen, X.D. Verification of Ganoderma (lingzhi) commercial products by Fourier transform infrared spectroscopy and two-dimensional IR correlation spectroscopy. J. Mol. Struct. 2014, 1069, 60–72. [Google Scholar] [CrossRef]
- Li, X.P.; Li, J.; Liu, H.; Wang, Y.Z. A new analytical method for discrimination of species in Ganodermataceae mushrooms. Int. J. Food Prop. 2020, 23, 227–240. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, A.T.L. Discrimination of wild-grown and cultivated Ganoderma lucidum by Fourier transform infrared spectroscopy and chemometric methods. Am. J. Anal. Chem. 2015, 6, 480–491. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, A.T.L. Chemometric feature selection and classification of Ganoderma lucidum spores and fruiting body using ATR-FTIR spectroscopy. Am. J. Anal. Chem. 2015, 6, 830–840. [Google Scholar] [CrossRef]
- Baeva, E.; Bleha, R.; Lavrova, E.; Sushytskyi, L.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Polysaccharides from basidiocarps of cultivating mushroom Pleurotus ostreatus: Isolation and structural characterization. Molecules 2019, 24, 2740. [Google Scholar] [CrossRef]
- Margreth, M.; Schlink, R.; Steinbach, A. Water determination by Karl Fischer titration. In Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing; Gad, S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–34. [Google Scholar]
- McCleary, B.V.; Draga, A. Measurement of β-glucan in mushrooms and mycelia products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kvasnička, F.; Kouřimská, L.; Bleha, R.; Škvorová, P.; Kulma, M.; Rajchl, A. Electrophoretic determination of chitin in insects. J. Chromatogr. A 2023, 1695, 463952. [Google Scholar] [CrossRef] [PubMed]
- Tshinyangu, K.K.; Hennebert, G.L. Protein and chitin nitrogen contents and protein content in Pleurotus ostreatus var. columbinus. Food Chem. 1996, 57, 223–227. [Google Scholar] [CrossRef]
- Gomba, G.K.; Synytsya, A.; Švecová, P.; Coimbra, M.A.; Čopíková, J. Distinction of fungal polysaccharides by N/C ratio and mid infrared spectroscopy. Int. J. Biol. Macromol. 2015, 80, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kovářiková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Prieto, A.; Martínez, A.T. Structural characterization of extracellular polysaccharides produced by fungi from the genus Pleurotus. Carbohydr. Res. 1996, 281, 143–154. [Google Scholar] [CrossRef]
- Šandula, J.; Kogan, G.; Kacurakova, M.; Machova, E. Microbial (1→3)-β-D-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohydr. Res. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. 2021, 12, 100168. [Google Scholar] [CrossRef]
- Vasylieva, A.; Doroshenko, I.; Vaskivskyi, Y.; Chernolevska, Y.; Pogorelov, V. FTIR study of condensed water structure. J. Mol. Struct. 2012, 1167, 232–238. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. App. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Su, H.G.; Peng, X.R.; Shi, Q.Q.; Huang, Y.J.; Zhou, L.; Qiu, M.H. Lanostane triterpenoids with anti-inflammatory activities from Ganoderma lucidum. Phytochemistry 2020, 173, 112256. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Shi, L.S.; Kuo, S.C. Cytotoxicity of Ganoderma lucidum triterpenes. J. Nat. Prod. 2001, 64, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Boukaoud, A.; Chiba, Y.; Sebbar, D. A periodic DFT study of IR spectra of amino acids: An approach toward a better understanding of the NH and OH stretching regions. Vibr. Spectrosc. 2021, 116, 103280. