Long COVID-19 Myocarditis and Various Heart Failure Presentations: A Case Series
Abstract
1. Introduction
2. Materials and Methods
3. Case Presentation
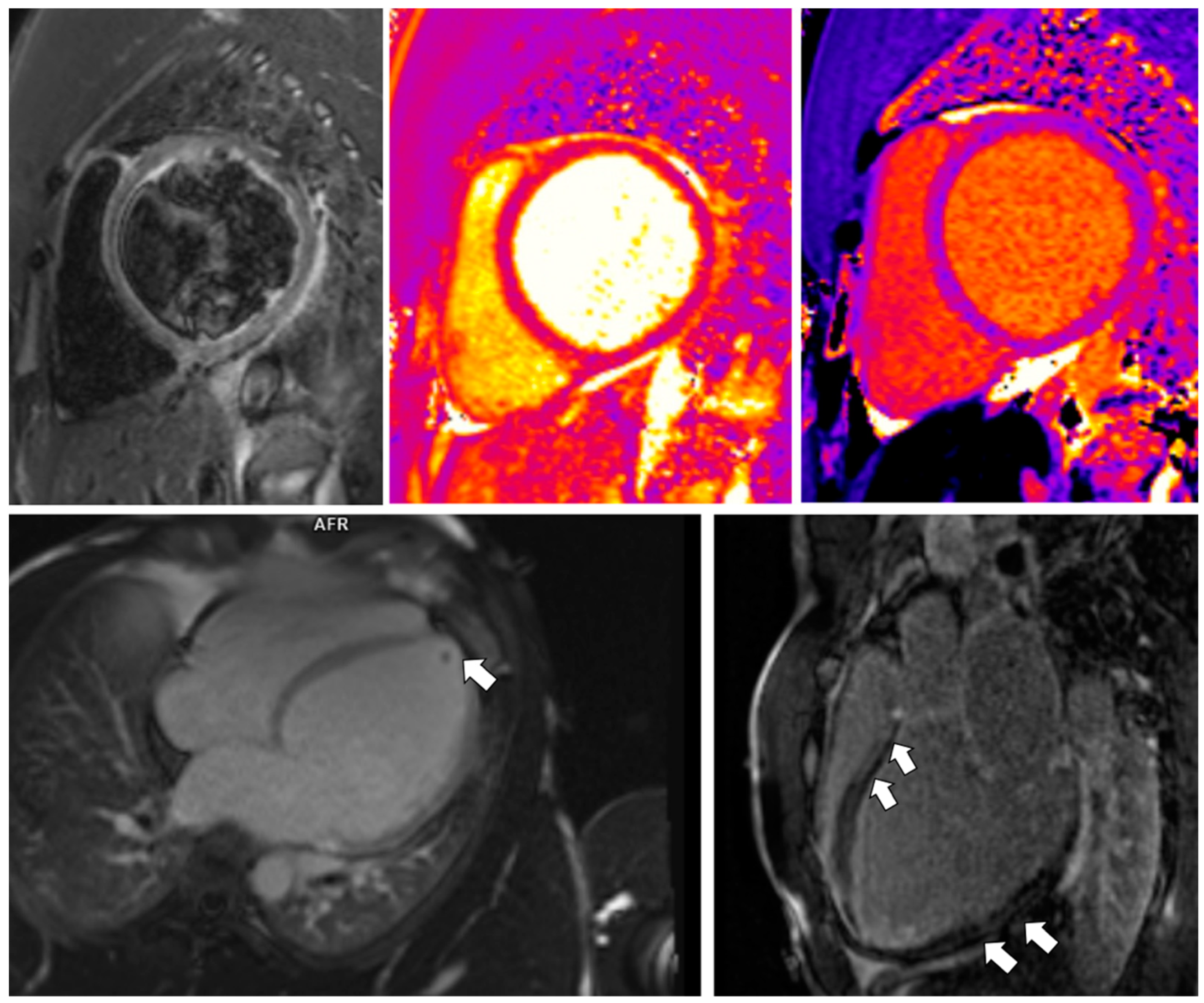
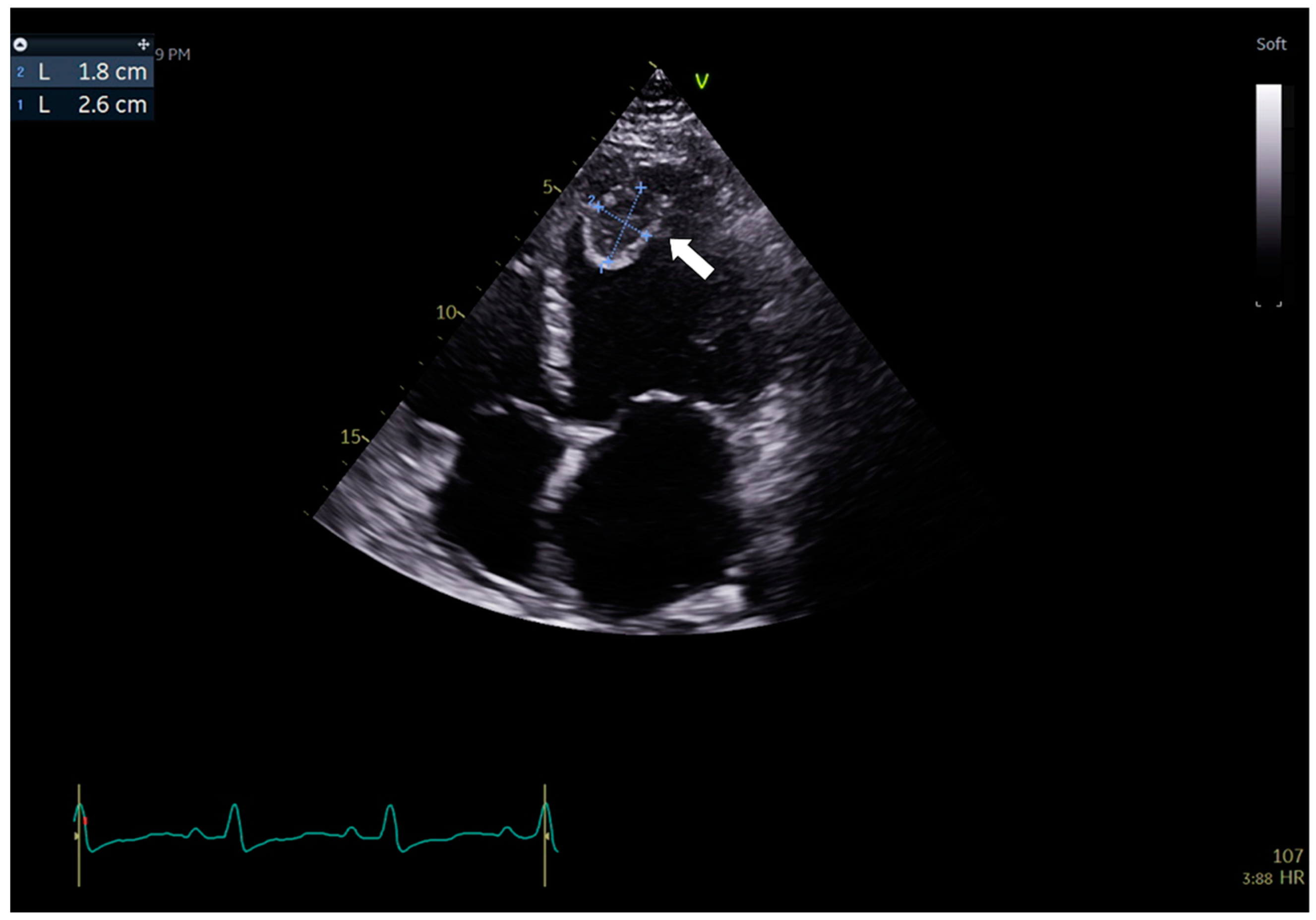
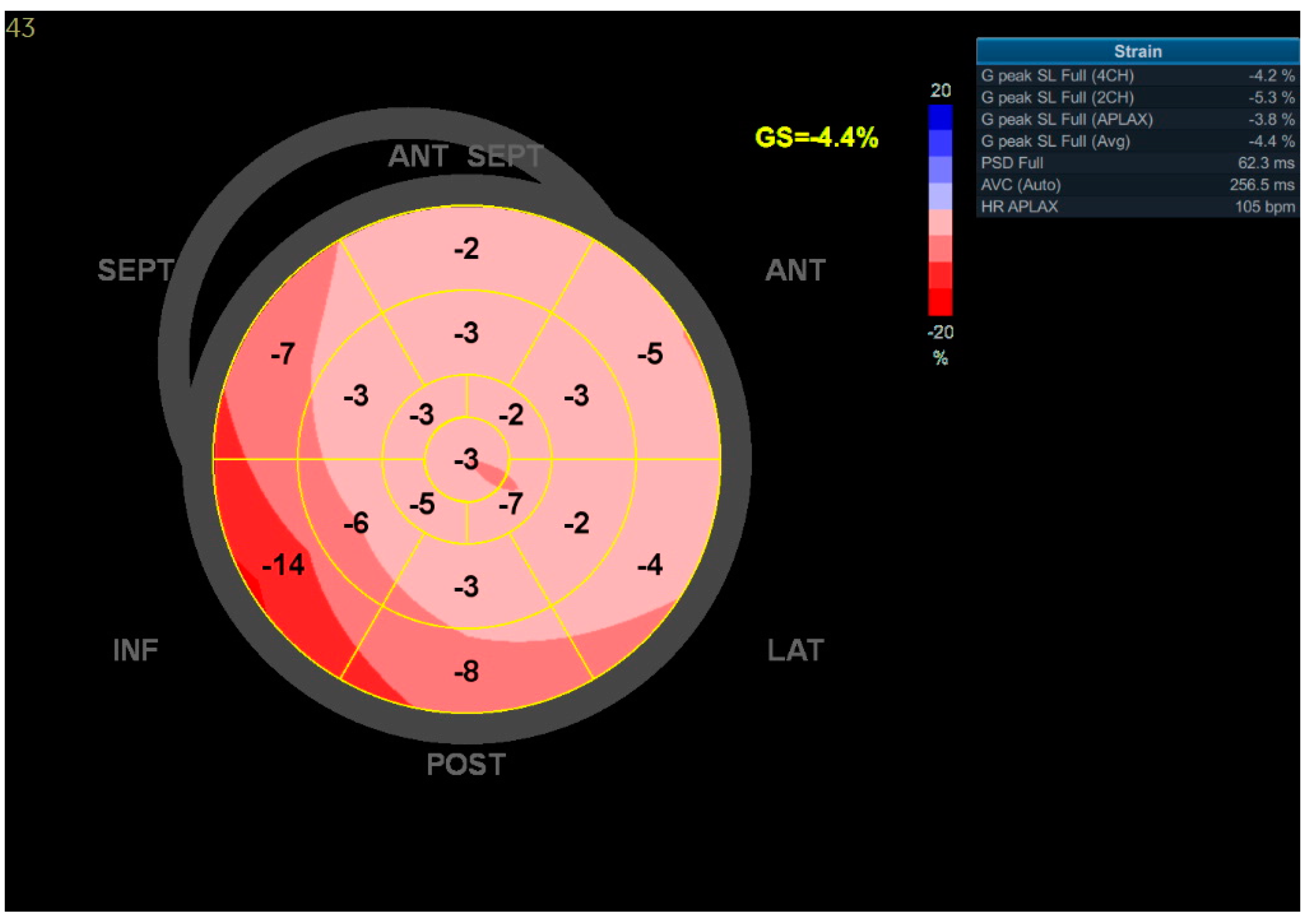
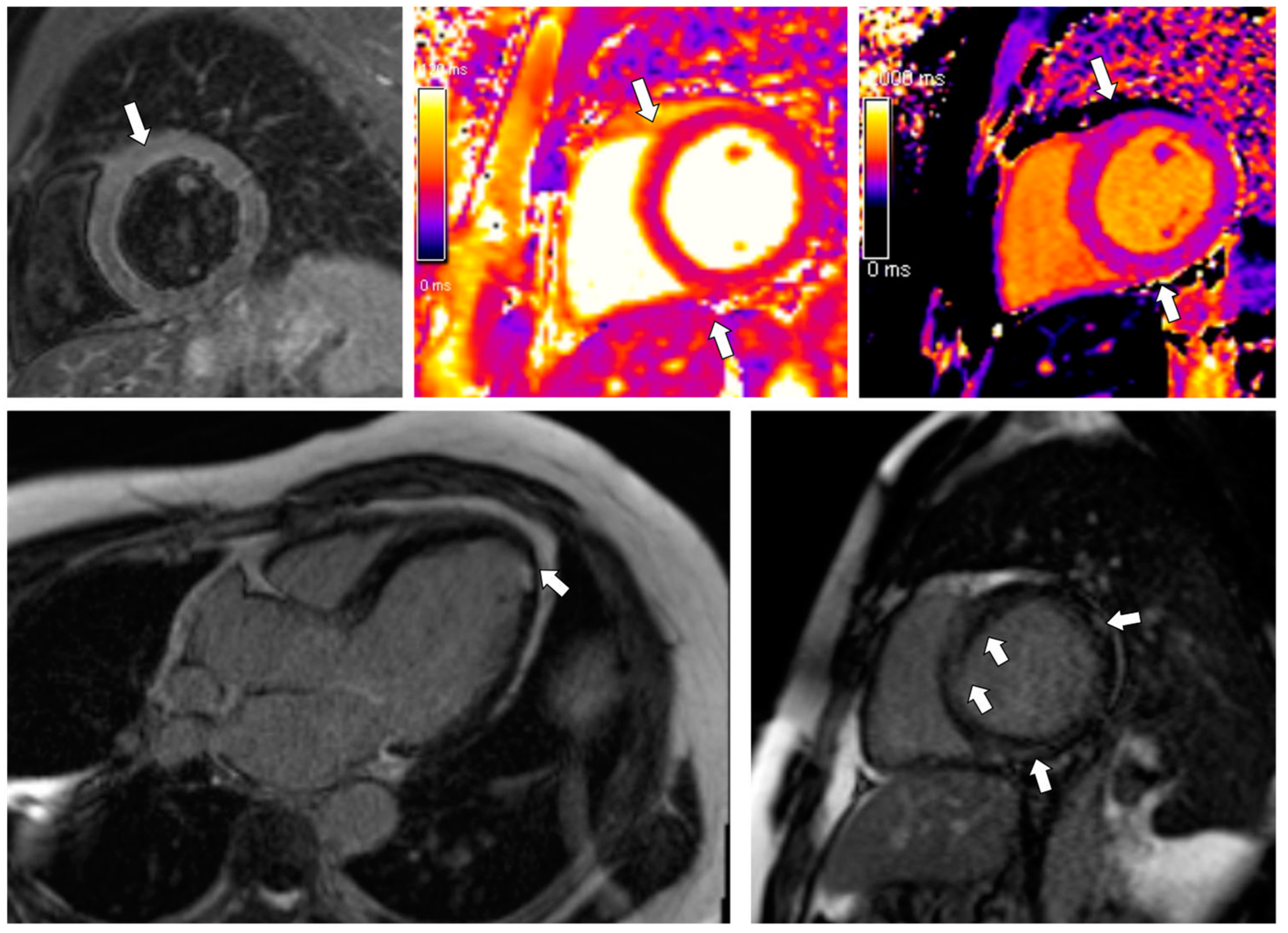
3.1. Case 1
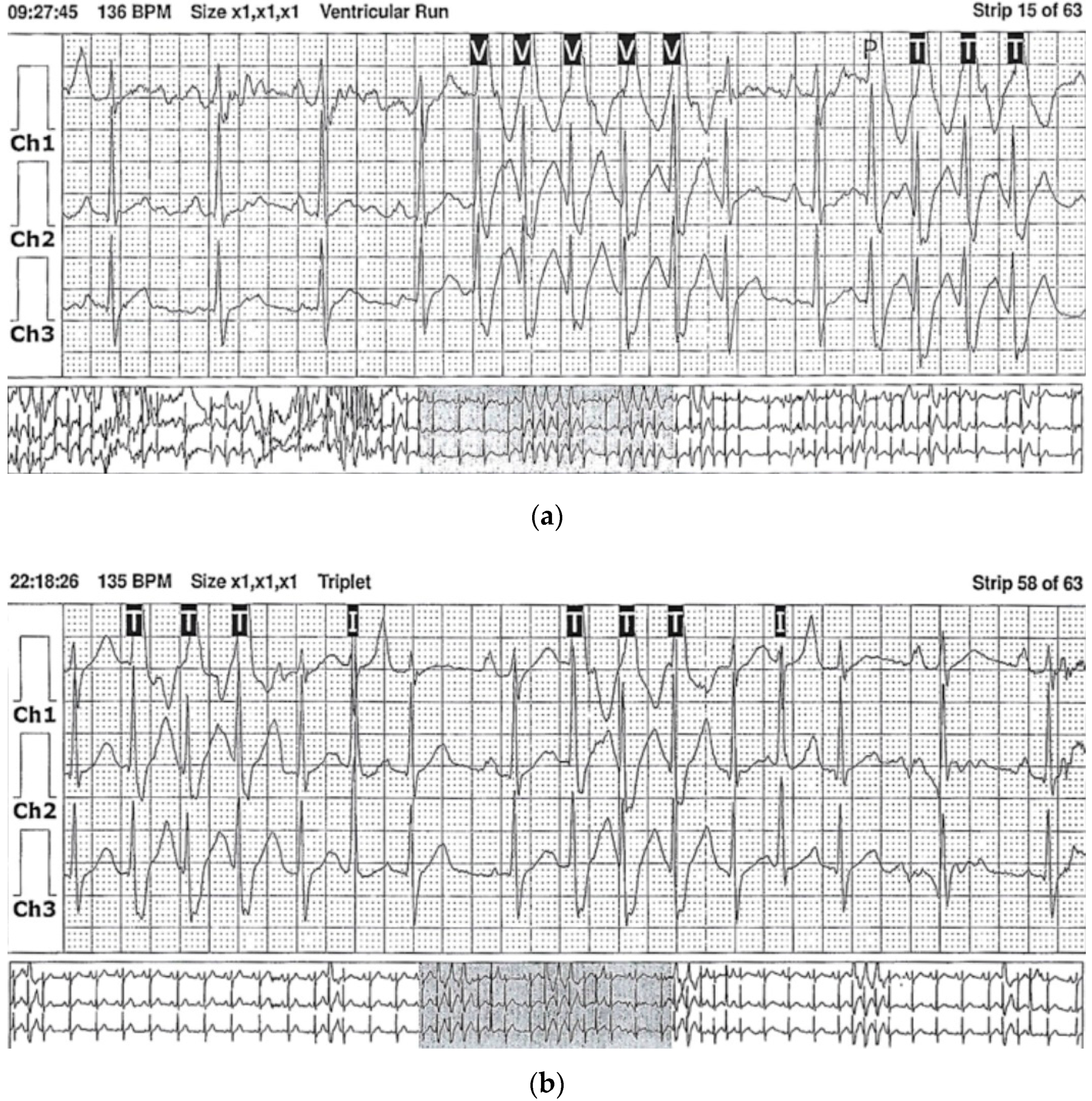
3.2. Case 2
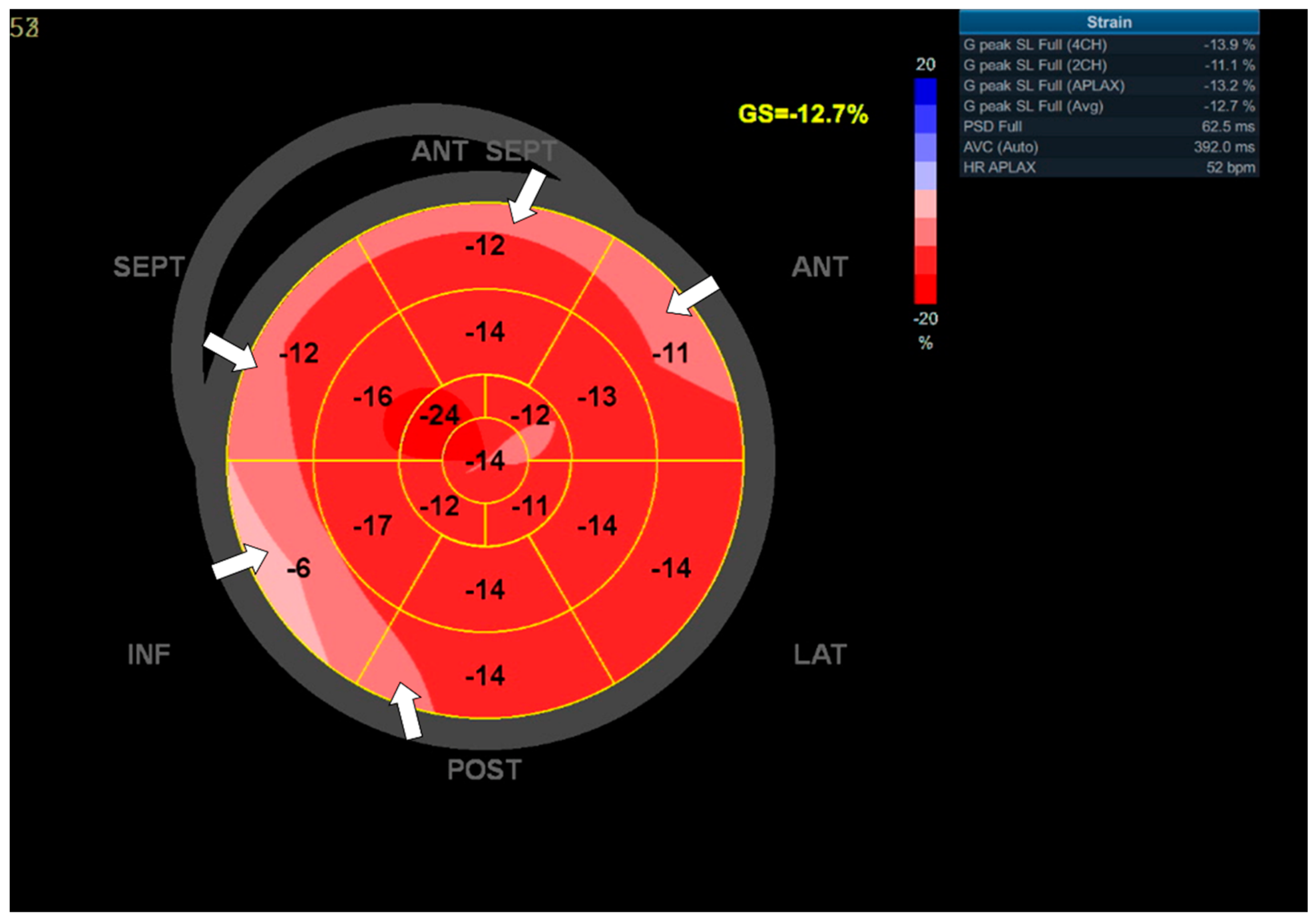
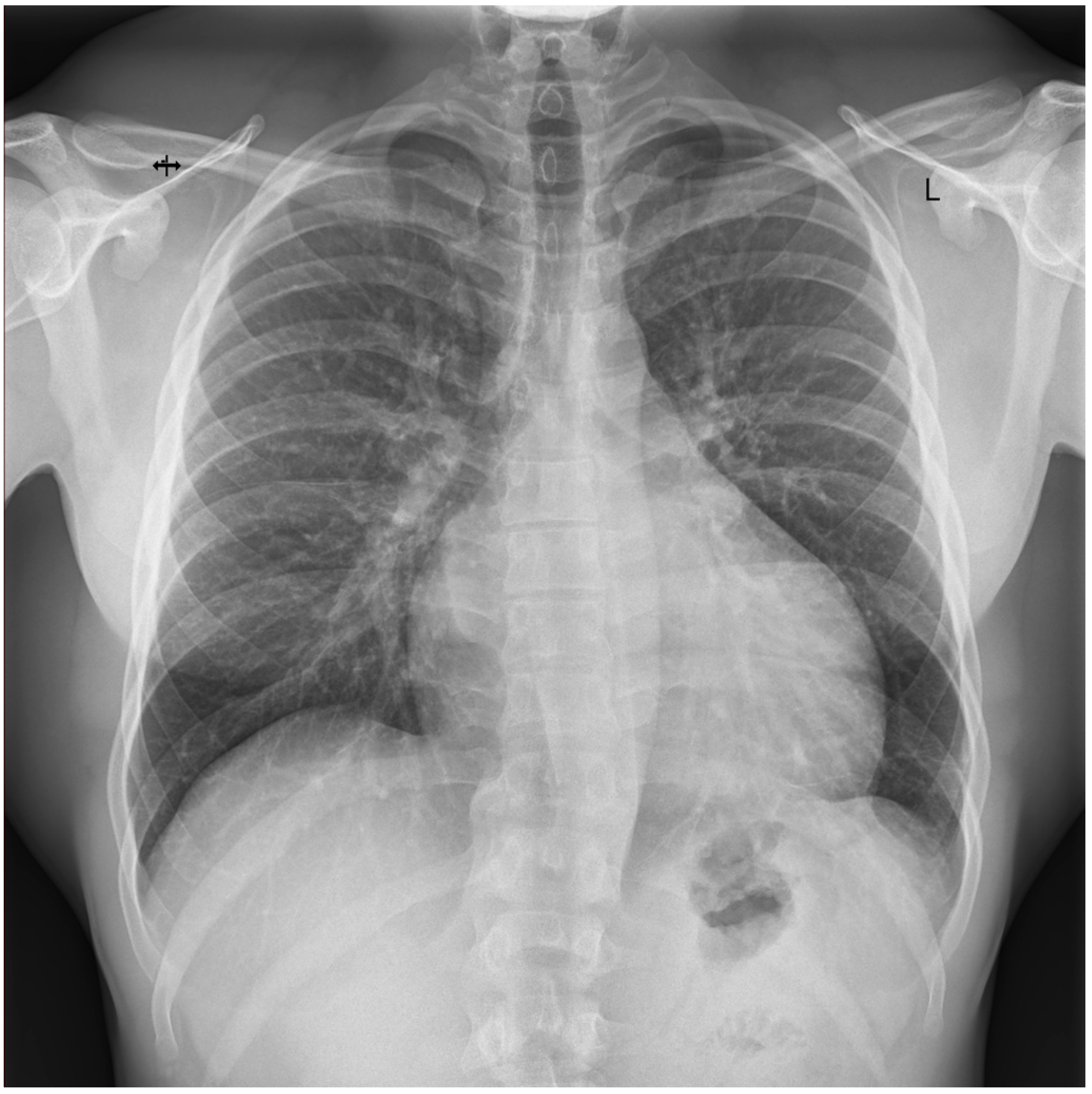
3.3. Case 3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rando, H.M.; Bennett, T.D.; Byrd, J.B.; Bramante, C.; Callahan, T.J.; Chute, C.G.; Davis, H.E.; Deer, R.; Gagnier, J.; Koraishy, F.M.; et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. Preprint. medRxiv 2021. [Google Scholar] [CrossRef]
- Datta, S.D.; Talwar, A.; Lee, J.T. A Proposed Framework and Timeline of the Spectrum of Disease Due to SARS-CoV-2 Infection: Illness Beyond Acute Infection and Public Health Implications. JAMA 2020, 324, 2251–2252. