Foliar Lesions Induced by Pestalotiopsis arengae in Oil Palm (O × G) in the Colombian Southwest Palm Zone
Abstract
:1. Introduction
2. Materials and Methods
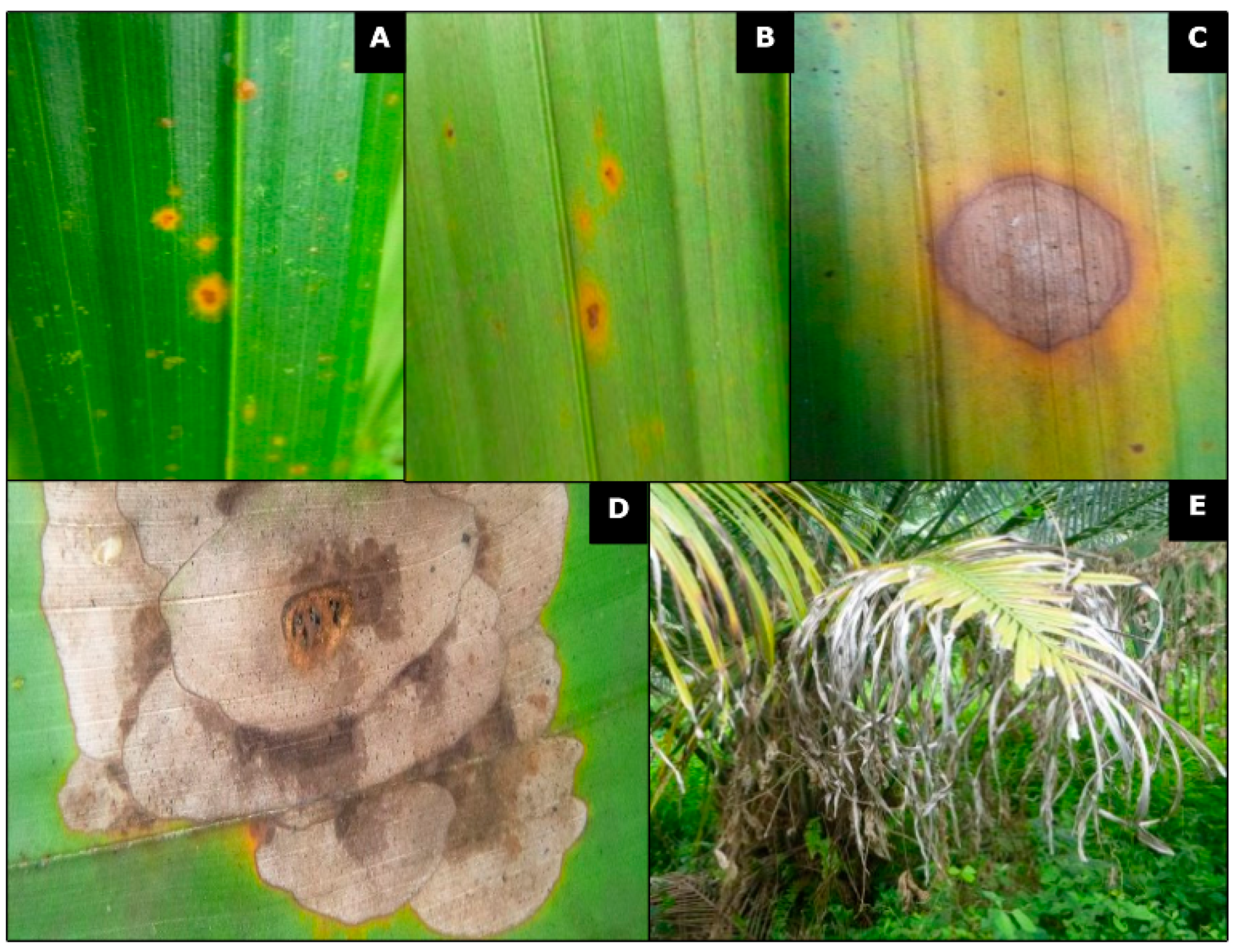
2.1. Description of Symptoms and Foliar Tissue Sampling
2.2. Isolation and Purification of Microorganisms
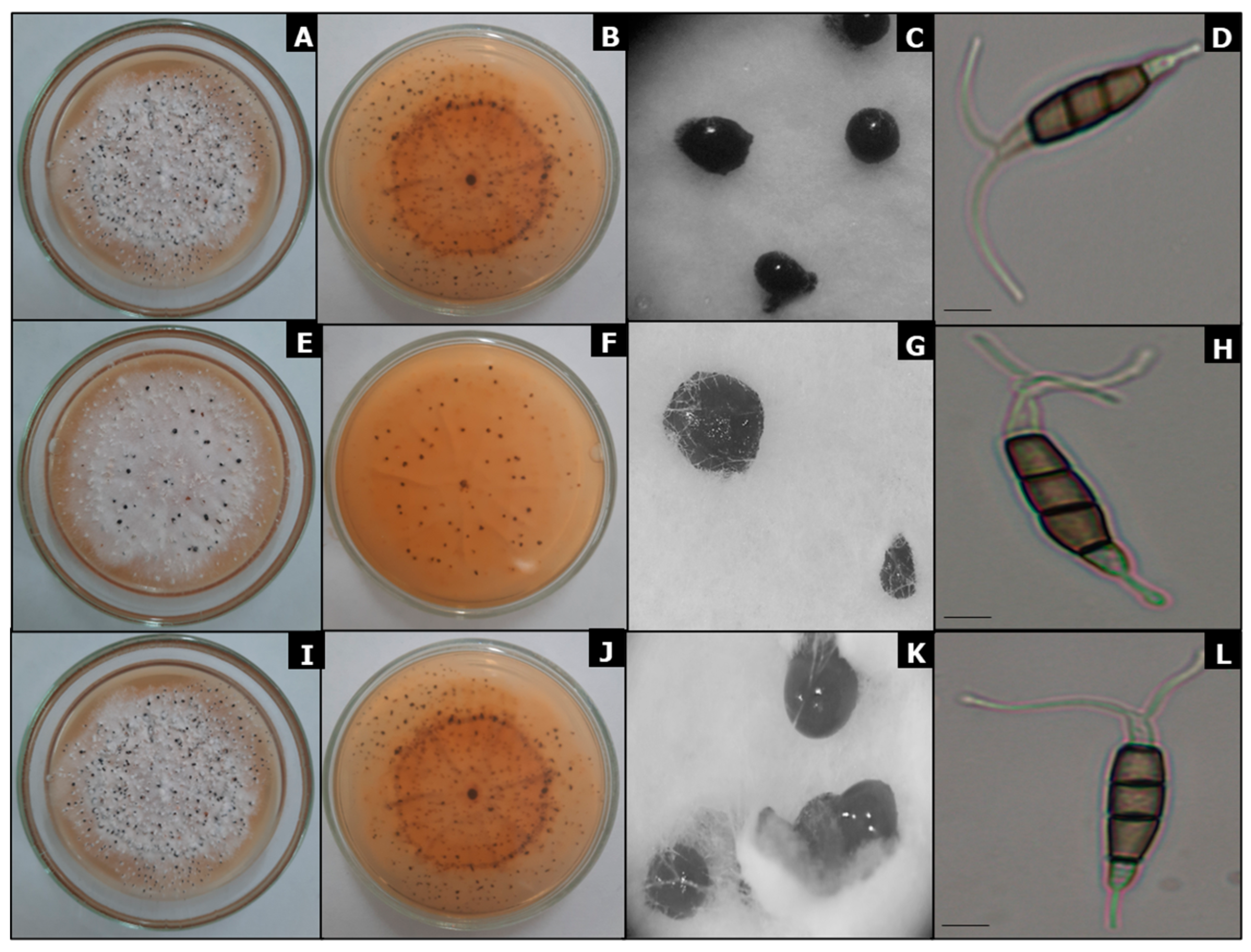
2.3. Culture and Morphological Characterization
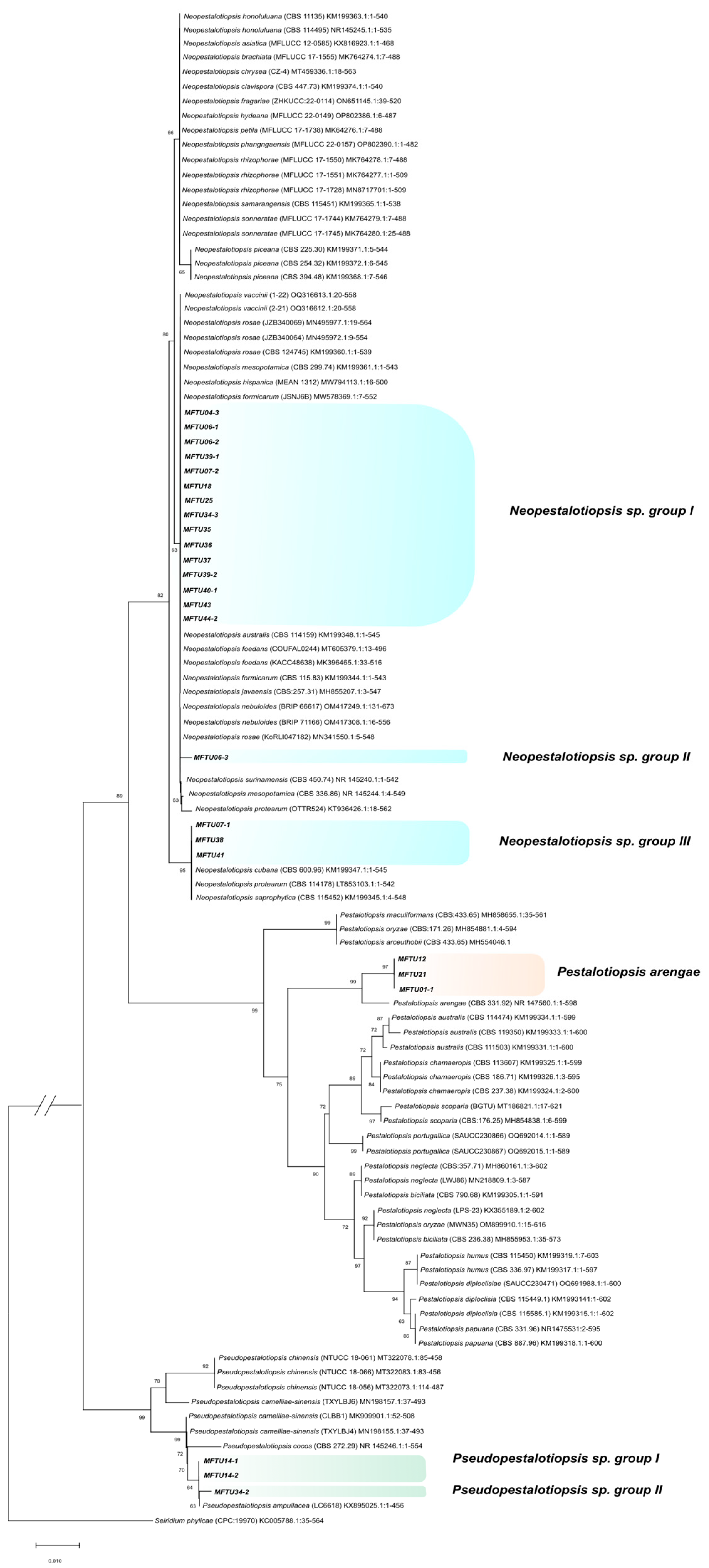
2.4. Molecular Identification and Phylogenetic Analysis
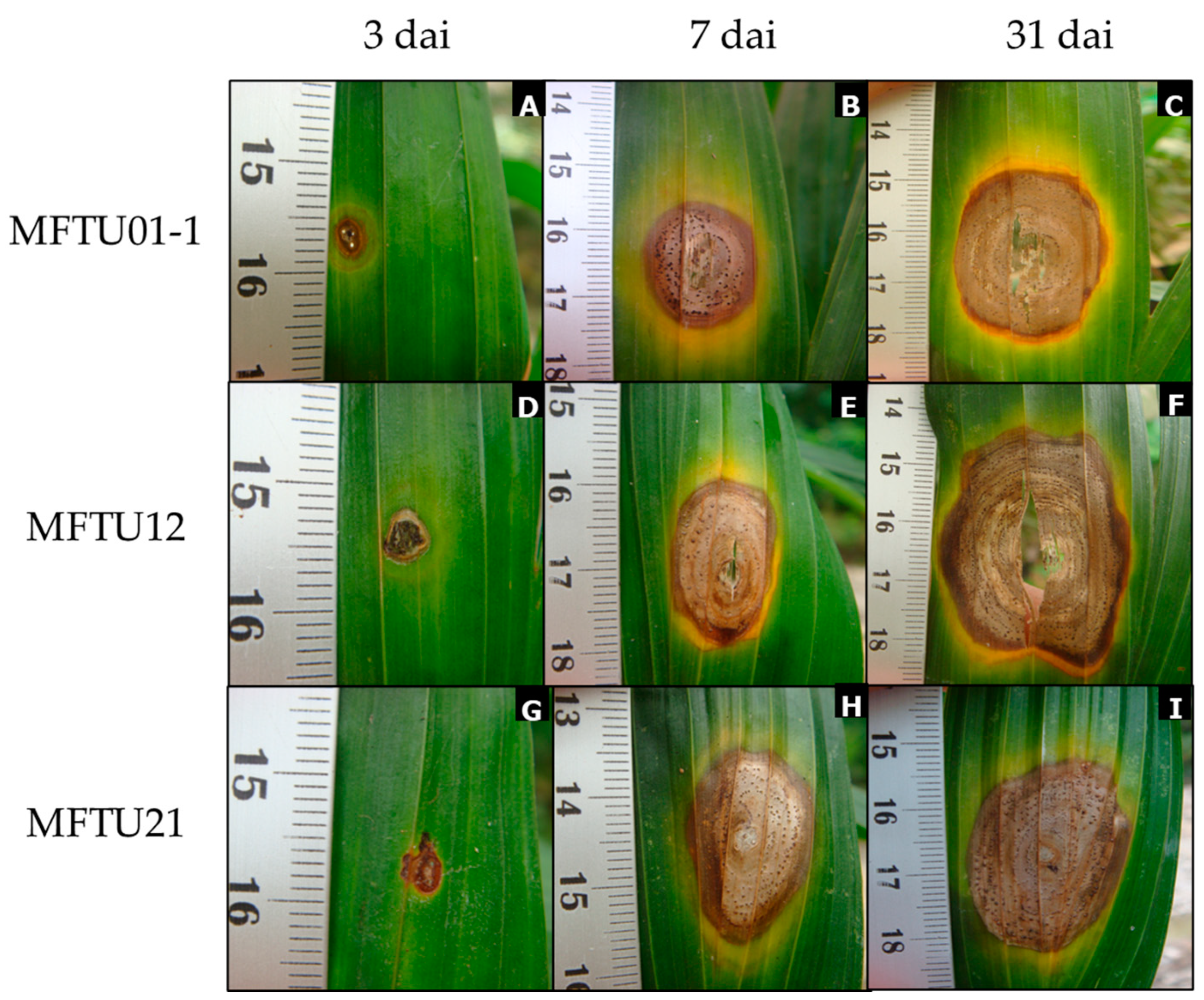
2.5. Pathogenicity Tests
2.6. Primer Design and Molecular Detection of Pestalotiopsis Arengae in Foliar Tissues
3. Results
3.1. Description of Symptoms in Naturally Infected Farms
3.2. Isolation of Microorganisms
3.3. Cultural Characterization
3.4. Morphological Characterization
3.5. Molecular Characterization
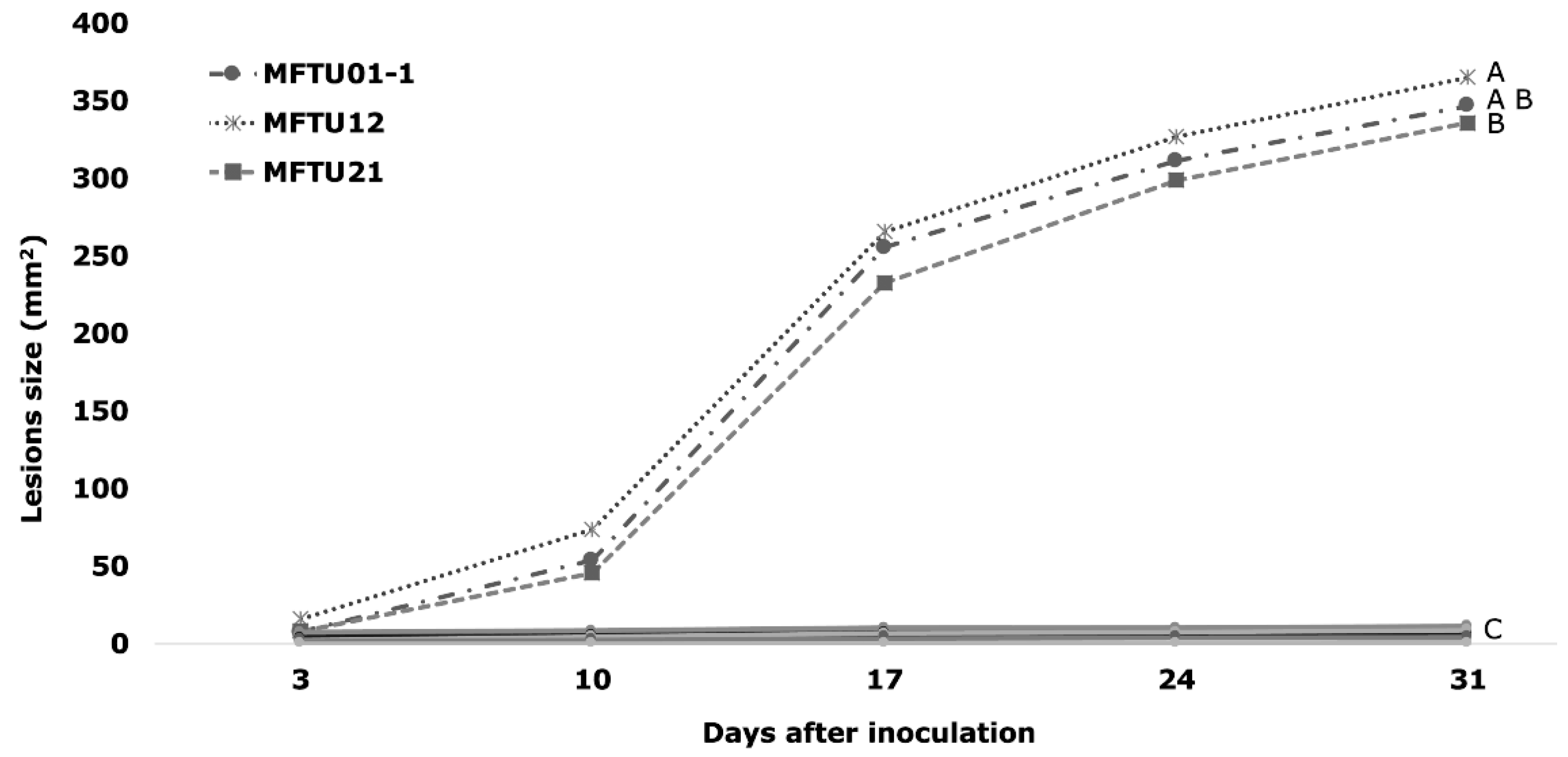
3.6. Pathogenicity Tests
3.7. Molecular Detection of Pestalotiopsis arengae in Foliar Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corley, R.; Tinker, B. Origin and development of oil palm. In The Oil Palm, 4th ed.; Bath Press: Bath, UK; Blackwell Science Ltd.: Chichester, UK, 2003; p. 604. [Google Scholar]
- Potter, L. Colombia’s oil palm development in times of war and ‘peace’: Myths, enablers and the disparate realities of land control. J. Rural. Stud. 2020, 78, 491–502. [Google Scholar] [CrossRef]
- Federación Nacional de Cultivadores de Palma de Aceite, F Minianuario estadístico 2022: Principales Cifras de la Agroindustria de Palma de Aceite en Colombia. Colombia. 19 May 2022. pp. 2344–8482. Available online: https://repositorio.fedepalma.org/handle/123456789/141446 (accessed on 10 March 2023).
- Sarria, G.; Torres, G.; Velez, D.; Rodríguez, J.; Noreña, C.; Varón, F.; Coffey, M.D.; Elliott, M.L.; Martínez, G.; Castro, B.L. Caracterización morfológica y molecular de Phytophthora palmivora agente causal de las lesiones iniciales de la pudrición del cogollo (PC) de la palma de aceite en Colombia. Fitopatol Colomb. 2008, 32, 39–44. [Google Scholar]