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Forfang, K.; Zimmermann, B.; Kosa, G.; Kohler, A.; Shapaval, V. FTIR spectroscopy for evaluation and monitoring of lipid extraction efficiency for oleaginous fungi. PLoS ONE 2017, 12, e0170611. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Zhu, Q.; Tamrakar, S.; Amen, Y.; Mori, Y.; Suhara, H.; Kaneko, S.; Kawashima, H.; Okuzono, K.; Inoue, Y.; et al. Changes in content of triterpenoids and polysaccharides in Ganoderma lingzhi at different growth stages. J. Nat. Med. 2018, 72, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tang, Q.; Tang, C.; Liu, Y.; Ma, F.; Zhang, X.; Zhang, J. Triterpenes and soluble polysaccharide changes in lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes), during fruiting growth. Int. J. Med. Mushrooms 2018, 20, 859–871. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Chang, M.; Cheng, Y.; Geng, X.; Meng, J.; Zhu, M. Comparison of physicochemical and biochemical properties of natural and arginine-modified melanin from medicinal mushroom Ganoderma lucidum. J. Basic Microbiol. 2020, 60, 1014–1028. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, H.I.; Kim, J.H.; Kwon, O.C.; Son, E.S.; Lee, C.S.; Park, Y.J. Effects of ganodermanondiol, a new melanogenesis inhibitor from the medicinal mushroom Ganoderma lucidum. Int. J. Mol. Sci. 2016, 17, 1798. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Z.; Xu, P.; Wang, Y.; Gu, Z.; Qian, Z.; Shi, G.; Zhang, K. Methyl lucidenate F isolated from the ethanol-soluble-acidic components of Ganoderma lucidum is a novel tyrosinase inhibitor. Biotechnol. Bioproc. Eng. 2011, 16, 457–461. [Google Scholar] [CrossRef]
- He, H.; Yao, G.; Ma, Y.; Feng, N.; Zhou, S.; Huang, Q. Experimental and theoretical study of the Raman spectra of ganoderic acid T. J. Struct. Chem. 2019, 60, 1407–1415. [Google Scholar] [CrossRef]
- Yao, G.; Ma, Y.; Muhammad, M.; Huang, Q. Understanding the infrared and Raman spectra of ganoderic acid A: An experimental and DFT study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Sun, H.H. New ganoderic acids, bioactive triterpenoid metabolites from the mushroom Ganoderma lucidum. Nat. Prod. Res. 2006, 20, 985–991. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.; Lindequist, U.; Mentel, R.; Gördes, D.; Schmidt, E.; Thurow, K.; Lalk, M. Antiviral Terpenoid constituents of Ganoderma pfeifferi. J. Nat. Prod. 2005, 68, 1728–1731. [Google Scholar] [CrossRef]
- Ma, L.F.; Yan, J.J.; Lang, H.Y.; Jin, L.C.; Qiu, F.J.; Wang, Y.J.; Xi, Z.F.; Shan, W.G.; Zhan, Z.J.; Ying, Y.M. Bioassay-guided isolation of lanostane-type triterpenoids as α-glucosidase inhibitors from Ganoderma hainanense. Phytochem. Lett. 2019, 29, 154–159. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, X.M.; Qiu, M.H. Two novel lanostane triterpenoids from Ganoderma sinense. Molecules 2007, 12, 2038–2046. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, W.; Liu, H. Extraction, characterization and stability of a melanin-like pigment from Ganoderma lucidum fruit bodies. Acta Edulis Fungi 2017, 24, 93–97. [Google Scholar]
- Wang, T.; Deng, L.; Li, S.; Tan, T. Structural characterization of a water-insoluble α-(1 → 3)-D-glucan isolated from the Penicillium chrysogenum. Carbohydr. Polym. 2007, 67, 133–137. [Google Scholar] [CrossRef]
- Unursaikhan, S.; Xu, X.; Zeng, F.; Zhang, L. Antitumor activities of O-sulphonated derivatives of (1 → 3)-glucans from different Lentinus edodes. Biosci. Biotechnol. Biochem. 2006, 70, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Mi, Z.Y.; Tang, Y.; Wang, G.; Li, D.S.; Tang, Y.J. Lanostanoids isolated from Ganoderma lucidum mycelium cultured by submerged fermentation. Helv. Chim. Acta 2009, 92, 1586–1593. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Ren, W.; Zhou, Y.; Ma, J.; Ruan, Y.; Wen, C.N. Triterpenoids from the spores of Ganoderma lucidum. N. Am. J. Med. Sci. 2011, 3, 495. [Google Scholar] [CrossRef]