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Zhao, Y.M.; Yan, W.; Li, C.; Lu, Q.D.; Liu, L.; Ni, S.Y.; Mei, H.; Yuan, K.; Shi, L.; et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: Call for research priority and action. Mol. Psychiatry 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Tuvali, O.; Tshori, S.; Derazne, E.; Hannuna, R.R.; Afek, A.; Haberman, D.; Sella, G.; George, J. The Incidence of Myocarditis and Pericarditis in Post COVID-19 Unvaccinated Patients-A Large Population-Based Study. J. Clin. Med. 2022, 11, 2219. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Karadavut, S.; Altintop, I. Long-term cardiovascular adverse events in very elderly COVID-19 patients. Arch. Gerontol. Geriatr. 2022, 100, 104628. [Google Scholar] [CrossRef]
- Eiros, R.; Barreiro-Pérez, M.; Martín-García, A.; Almeida, J.; Villacorta, E.; Pérez-Pons, A.; Merchán, S.; Torres-Valle, A.; Sánchez-Pablo, C.; González-Calle, D.; et al. Pericarditis and myocarditis long after SARS-CoV-2 infection: A cross-sectional descriptive study in health-care workers. Rev. Esp. Cardiol. 2022, 75, 735–747. [Google Scholar] [CrossRef]
- Rohun, J.; Daniłowicz-Szymanowicz, L. Myocarditis in the COVID-19 pandemic era with particular attention to the elderly—the literature review. Geriatria 2022, 16, 163–170. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr. Myocarditis. N Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef]