- Martínez, G.; Arango, M.; Torres, G.; Sarria, G.; Vélez, D.; Rodríguez, J.; Mestizo, Y.; Aya, H.; Noreña, C.; Varón, F.; et al. Advances in the Research of the Two Most Important Diseases on Oil Palm in Colombia: Bud Rot and Lethal Wilt. Palmas 2013, 34, 39–47. [Google Scholar]
- Meunier, J. Una posible solucion genetica para el control de la pudricion de cogollo en la palma aceitera. Hibrido interespecífico Elaeis oleifera × Elaeis guineensis. Palmas 1991, 12, 39–42. [Google Scholar]
- Bastidas, S.; Peña, E.; Reyes, R. Avances sobre el comportamiento de los híbridos de primera generación de retrocruzamiento entre palma Nolí (Elaeis oleifera) y palma africana (Elaeis guineensis). Corporación Colomb. Investig. Agropecu. CORPOICA Rev. Reg. Noved. Técnicas 2003, 3, 32–36. [Google Scholar]
- Torres, V.; Rey, B.; Gelves, R.; Santacruz, A.L. Evaluación del comportamiento de los híbridos interespecíficos elaeis oleífera × Elaeis guineensis, en la plantación de Guaicaramo S. A. Rev. Palmas 2004, 25, 350–357. [Google Scholar]
- Zambrano, J.E. Los híbridos interespecíficos elaeis oleífera HBK. x Elaeis guineensis Jacq.: Una alternativa de renovación para la Zona Oriental de Colombia. Rev. Palmas 2004, 25, 339–349. [Google Scholar]
- Chinchilla, C.; Alvarado, A.; Albertazzi, H.; Torres, R. Tolerancia y resistencia a las pudriciones del cogollo en fuentes de diferente origen de Elaeis guineensis. Rev. Palmas 2007, 28, 273–284. [Google Scholar]
- Sánchez, A. Estado fitosanitario de los cultivos de coco y palma africana en los litorales del Atlántica y Pacifico (Colombia). Inst. Fom. Algodón. Programa Fitopatol. 1962, 55. [Google Scholar]
- Jiménez, O.D.; Reyes, A. Estudio de una necrosis foliar que afecta varias plantaciones de palma de aceite (Elaeis guineensis Jacq.) en Colombia. Rev. Fitopatol. Colomb. 1977, 6, 15–32. [Google Scholar]
- Genty, P.; Garzon, A.; Garcia, R. Daños y control del complejo Leptopharsa-Pestalotiopsis en la palma africana. Rev. Palmas 1984, 5, 9–15. [Google Scholar]