- Hsu, C.L.; Yen, G.C. Ganoderic acid and lucidenic acid (triterpenoid). Enzymes 2014, 36, 33–56. [Google Scholar] [CrossRef]
- Peng, X.; Liu, J.; Xia, J.; Wang, C.; Li, X.; Deng, Y.; Bao, N.M.; Zhang, Z.R.; Qiu, M.H. Lanostane triterpenoids from Ganoderma hainanense JD Zhao. Phytochemistry 2015, 114, 137–145. [Google Scholar] [CrossRef]
- Gu, L.; Zheng, Y.; Lian, D.; Zhong, X.; Liu, X. Production of triterpenoids from Ganoderma lucidum: Elicitation strategy and signal transduction. Process. Biochem. 2018, 69, 22–32. [Google Scholar] [CrossRef]
- Cîntă-Pînzaru, S.; Dehelean, C.A.; Soica, C.; Culea, M.; Borcan, F. Evaluation and differentiation of the Betulaceae birch bark species and their bioactive triterpene content using analytical FT-vibrational spectroscopy and GC-MS. Chem. Central J. 2012, 6, 67. [Google Scholar] [CrossRef]
- Dehelean, C.; Pînzaru, S.C.; Peev, C.; Soica, C.; Antal, D.S. Characterization of birch tree leaves, buds and bark dry extracts with antitumor activity. J. Optoelectron. Adv. Mater. 2007, 9, 783. [Google Scholar]
- Melo, I.R.S.; Teixeira, A.M.R.; Junior, D.S.; Santos, H.S.; Albuquerque, M.R.J.R.; Bandeira, P.N.; Rodrigues, A.S.; Braz-Filho, R.; Gusmao, G.O.M.; Silva, J.H.; et al. FT-Raman and FTIR-ATR spectroscopies and DFT calculations of triterpene acetyl aleuritolic acid. J. Mol. Struct. 2014, 1058, 221–227. [Google Scholar] [CrossRef]
- Max, J.J.; Chapados, C. Infrared spectroscopy of aqueous carboxylic acids: Comparison between different acids and their salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- Chen, D.H.; Shiou, W.Y.; Wang, K.C.; Huang, S.Y.; Shie, Y.T.; Tsai, C.M.; Shie, J.F.; Chen, K.D. Chemotaxonomy of triterpenoid pattern of HPLC of Ganoderma lucidum and Ganoderma tsugae. J. Chin. Chem. Soc. 1999, 46, 47–51. [Google Scholar] [CrossRef]
- Kubota, T.; Asaka, Y.; Miura, I.; Mori, H. Structures of ganoderic acid A and B, two new lanostane type bitter triterpenes from Ganoderma lucidum (FR.) KARST. Helv. Chim. Acta 1982, 65, 611–619. [Google Scholar] [CrossRef]
- Fǎlǎmaş, A.; Pînzaru, S.C.; Dehelean, C.A.; Peev, C.I.; Soica, C. Betulin and its natural resource as potential anticancer drug candidate seen by FT-Raman and FT-IR spectroscopy. J. Raman Spectrosc. 2011, 42, 97–107. [Google Scholar] [CrossRef]
- Agarwal, Y.K.; Verma, P.K.; Khan, E.H.; Shafi, S. Infrared spectra of C6-spiro steroidal tetrazines. Indian J. Phys. 2003, 77, 215–221. Available online: http://hdl.handle.net/10821/6697 (accessed on 25 December 2023).
- Castaneda, S.; Alvarenga, E.S.; Demuner, A.J.; Guimaraes, L.M. Vibrational spectra and theoretical calculations of a natural pentacyclic triterpene alcool isolated from Mucuna pruriens. Struct. Chem. 2020, 3, 599–607. [Google Scholar] [CrossRef]
- Jamróz, M.K.; Jamróz, M.H.; Dobrowolski, J.C.; Gliński, J.A.; Davey, M.H.; Wawer, I. Novel and unusual triterpene from Black Cohosh. Determination of structure of 9, 10-seco-9, 19-cyclolanostane xyloside (cimipodocarpaside) by NMR, IR and Raman spectroscopy and DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 107–112. [Google Scholar] [CrossRef]
- Chaman, M.; Verma, P.K. Vibrational spectra of cholesterol derivative and its assignments. Indian J. Phys. 2004, 78, 1129–1135. Available online: http://hdl.handle.net/10821/7110 (accessed on 25 December 2023).