- Jaiswal, V.; Sarfraz, Z.; Sarfraz, A.; Mukherjee, D.; Batra, N.; Hitawala, G.; Yaqoob, S.; Patel, A.; Agarwala, P.; Ruchika, F.N.U.; et al. COVID-19 Infection and Myocarditis: A State-of-the-Art Systematic Review. J. Prim. Care Community Health 2021, 12, 21501327211056800. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc. Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P.; et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, K.; Suh, Y.J. Prevalence of abnormal cardiovascular magnetic resonance findings in recovered patients from COVID-19: A systematic review and meta-analysis. J. Cardiovasc. Magn. Reson. 2021, 23, 100. [Google Scholar] [CrossRef]
- Nicol, M.; Cacoub, L.; Baudet, M.; Nahmani, Y.; Cacoub, P.; Cohen-Solal, A.; Henry, P.; Adle-Biassette, H.; Logeart, D. Delayed acute myocarditis and COVID-19-related multisystem inflammatory syndrome. ESC Heart Fail 2020, 7, 4371–4376. [Google Scholar] [CrossRef] [PubMed]
- Spano, G.; Fischer, K.; Maillat, C.; Vicario, G.; Huber, A.T.; Gräni, C. Delayed isolated peri-myocarditis in a COVID-19 patient with respiratory symptoms but without lung involvement. Int. J. Cardiovasc. Imaging 2020, 36, 2279–2280. [Google Scholar] [CrossRef]
- Bajaj, R.; Sinclair, H.C.; Patel, K.; Low, B.; Pericao, A.; Manisty, C.; Guttmann, O.; Zemrak, F.; Miller, O.; Longhi, P.; et al. Delayed-onset myocarditis following COVID-19. Lancet Respir. Med. 2021, 9, e32–e34. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshni, S.; Westra, J.; Kuo, Y.F.; Baillargeon, J.G.; Khalife, W.; Raji, M. COVID-19 Infection and Incidence of Myocarditis: A Multi-Site Population-Based Propensity Score-Matched Analysis. Cureus 2022, 14, e21879. [Google Scholar] [CrossRef]
- Boehmer, T.K.; Kompaniyets, L.; Lavery, A.M.; Hsu, J.; Ko, J.Y.; Yusuf, H.; Romano, S.D.; Gundlapalli, A.V.; Oster, M.E.; Harris, A.M. Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data—United States, March 2020-January 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1228–1232. [Google Scholar] [CrossRef]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J. Am. Coll. Cardiol. 2007, 50, 1914–1931. [Google Scholar] [CrossRef]
