Left Atrial Diameter and the Risk of Thromboembolism in Patients with Left Ventricular Noncompaction
Abstract
1. Introduction
2. Methods
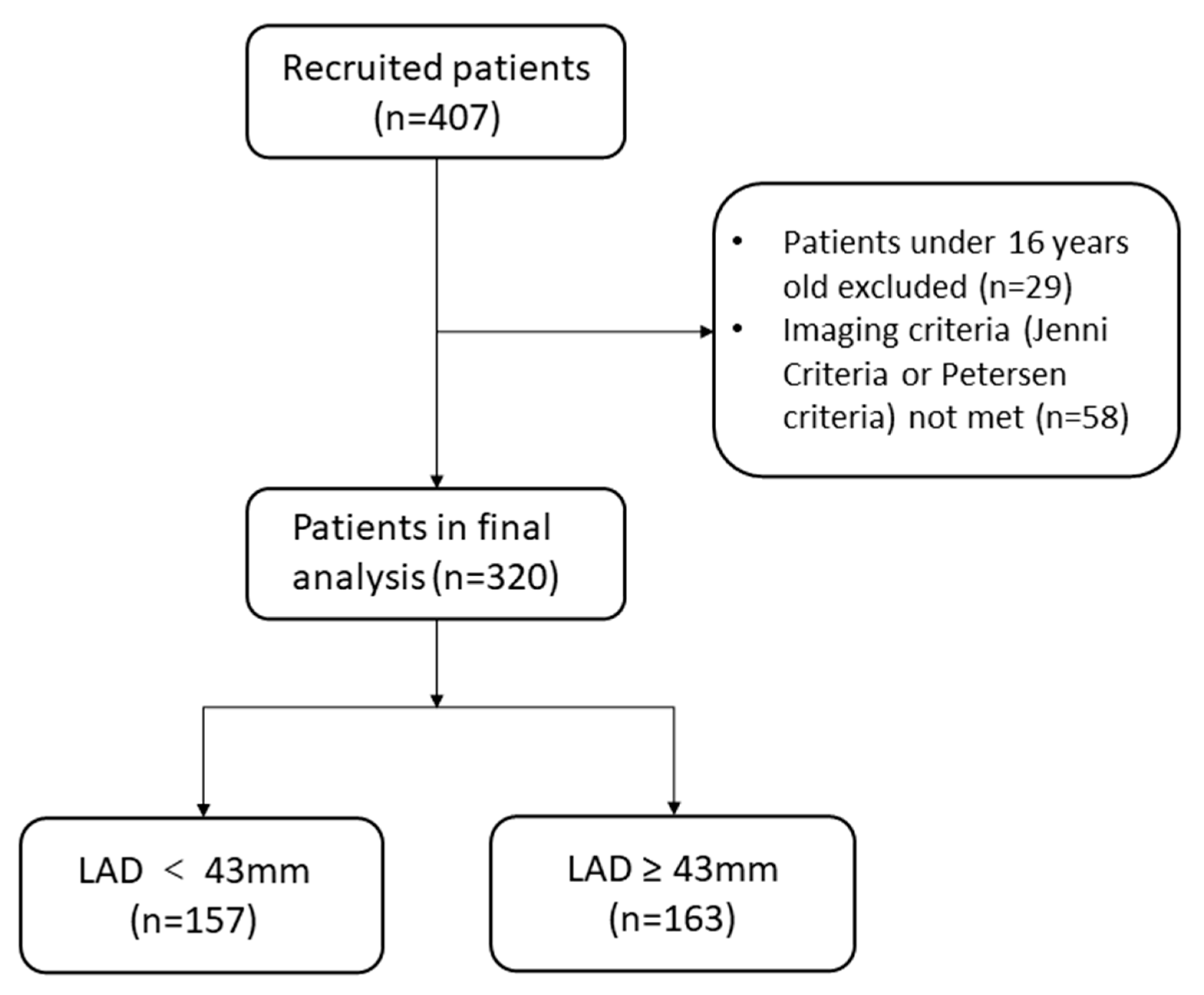
2.1. Study Design and Population
2.2. Baseline Data Collection
2.3. Measurements
2.4. Follow-Up and Outcomes
2.5. Statistical Analysis
3. Results
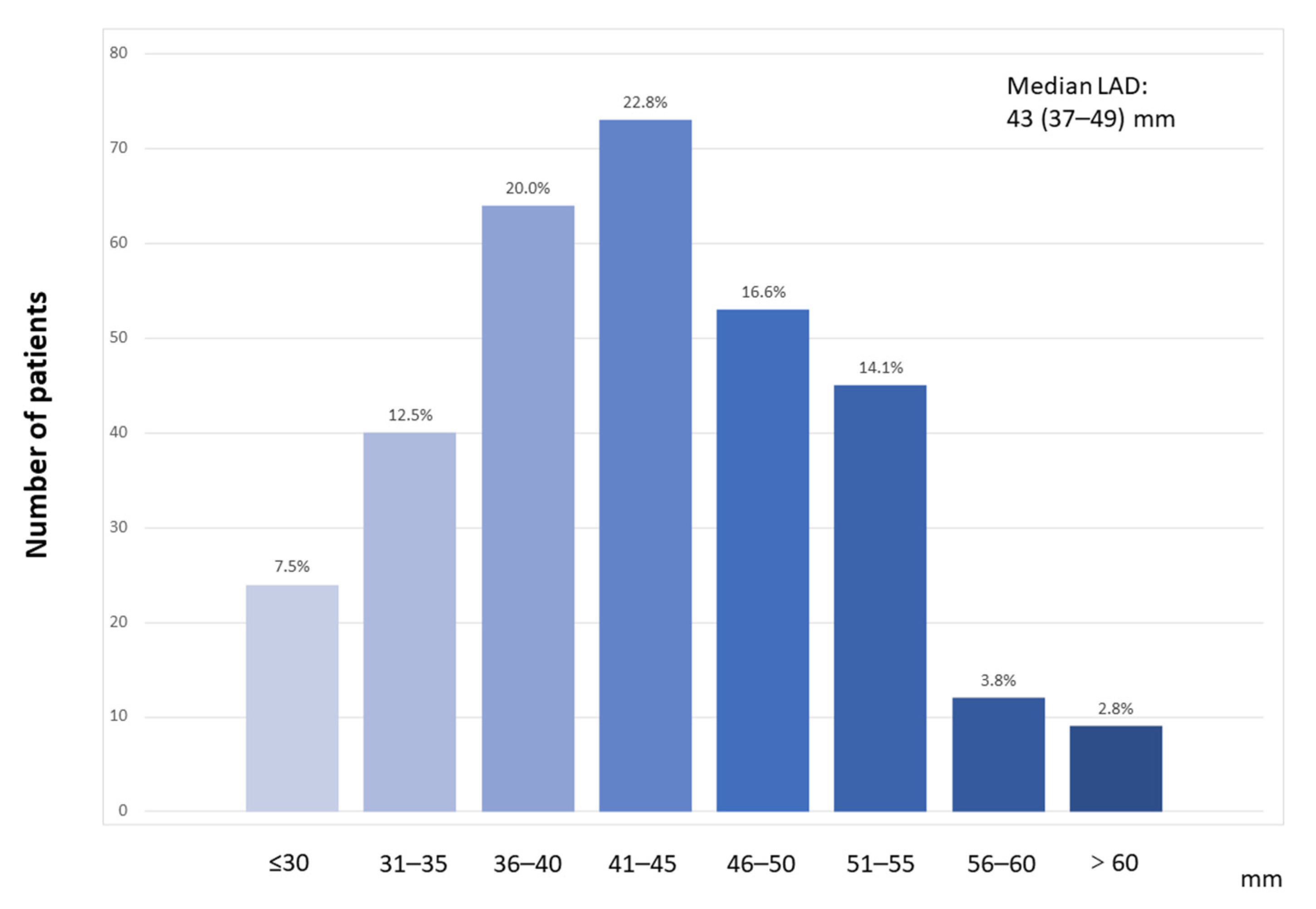
3.1. Baseline Characteristics
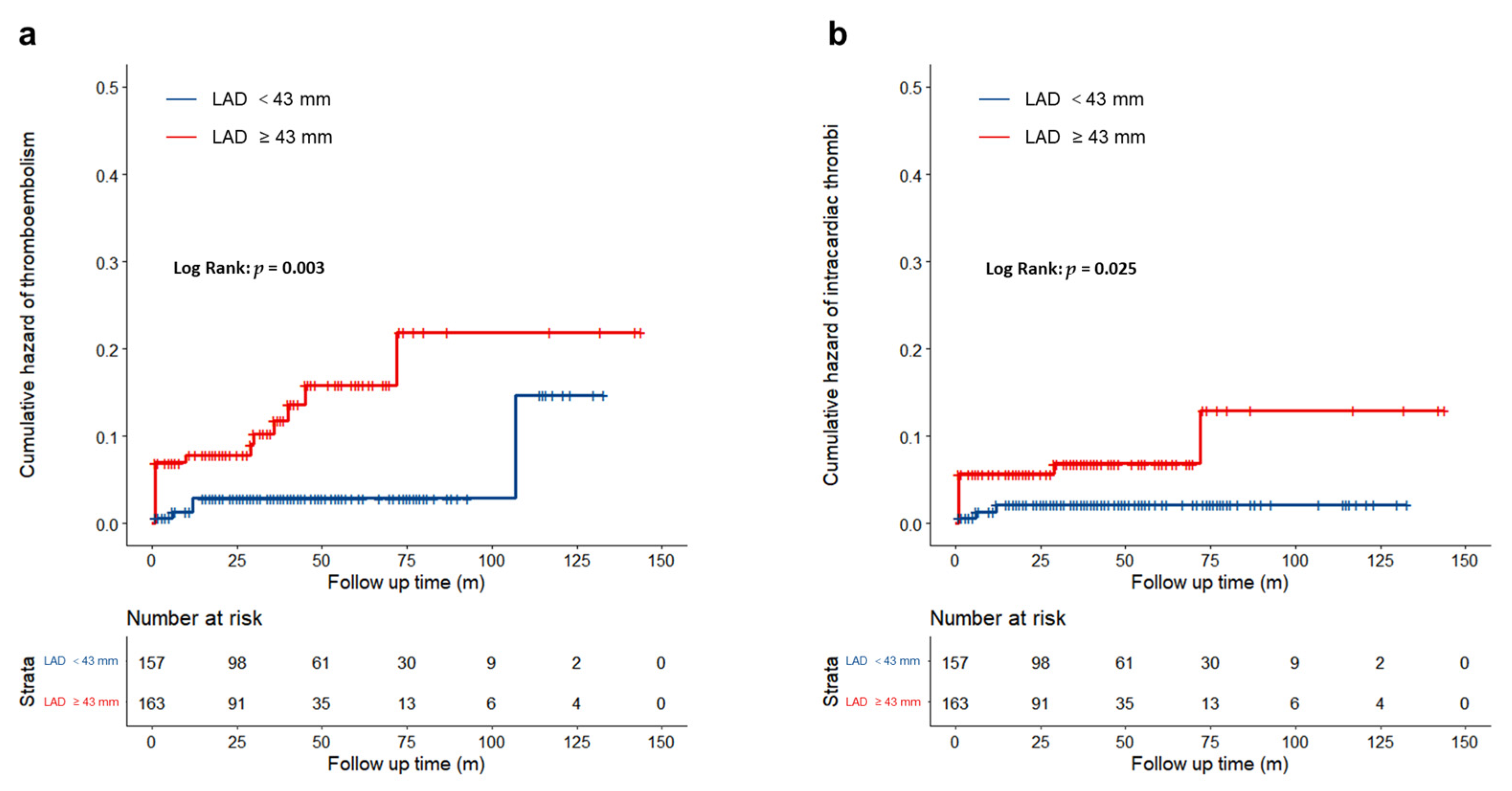
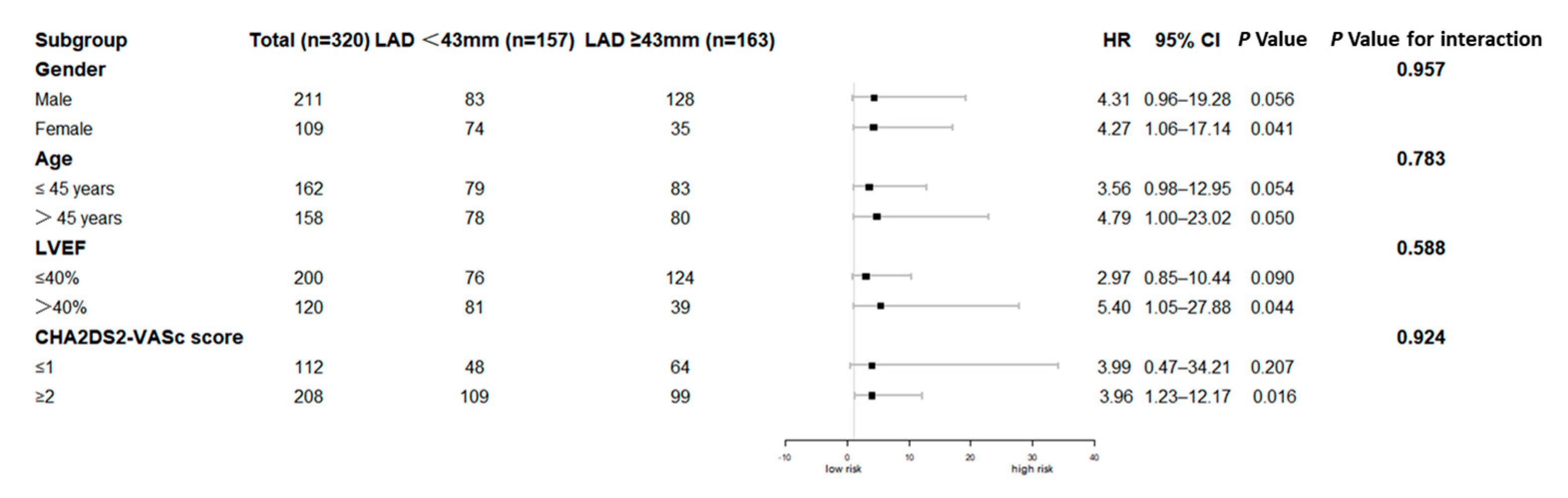
3.2. Clinical Outcomes
4. Discussion
4.1. Main Findings
4.2. Thromboembolism in Patients with LVNC
4.3. Left Atrial Dilation and Thrombotic Risk
4.4. Clinical Implications and Future Directions
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Casas, G.; Limeres, J.; Oristrell, G.; Gutierrez-Garcia, L.; Andreini, D.; Borregan, M.; Larrañaga-Moreira, J.M.; Lopez-Sainz, A.; Codina-Solà, M.; Teixido-Tura, G.; et al. Clinical Risk Prediction in Patients With Left Ventricular Myocardial Noncompaction. J. Am. Coll. Cardiol. 2021, 78, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.R.; Lyle, M.; Miranda, W.R.; Farwati, M.; Isath, A.; Patlolla, S.H.; Hodge, D.O.; Asirvatham, S.J.; Kapa, S.; Deshmukh, A.J.; et al. Long-Term Survival of Patients With Left Ventricular Noncompaction. J. Am. Heart Assoc. 