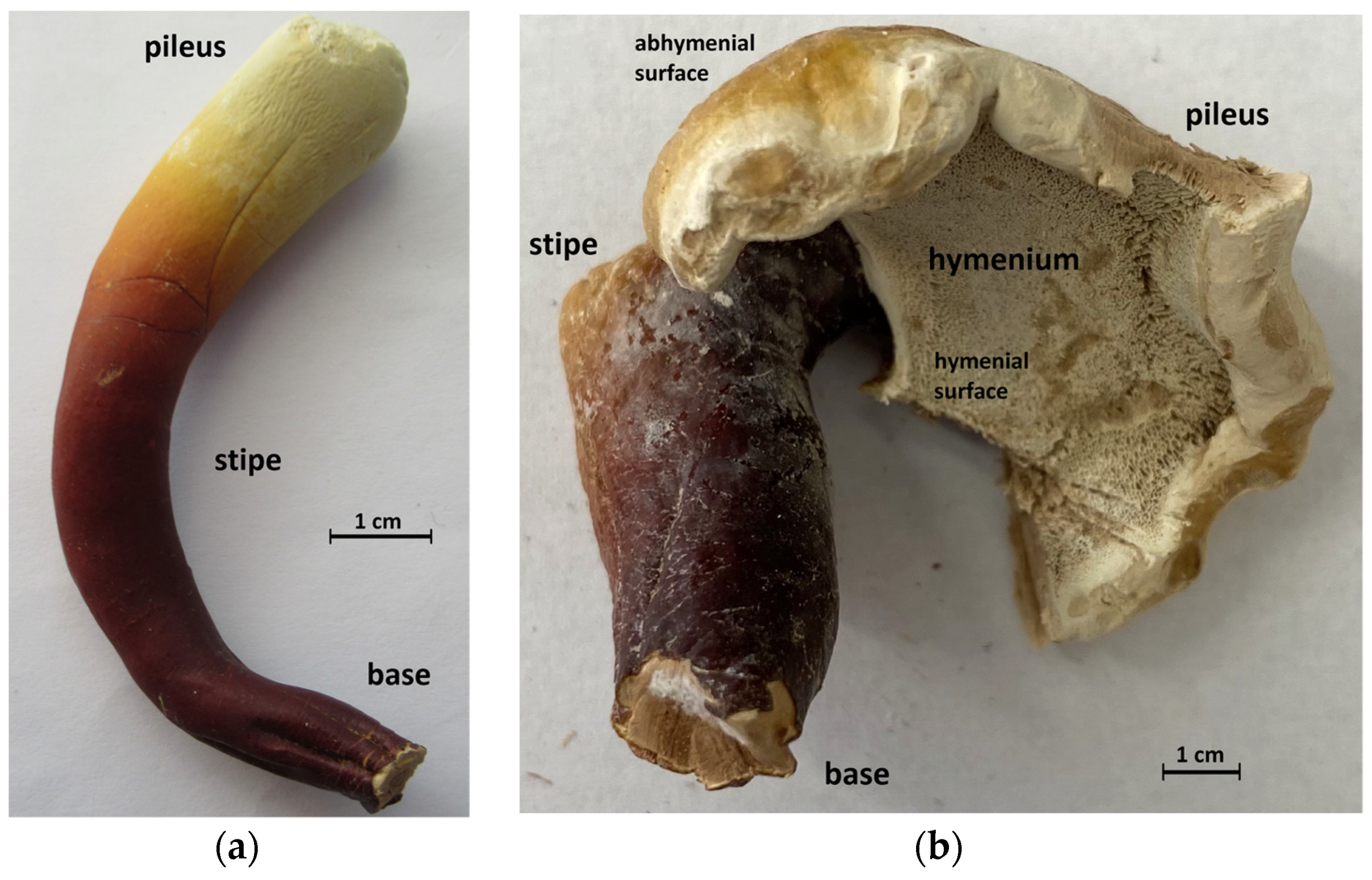
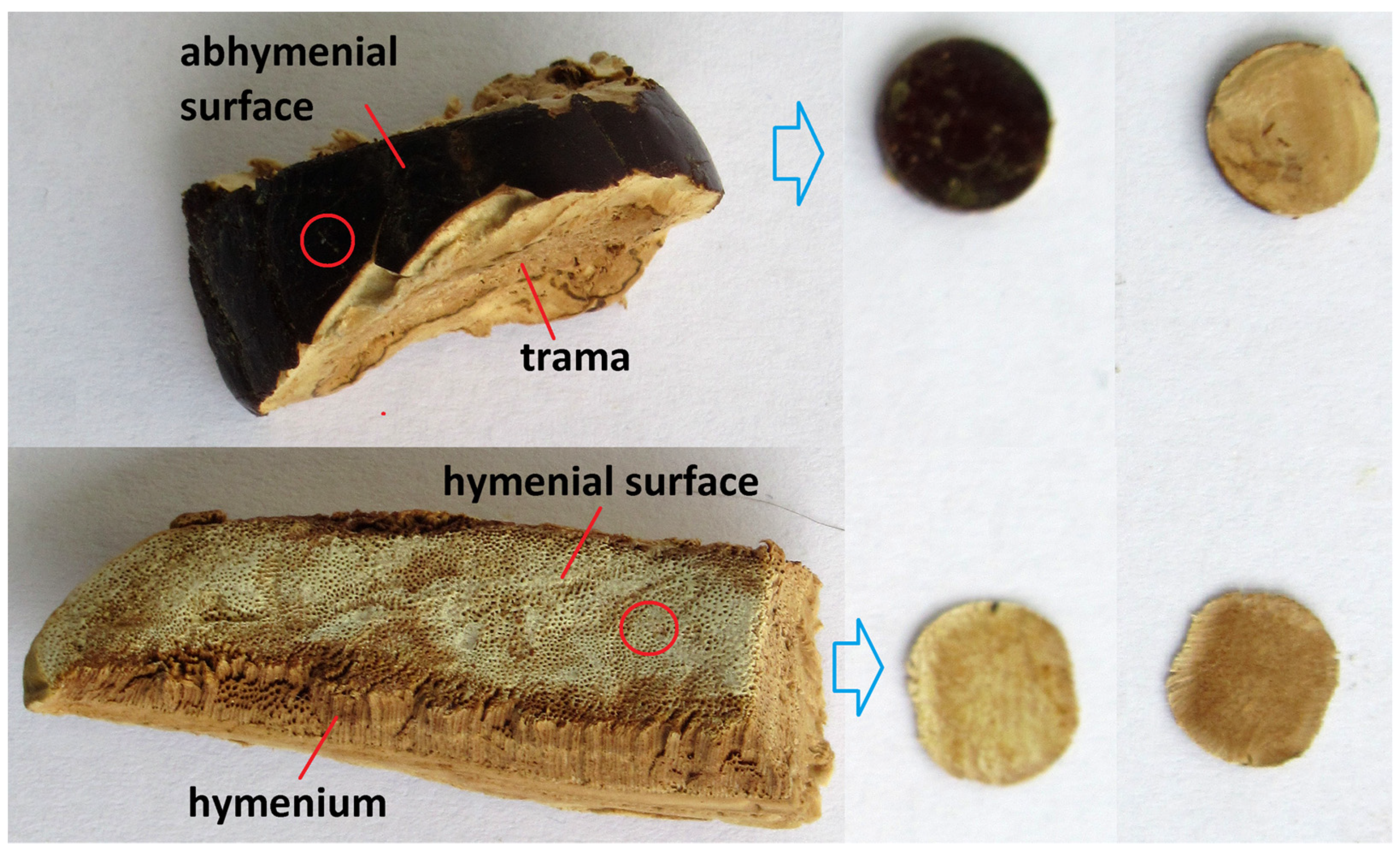

| Sample | Basidiocarps—Slices | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Species | Strains | Young | Mature | Base | Stipes | Pilei | ||||||
| Surface | |||||||||||||
| Surface | Trama | Surface | Trama | Non-differentiated | Abhymenial | Hymenial | Trama | Hymenium | |||||
| 1 | G. applanatum | 2023 | + | + | + | + | + | + | |||||
| 2 | G. applanatum | + | + | + | + | + | + | ||||||
| 3 | G. lucidum | KZ 76 | + | + | + | + | + | ||||||
| 4 | G. lingzhi | + | + | + | + | + | |||||||
| 5 | G. oregonense | + | + | + | + | + | + | ||||||
| 6 | G. pffeiferi | + | + | + | + | + | |||||||
| 7 | G. resinaceum | + | + | + | + | + | + | + | + | ||||
| 8 | G. tsugae | + | + | + | + | + | |||||||
| 9 | G. sp. | 2a | + | + | + | + | + | + | + | ||||
| 10 | G. sp. | 2b | + | + | + | ||||||||
| 11 | G. sp. | 338 | + | + | + | ||||||||
| 12 | G. sp. | 338a | + | + | + | ||||||||
| 13 | G. sp. | 338b | + | + | + | + | + | ||||||
| 14 | G. sp. | 780 | + | + | + | + | + | + | + | ||||