- Reyes, A. Añublo foliar de la palma africana (Elaeis guineensis Jacq) en Colombia: Importancia económica, etiología y control. Rev. Palmas 1988, 9, 33–39. [Google Scholar]
- Guzmán, L.; Calvache, H.; Aldana, J.; Méndez, A. Manejo de Leptopharsa gibbicarina Froeschner (Hemíptera: Tingidae) con la hormiga Crematogaster sp. en una plantación de palma de aceite. Rev Palmas 1997, 18, 19–26. [Google Scholar]
- Méndez, A. Manejo integrado de la Pestalotiopsis en una plantación comercial de palma de aceite. Rev. Palmas 2000, 21, 1–5. [Google Scholar]
- Aldana de La Torre, R.C.; Aldana de La Torre, J.A. Reconocimiento y manejo de insectos defoliadores y asociados a la pestalotiopsis. Tecnologías para la agroindustria de la palma de aceite: Guía para facilitadores. Palmas 2011, 180, 71–111. [Google Scholar]
- Barrios, C.; Bustillo-Pardey, A.E.; Ocampo, K.; Reina, M.A.; Alvarado, H.L. Eficacia de hongos entomopatógenos en el control de L. gibbicarina (Hemipetra: Tingidae) en palma de aceite. Rev. Colomb. Entomol. 2016, 42, 22–27. [Google Scholar] [CrossRef]
- Jeewon, R.; Liew, E.C.Y.; Simpson, J.A.; Hodgkiss, J.H.K. Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Mol. Phylogenet. Evol. 2003, 27, 372–383. [Google Scholar] [CrossRef]
- Jeewon, R.; Liew, E.C.Y.; Hyde, K.D. Phylogenetic evaluation of species nomenclature of Pestalotiopsis in relation to host association. Fungal. Divers. 2004, 17, 39–55. [Google Scholar]
- Maharachchikumbura, S.S.N.; Guo, L.D.; Cai, L.; Chukeatirote, E.; Wu, W.P.; Sun, X.; Crous, P.W.; Bhat, D.J.; McKenzie, E.H.C.; Bahkali, A.H.; et al. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers. 2012, 56, 95–129. [Google Scholar] [CrossRef]
- Ayoubi, N.; Soleimani, M.J. Strawberry Fruit Rot Caused by Neopestalotiopsis iranensis sp. nov., and N. mesopotamica. Curr. Microbiol. 2015, 72, 329–336. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Bate-Smith, E. Leuco-anthocyanins. 3. The nature and systematic distribution of tannin in dicotyledonous plants. Bot. J. 1957, 55, 669–705. [Google Scholar] [CrossRef]