2021, 10, e015563. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.; Jenni, R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur. Heart J. 2011, 32, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Finsterer, J. Thrombi in left ventricular hypertrabeculation/noncompaction--review of the literature. Acta Cardiol. 2004, 59, 341–344. [Google Scholar] [CrossRef]
- Hamatani, Y.; Ogawa, H.; Takabayashi, K.; Yamashita, Y.; Takagi, D.; Esato, M.; Chun, Y.H.; Tsuji, H.; Wada, H.; Hasegawa, K.; et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci. Rep. 2016, 6, 31042. [Google Scholar] [CrossRef]
- Iliadis, C.; Baldus, S.; Kalbacher, D.; Boekstegers, P.; Schillinger, W.; Ouarrak, T.; Zahn, R.; Butter, C.; Zuern, C.S.; von Bardeleben, R.S.; et al. Impact of left atrial diameter on outcome in patients undergoing edge-to-edge mitral valve repair: Results from the German TRAnscatheter Mitral valve Interventions (TRAMI) registry. Eur. J. Heart Fail. 2020, 22, 1202–1210. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.; Schneider, J.; Attenhofer Jost, C.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart (Br. Card. Soc.) 2001, 86, 666–671. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef]
- Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; Hindricks, G.; Prendergast, B.; et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar]
- Ma, H.; Bandos, A.I.; Rockette, H.E.; Gur, D. On use of partial area under the ROC curve for evaluation of diagnostic performance. Stat. Med. 2013, 32, 3449–3458. [Google Scholar] [CrossRef]
- Kayvanpour, E.; Sedaghat-Hamedani, F.; Gi, W.T.; Tugrul, O.F.; Amr, A.; Haas, J.; Zhu, F.; Ehlermann, P.; Uhlmann, L.; Katus, H.A.; et al. Clinical and genetic insights into non-compaction: A meta-analysis and systematic review on 7598 individuals. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2019, 108, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Finsterer, J. Left ventricular hypertrabeculation/noncompaction. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2004, 17, 91–100. [Google Scholar] [CrossRef]
- Benjamin, E.J.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A.; Levy, D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation 1995, 92, 835–841. [Google Scholar] [CrossRef]