| 15 | G. sp. | KZ 74 | + | + | + | + | + | ||||||
| Analytical Method | Contents (% w/w) | Parts of Basidiocarps | ||
|---|---|---|---|---|
| Bases | Stipes | Pilei | ||
| Organic elemental analysis (OEA) | N | 2.26 | 1.81 | 6.37 |
| C | 47.46 | 51.66 | 46.78 | |
| H | 6.74 | 7.19 | 6.99 | |
| S | 0.17 | 0.15 | 0.37 | |
| Karl Fischer titration | Moisture | 11.83 ± 0.27 | 11.09 ± 0.96 | 10.42 ± 0.27 |
| Photometry (Megazyme set) | Total d-glucans * | 54.78 ± 1.94 | 35.84 ± 0.83 | 30.07 ± 0.94 |
| α-d-Glucans * | 9.99 ± 1.99 | 5.59 ± 0.63 | 5.72 ± 0.19 | |
| β-d-Glucans * | 44.79 ± 1.01 | 30.26 ± 0.44 | 24.35 ± 1.04 | |
| CITC-CZE | Chitin * | 15.20 ± 2.27 | 2.92 ± 0.56 | 6.93 ± 0.56 |
| N (chitin) | 0.93 | 0.18 | 0.43 | |
| OEA + CITC-CZE | N (proteins) | 1.33 | 1.63 | 5.94 |
| Proteins * | 9.94 | 12.06 | 43.63 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Synytsya, A.; Bleha, R.; Skrynnikova, A.; Babayeva, T.; Čopíková, J.; Kvasnička, F.; Jablonsky, I.; Klouček, P. Mid-Infrared Spectroscopic Study of Cultivating Medicinal Fungi Ganoderma: Composition, Development, and Strain Variability of Basidiocarps. J. Fungi 2024, 10, 23. https://doi.org/10.3390/jof10010023