- Das, R.; Chutia, M.; Das, K.; Jha, D.K. Factors affecting sporulation of Pestalotiopsis disseminata causing grey blight disease of Persea bombycina Kost., the primary food plant of muga silkworm. Crop. Prot. 2010, 29, 963–968. [Google Scholar] [CrossRef]
- Solarte, F.; Muñoz, C.G.; Maharachchikumbura, S.S.N.; Álvarez, E. Diversity of neopestalotiopsis and pestalotiopsis spp., causal agents of guava scab in Colombia. Plant Dis. 2018, 102, 49–59. [Google Scholar] [CrossRef]
- Ismail, A.M.; Cirvilleri, G.; Polizzi, G. Characterisation and pathogenicity of Pestalotiopsis uvicola and Pestalotiopsis clavispora causing grey leaf spot of mango (Mangifera indica L.) in Italy. Eur. J. Plant. Pathol. 2013, 135, 619–625. [Google Scholar] [CrossRef]
- Keith, L.M.; Velasquez, M.E.; Zee, F.T.; Plant, T.; Resource, G.; Unit, M. Identification and Characterization of Pestalotiopsis spp. Causing Scab Disease of Guava, Psidium guajava, in Hawaii. Plant Dis. 2006, 90, 16–23. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Guo, L.; Chukeatirote, E.; Mckenzie, E.H.C.; Hyde, K.D. A destructive new disease of Syzygium samarangense in Thailand caused by the new species Pestalotiopsis samarangensis. Trop. Plant. Pathol. 2013, 38, 227–235. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Larignon, P.; Hyde, K.D.; Al-sadi, A.M.; Liu, Z.Y. Characterization of Neopestalotiopsis, Pestalotiopsis and Truncatella species associated with grapevine trunk diseases in France. Phytopathol. Mediterr. 2016, 55, 380–390. [Google Scholar]
- Maharachchikumbura, S.; Guo, L.; Liu, Z.; Hyde, K. Pseudopestalotiopsis ignota and Ps. camelliae spp. nov. associated with grey blight disease of tea in China. Mycol. Prog. 2016, 15, 1–7. [Google Scholar] [CrossRef]
- Jimenéz, O. El Añublo Foliar de la Palma Africana en Colombia. Palmas 1984, 5, 89–92. [Google Scholar]