- Ojha, V.; Verma, M.; Pandey, N.N.; Mani, A.; Malhi, A.S.; Kumar, S.; Jagia, P.; Roy, A.; Sharma, S. Cardiac Magnetic Resonance Imaging in Coronavirus Disease 2019 (COVID-19): A Systematic Review of Cardiac Magnetic Resonance Imaging Findings in 199 Patients. J. Thorac. Imaging 2021, 36, 73–83. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Malipiero, G.; Marcolongo, R.; Iliceto, S. Clinically suspected myocarditis with pseudo-infarct presentation: The role of endomyocardial biopsy. J. Thorac. Dis. 2017, 9, 423–427. [Google Scholar] [CrossRef]
- Francone, M.; Chimenti, C.; Galea, N.; Scopelliti, F.; Verardo, R.; Galea, R.; Carbone, I.; Catalano, C.; Fedele, F.; Frustaci, A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc. Imaging 2014, 7, 254–263. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J. Association of cardiac injury with mortality in hospitalised patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–881. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Khan, M.H.; Aqtash, O.; Harris, D.M.; Costea, A.I.; Gerson, M.C. Ventricular Tachycardia or Fibrillation Storm in Coronavirus Disease. Case Rep. Cardiol. 2022, 2022, 1157728. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.P.; Coromilas, E.J.; Wan, E.Y.; Rubin, G.A.; Garan, H.; Dizon, J.M. Malignant ventricular arrhythmias in patients with severe acute respiratory distress syndrome due to COVID-19 without significant structural heart disease. HeartRhythm Case Rep. 2020, 6, 858–862. [Google Scholar] [CrossRef]
- Ingul, C.B.; Grimsmo, J.; Mecinaj, A.; Trebinjac, D.; Berger Nossen, M.; Andrup, S.; Grenne, B.; Dalen, H.; Einvik, G.; Stavem, K.; et al. Cardiac Dysfunction and Arrhythmias 3 Months After Hospitalization for COVID-19. J. Am. Heart Assoc. 2022, 11, e023473. [Google Scholar] [CrossRef]
- Coromilas, E.J.; Kochav, S.; Goldenthal, I.; Biviano, A.; Garan, H.; Goldbarg, S.; Kim, J.H.; Yeo, I.; Tracy, C.; Ayanian, S.; et al. Worldwide survey of COVID-19-associated arrhythmias. Circ. Arrhythm. Electrophysiol. 2021, 14, e009458. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Desai, A.D.; Boursiquot, B.C.; Melki, L.; Wan, E.Y. Management of Arrhythmias Associated with COVID-19. Curr. Cardiol. Rep. 2020, 23, 2. [Google Scholar] [CrossRef]
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| BP | 120/73 | 120/70 | 119/70 |
| HR | 80 | 98 | 88 |