Synytsya A, Bleha R, Skrynnikova A, Babayeva T, Čopíková J, Kvasnička F, Jablonsky I, Klouček P. Mid-Infrared Spectroscopic Study of Cultivating Medicinal Fungi Ganoderma: Composition, Development, and Strain Variability of Basidiocarps. Journal of Fungi. 2024; 10(1):23. https://doi.org/10.3390/jof10010023
Chicago/Turabian StyleSynytsya, Andriy, Roman Bleha, Anastasia Skrynnikova, Tamilla Babayeva, Jana Čopíková, František Kvasnička, Ivan Jablonsky, and Pavel Klouček. 2024. "Mid-Infrared Spectroscopic Study of Cultivating Medicinal Fungi Ganoderma: Composition, Development, and Strain Variability of Basidiocarps" Journal of Fungi 10, no. 1: 23. https://doi.org/10.3390/jof10010023
APA StyleSynytsya, A., Bleha, R., Skrynnikova, A., Babayeva, T., Čopíková, J., Kvasnička, F., Jablonsky, I., & Klouček, P. (2024). Mid-Infrared Spectroscopic Study of Cultivating Medicinal Fungi Ganoderma: Composition, Development, and Strain Variability of Basidiocarps. Journal of Fungi, 10(1), 23. https://doi.org/10.3390/jof10010023