- Shen, H.; Zhang, J.; Lin, B.; Pu, X. First Report of Pestalotiopsis microspore Causing Leaf Spot of Oil Palm (Elaeis guineensis) in China. Am. Phytopathol. Soc. 2014, 98, 1429. [Google Scholar]
- Suwannarach, N.; Sujarit, K.; Kumla, J.; Bussaban, B.; Lumyong, S. First report of leaf spot disease on oil palm caused by Pestalotiopsis theae in Thailand. J. Gen. Plant. Pathol. 2013, 79, 277–279. [Google Scholar] [CrossRef]
- Fröhlich, J.; Hyde, D.K.; Guest, D.I. Fungi associated with leaf spots of palms in north Queensland, Australia. Mycol. Res. 1997, 101, 721–732. [Google Scholar] [CrossRef]
- Loredo, J.; Mena, J.; Ríos, J. Manual De Prácticas Del Laboratorio De Fitopatología; Universidad Autónoma De Sinaloa Unidad Regional Norte: Los Mochis, Mexico, 2009; p. 81. [Google Scholar]
- Khadidja, K. Isolation, Characterization of Phytopathogenic Fungi Alternaria sp., and Physico-Chemical Study; Faculty of Natural and Life Science Departement of Biologie: Mostaghanem, Algeria, 2020. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera de Imperfect Fungi, 4th ed.; The American Phytopathological Society: St. Paul. MN, USA, 1988; 218p. [Google Scholar]
- Agrios, G.N. Plant Pathology; Elsevier Academia Press: Cambridge, MA, USA, 2005; p. 922. [Google Scholar]
- Steyaert, R. Contribution a l’etude monographique de Pestalotia de Not. et Monochaetia Sacc. (Truncatella gen. nov. et Pestalotiopsis gen. nov.). Plant Dis. 1949, 19, 285–354. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user friendly biological seque. In Nucleid Acids Symposium Series; Oxford University Press: Oxford, UK, 1999; pp. 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Sosa, N.T.; Alvarez, R.; Cabrera, M. Ocurrencia de Pestalotia sp. causando lesiones necróticas en plantas de Jazmín del Cabo (Gardenia Augusta); Universidad Nacional del Nordeste Comunicación es Científicas y Tecnológicas: Corrientes, Argentina, 2003; pp. 1–3. Available online: https://www.unne.edu.ar/unnevieja/Web/cyt/cyt/2003/comunicaciones/05-Agrarias/A-019.pdf (accessed on 22 February 2018).