| SpO2 | 97 | 99 | 95 |
| hsTnI (ng/mL; normal < 0.1) | <0.005 | 0.1174 | 0.014 |
| BNP (pg/mL; normal < 73) | 46 | 432 | 3004 |
| CRP (mg/L; normal 0.0–5.0) | <0.4 | 0.94 | 63.33 |
| WBC (×109/L; normal 4–10) | 5.08 | 11.07 | 13.87 |
| Creatinine (mg/dL; normal 0.55–1.02) | 1.02 | 1.56 | 1.4 |
| eGFR (mL/min/1.73 m2; normal > 60) | 88 | 52 | 53 |
| ALT (U/L; normal 0–55) | 26 | 137 | 295 |
| GGT (U/L; normal 12–64) | 9 | 255 | 152 |
| Case 1 (CMR 1) | Case 1 (CMR 2) | Case 2 | Case 3 | |
|---|---|---|---|---|
| LVEDV (mL; normal 83–207) | 206 | 227 | 489 | 215 |
| LVEDVI (mL/m2; normal 47–107) | 99 | 110 | 242 | 106 |
| LVEF (%; normal 51–76) | 49 | 53 | 14 | 26 |
| LVSV (mL; normal 55–127) | 100 | 121 | 67 | 55 |
| RVEF (%, normal: 42–72) | 53 | 47 | 21 | 34 |
| Native T1 (ms; normal 993 ± 21) | not measured | 971 | 1014 | 1012–1080 |
| T2 (ms; normal 44 ± 2.4 ms) | not measured | 47 | 46 | 48 with local increase subepicardially up to 53 ms |
| LGE (segments) | subepicardial
| not detected | intramural-subepicardial
| intramural—subepicardial
|
| ECV (%; normal 25.3 ± 3.5) | not calculated | 26 | 30 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohun, J.; Dorniak, K.; Faran, A.; Kochańska, A.; Zacharek, D.; Daniłowicz-Szymanowicz, L. Long COVID-19 Myocarditis and Various Heart Failure Presentations: A Case Series. J. Cardiovasc. Dev. Dis. 2022, 9, 427. https://doi.org/10.3390/jcdd9120427
Rohun J, Dorniak K, Faran A, Kochańska A, Zacharek D, Daniłowicz-Szymanowicz L. Long COVID-19 Myocarditis and Various Heart Failure Presentations: A Case Series. Journal of Cardiovascular Development and Disease. 2022; 9(12):427. https://doi.org/10.3390/jcdd9120427
Chicago/Turabian StyleRohun, Justyna, Karolina Dorniak, Anna Faran, Anna Kochańska, Dariusz Zacharek, and Ludmiła Daniłowicz-Szymanowicz. 2022. "Long COVID-19 Myocarditis and Various Heart Failure Presentations: A Case Series" Journal of Cardiovascular Development and Disease 9, no. 12: 427. https://doi.org/10.3390/jcdd9120427
APA StyleRohun, J., Dorniak, K., Faran, A., Kochańska, A., Zacharek, D., & Daniłowicz-Szymanowicz, L. (2022). Long COVID-19 Myocarditis and Various Heart Failure Presentations: A Case Series. Journal of Cardiovascular Development and Disease, 9(12), 427. https://doi.org/10.3390/jcdd9120427