- Jayawardena, R.S.; Zhang, W.; Liu, M.; Maharachchikumbura, S.S.N.; Zhou, Y.; Huang, J.; Nilthong, S.; Wang, Z.; Li, X.; Yan, J.; et al. Identification and characterization of Pestalotiopsis-like fungi related to grapevine diseases in China. Fungal. Biol. 2015, 119, 348–361. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Groenewald, J.; Xu, Z.; Crous, P.W. Pestalotiopsis revisited. Stud Mycol. 2014, 79, 121–186. [Google Scholar] [CrossRef]
- Liu, F.; Hou, L.; Raza, M.; Cai, L. Pestalotiopsis and allied genera from Camellia, with description of 11 new species from China. Sci. Rep. 2017, 7, 866. [Google Scholar] [CrossRef]
- Monclova-Santana, C. Pathogenic Threats of ten Endangered Plant Species of the Karst Region of Puerto Rico; University of Puerto Rico, Mayagüez Campus: Mayagüez, Puerto Rico, 2015. [Google Scholar]
- Pineda, B.; Martínez, G. Reconocimiento de enfermedades en la palma de aceite. Tecnologías para la agroindustria de la palma de aceite: Guía para facilitadores. Rev. Cenipalma. 2013, 138. [Google Scholar]
- Martínez, L.; Plata, A. Lepidoptera vectors of Pestalotiopsis fungal disease: First record in oil palm plantations from Colombia [Internet]. Trop. Insect Sci. 2013, 33, 239–246. Available online: https://aplicaciones.msp.gob.ec/upload/upload/00000403_2011_00000403.PDF (accessed on 14 November 2022). [CrossRef]
- Turner, P. Oil Palm Disease and Disorders; Oxford University Press: Kuala Lumpur, Malaysia, 1981; p. 280. [Google Scholar]
- Sánchez, A. Enfermedades de la palma de aceite en América Latina. Palmas 1990, 1, 5–38. [Google Scholar]
- Elliott, M.L. Pestalotiopsis (Pestalotia) Diseases of Palm; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2019; Volume 2006, pp. 2–4. [Google Scholar]
- Maharachchikumbura, S.S.N.; Guo, L.-D.; Chukeatirote, E.; Bahkali, A.H.; Hyde, K.D. Pestalotiopsis—Morphology, phylogeny, biochemistry and diversity. Fungal. Divers. 2011, 50, 173–175. [Google Scholar] [CrossRef]
- Jeewon, R.; Hyde, K.D. Establishing species boundaries and new taxa among fungi: Recommendations to resolve taxonomic ambiguities. Mycosphere 2016, 7, 1669–1677. [Google Scholar] [CrossRef]
- Gerardo-Lugo, S.S.; Tovar-Pedraza, J.M.; Maharachchikumbura, S.S.N.; Apodaca-Sánchez, M.A.; Correia, K.C.; Sauceda-Acosta, C.P.; Camacho-Tapia, M.; Hyde, K.D.; Marraiki, N.; Elgorban, A.M.; et al. Characterization of neopestalotiopsis species associated with mango grey leaf spot disease in Sinaloa, Mexico. Pathogens 2020, 9, 788. [Google Scholar] [CrossRef]


| Isolate Code | Genus | Host A | Source | Geographic Origin |
|---|---|---|---|---|
| MFTU01-1 | Pestalotiopsis sp. | Sinú (cerete) × Deli Yangambí | Leaf | Tumaco, Nariño, Colombia |
| MFTU04-3 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Leaf | Tumaco, Nariño, Colombia |
| MFTU06-1 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Leaf | Tumaco, Nariño, Colombia |
| MFTU06-2 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Leaf | Tumaco, Nariño, Colombia |
| MFTU06-3 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Leaf | Tumaco, Nariño, Colombia |
| MFTU07-1 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Leaf | Tumaco, Nariño, Colombia |
| MFTU07-2 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Leaf | Tumaco, Nariño, Colombia |
| MFTU12 | Pestalotiopsis sp. | Sinú (cerete) × Deli Yangambí | Leaf | Tumaco, Nariño, Colombia |
| MFTU14-1 | Pseudopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU14-2 | Pseudopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU18 | Neopestalotiopsis sp. | Manaos × Compacta | Leaf | Tumaco, Nariño, Colombia |
| MFTU21 | Pestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU25 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU34-2 | Pseudopestalotiopsis sp. | Brazil × 7 polen africans | Leaf | Tumaco, Nariño, Colombia |
| MFTU34-3 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Leaf | Tumaco, Nariño, Colombia |
| MFTU35 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU36 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU37 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU38 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU39-1 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU39-2 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU40-1 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU41 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU43 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| MFTU44-2 | Neopestalotiopsis sp. | Coarí × La Mé | Leaf | Tumaco, Nariño, Colombia |
| Isolate Code | Genus/Species | Colony Type C | Conidia Length (µm) | Conidia Width (µm) | Length of Basal Appendage (µm) | Median Cell Length (μm) | No. of Apical Appendages (Range) | Length of Apical Appendages (µm) | Color of Middle Cells | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFTU01-1 | Pestalotiopsis sp. | 3 | 28.1 ± 1.6 B | a D | 6.6 ± 0.5 | a | 5.6 ± 1.3 | b | 18.2 ± 1.2 | b | 3–2 | 15.1 ± 2.6 | c | Concolorous |
| MFTU04-3 | Neopestalotiopsis sp. | 5 | 24.4 ± 1.9 | b | 6.3 ± 0.6 | b | 5.4 ± 1.1 | ab | 16.3 ± 1.3 | c | 3–2 | 19.8 ± 3.5 | b | Versicolorous |
| MFTU06-1 | Neopestalotiopsis sp. | 3 | 24.4 ± 1.1 | b | 6.5 ± 0.4 | b | 6.2 ± 0.9 | ab | 16.0 ± 1.3 | c | 3–2 | 23.3 ± 2.9 | b | Versicolorous |
| MFTU06-2 | Neopestalotiopsis sp. | 2 | 28.8 ± 2.8 | b | 7.1 ± 0.5 | b | 5.1 ± 1.0 | ab | 18.7 ± 1.3 | c | 3–1 | 12.7 ± 2.7 | b | Versicolorous |
| MFTU06-3 | Neopestalotiopsis sp. | 1 | 21.7 ± 1.8 | b | 5.9 ± 0.4 | b | 5.7 ± 1.1 | ab | 13.9 ± 1.2 | c | 3–2 | 17.0 ± 2.5 | b | Versicolorous |
| MFTU07-1 | Neopestalotiopsis sp. | 2 | 22.0 ± 1.7 | b | 5.7 ± 0.4 | b | 4.6 ± 0.7 | ab | 14.8 ± 1.2 | c | 3–2 | 16.0 ± 2.2 | b | Versicolorous |
| MFTU07-2 | Neopestalotiopsis sp. | 2 | 23.2 ± 1.5 | b | 5.7 ± 0.3 | b | 5.1 ± 0.9 | ab | 15.1 ± 1.1 | c | 3–2 | 18.0 ± 2.8 | b | Versicolorous |
| MFTU12 | Pestalotiopsis sp. | 3 | 28.1 ± 2.3 | a | 6.5 ± 0.4 | a | 3.7 ± 0.6 | b | 17.8 ± 1.3 | b | 3–2 | 14.7 ± 2.4 | c | Concolorous |
| MFTU14-1 | Pseudopestalotiopsis sp. | 4 | 26.1 ± 1.5 | a | 7.6 ± 0.5 | a | 4.2 ± 0.3 | a | 18.9 ± 1.0 | a | 4–2 | 19.2 ± 3.0 | a | Concolorous |
| MFTU14-2 | Pseudopestalotiopsis sp. | 4 | 27.1 ± 1.4 | a | 6.7 ± 0.4 | a | 4.5 ± 0.5 | a | 18.8 ± 1.1 | a | 3–2 | 2.5 ± 3.8 | a | Concolorous |
| MFTU18 | Neopestalotiopsis sp. | 5 | 20.9 ± 1.4 | b | 6.2 ± 0.3 | b | 4.9 ± 0.8 | ab | 13.7 ± 0.9 | c | 4–2 | 14.6 ± 2.5 | b | Versicolorous |
| MFTU21 | Pestalotiopsis sp. | 3 | 28.6 ± 2.4 | a | 6.9 ± 0.4 | a | 4.7 ± 0.5 | b | 18.5 ± 1.2 | b | 3–2 | 15.7 ± 1.9 | c | Concolorous |
| MFTU25 | Neopestalotiopsis sp. | 1 | 21.1 ± 1.3 | b | 6.4 ± 0.4 | b | 3.7 ± 0.5 | ab | 14.1 ± 0.9 | c | 3–2 | 20.1 ± 4.5 | b | Versicolorous |
| MFTU34-2 | Pseudopestalotiopsis sp. | 4 | 31.7 ± 3.2 | a | 5.6 ± 0.5 | a | 6.6 ± 1.3 | a | 19.9 ± 1.9 | a | 3–2 | 22.9 ± 3.6 | a | Concolorous |
| MFTU34-3 | Neopestalotiopsis sp. | 2 | 21.8 ± 1.7 | b | 5.8 ± 0.5 | b | 5.5 ± 1.0 | ab | 14.0 ± 0.9 | c | 3–2 | 21.7 ± 2.9 | b | Versicolorous |
| MFTU35 | Neopestalotiopsis sp. | 1 | 22.9 ± 1.2 | b | 6.7 ± 0.5 | b | 5.1 ± 0.7 | ab | 14.9 ± 0.8 | c | 3–2 | 22.2 ± 5.0 | b | Versicolorous |
| MFTU36 | Neopestalotiopsis sp. | 1 | 21.4 ± 1.2 | b | 6.5 ± 0.4 | b | 4.9 ± 0.6 | ab | 14.2 ± 0.8 | c | 3–2 | 21.3 ± 2.3 | b | Versicolorous |
| MFTU37 | Neopestalotiopsis sp. | 1 | 19.8 ± 1.8 | b | 6.3 ± 0.4 | b | 5.4 ± 0.8 | ab | 13.3 ± 1.3 | c | 4–2 | 15.3 ± 2.8 | b | Versicolorous |
| MFTU38 | Neopestalotiopsis sp. | 1 | 20.6 ± 1.1 | b | 5.9 ± 0.5 | b | 5.6 ± 0.9 | ab | 13.6 ± 0.9 | c | 4–2 | 21.3 ± 3.6 | b | Versicolorous |
| MFTU39-1 | Neopestalotiopsis sp. | 1 | 21.9 ± 2.2 | b | 5.9 ± 0.5 | b | 5.7 ± 1.1 | ab | 14.5 ± 1.3 | c | 3–2 | 18.1 ± 2.9 | b | Versicolorous |
| MFTU39-2 | Neopestalotiopsis sp. | 1 | 21.7 ± 1.7 | b | 5.3 ± 0.4 | b | 5.6 ± 0.7 | ab | 13.8 ± 1.2 | c | 3–2 | 15.9 ± 1.8 | b | Versicolorous |
| MFTU40-1 | Neopestalotiopsis sp. | 6 | 27.8 ± 1.8 | b | 7.2 ± 0.7 | b | 3.5 ± 0.4 | ab | 19.1 ± 1.3 | c | 3–2 | 12.7 ± 1.9 | b | Versicolorous |
| MFTU41 | Neopestalotiopsis sp. | 2 | 23.5 ± 1.9 | b | 7.1 ± 0.5 | b | 3.4 ± 0.5 | ab | 15.9 ± 1.1 | c | 3–1 | 13.7 ± 1.8 | b | Versicolorous |
| MFTU43 | Neopestalotiopsis sp. | 1 | 21.5 ± 1.3 | b | 6.1 ± 0.3 | b | 4.9 ± 0.6 | ab | 14.1 ± 0.8 | c | 3–2 | 19.8 ± 2.7 | b | Versicolorous |
| MFTU44-2 | Neopestalotiopsis sp. | 1 | 21.9 ± 1.8 | b | 6.1 ± 0.4 | b | 5.9 ± 0.9 | ab | 14.4 ± 1.0 | c | 3–2 | 19.5 ± 2.9 | b | Versicolorous |
| Isolate Code | Genus/Species | Host | Geographic Origin | GenBank Accession Number | ||
|---|---|---|---|---|---|---|
| ITS | BTUB 2 | TEF1-α | ||||
| MFTU01-1 | Pestalotiopsis arengae | Sinú (cerete) × Deli Yangambí | Tumaco-Nariño, Colombia | MT952577 | MT957907 | MT957932 |
| MFTU04-3 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Tumaco-Nariño, Colombia | MT952578 | MT957908 | MT957933 |
| MFTU06-1 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Tumaco-Nariño, Colombia | MT952579 | MT957909 | MT957934 |
| MFTU06-2 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Tumaco-Nariño, Colombia | MT952580 | MT957910 | MT957935 |
| MFTU06-3 | N. surinamensis | Brazil × 7 polen africans | Tumaco-Nariño, Colombia | MT952581 | MT957911 | MT957936 |
| MFTU07-1 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Tumaco-Nariño, Colombia | MT952582 | MT957912 | MT957937 |
| MFTU07-2 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Tumaco-Nariño, Colombia | MT952583 | MT957913 | MT957938 |
| MFTU12 | P. arengae | Sinú (cerete) × Deli Yangambí | Tumaco-Nariño, Colombia | MT952584 | MT957914 | MT957939 |
| MFTU14-1 | Pseudopestalotiopsis cocos | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952585 | MT957915 | MT957940 |
| MFTU14-2 | Ps. Cocos | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952586 | MT957916 | MT957941 |
| MFTU18 | Neopestalotiopsis sp. | Manaos × Compacta | Tumaco-Nariño, Colombia | MT952587 | MT957917 | MT957942 |
| MFTU21 | P. arengae | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952588 | MT957918 | MT957943 |
| MFTU25 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952589 | MT957919 | MT957944 |
| MFTU34-2 | Ps. ampullacea | Brazil × 7 polen africans | Tumaco-Nariño, Colombia | MT952590 | MT957920 | MT957945 |
| MFTU34-3 | Neopestalotiopsis sp. | Brazil × 7 polen africans | Tumaco-Nariño, Colombia | MT952591 | MT957921 | MT957946 |
| MFTU35 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952592 | MT957922 | MT957947 |
| MFTU36 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952593 | MT957923 | MT957948 |
| MFTU37 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952594 | MT957924 | MT957949 |
| MFTU38 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952595 | MT957925 | MT957950 |
| MFTU39-1 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952596 | MT957926 | MT957951 |
| MFTU39-2 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952597 | MT957927 | MT957952 |
| MFTU40-1 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952598 | MT957928 | MT957953 |
| MFTU41 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952599 | MT957929 | MT957954 |
| MFTU43 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952600 | MT957930 | MT957955 |
| MFTU44-2 | Neopestalotiopsis sp. | Coarí × La Mé | Tumaco-Nariño, Colombia | MT952601 | MT957931 | MT957956 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancourt-Ortiz, W.F.; Medina-Cardenas, H.C.; Padilla-Agudelo, J.L.; Varon, F.H.; Mestizo-Garzón, Y.A.; Morales-Rodríguez, A.; Sarria-Villa, G.A. Foliar Lesions Induced by Pestalotiopsis arengae in Oil Palm (O × G) in the Colombian Southwest Palm Zone. J. Fungi 2024, 10, 24. https://doi.org/10.3390/jof10010024
Betancourt-Ortiz WF, Medina-Cardenas HC, Padilla-Agudelo JL, Varon FH, Mestizo-Garzón YA, Morales-Rodríguez A, Sarria-Villa GA. Foliar Lesions Induced by Pestalotiopsis arengae in Oil Palm (O × G) in the Colombian Southwest Palm Zone. Journal of Fungi. 2024; 10(1):24. https://doi.org/10.3390/jof10010024
Chicago/Turabian StyleBetancourt-Ortiz, William Fabian, Hector Camilo Medina-Cardenas, Jose Luis Padilla-Agudelo, Francia Helena Varon, Yuri Adriana Mestizo-Garzón, Anuar Morales-Rodríguez, and Greicy Andrea Sarria-Villa. 2024. "Foliar Lesions Induced by Pestalotiopsis arengae in Oil Palm (O × G) in the Colombian Southwest Palm Zone" Journal of Fungi 10, no. 1: 24. https://doi.org/10.3390/jof10010024
APA StyleBetancourt-Ortiz, W. F., Medina-Cardenas, H. C., Padilla-Agudelo, J. L., Varon, F. H., Mestizo-Garzón, Y. A., Morales-Rodríguez, A., & Sarria-Villa, G. A. (2024). Foliar Lesions Induced by Pestalotiopsis arengae in Oil Palm (O × G) in the Colombian Southwest Palm Zone. Journal of Fungi, 10(1), 24. https://doi.org/10.3390/jof10010024