- Guttmann, O.P.; Pavlou, M.; O’Mahony, C.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; Garcia-Pavia, P.; et al. Prediction of thrombo-embolic risk in patients with hypertrophic cardiomyopathy (HCM Risk-CVA). Eur. J. Heart Fail. 2015, 17, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Di Tullio, M.R.; Qian, M.; Thompson, J.L.P.; Labovitz, A.J.; Mann, D.L.; Sacco, R.L.; Pullicino, P.M.; Freudenberger, R.S.; Teerlink, J.R.; Graham, S.; et al. Left atrial volume and cardiovascular outcomes in systolic heart failure: Effect of antithrombotic treatment. ESC Heart Fail. 2018, 5, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.; Barnes, M.E.; Bailey, K.R.; Leibson, C.L.; Montgomery, S.C.; Takemoto, Y.; Diamond, P.M.; Marra, M.A.; Gersh, B.J.; Wiebers, D.O.; et al. Left atrial volume: Important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin. Proc. 2001, 76, 467–475. [Google Scholar] [CrossRef]
- Barnes, M.E.; Miyasaka, Y.; Seward, J.B.; Gersh, B.J.; Rosales, A.G.; Bailey, K.R.; Petty, G.W.; Wiebers, D.O.; Tsang, T.S. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin. Proc. 2004, 79, 1008–1014. [Google Scholar] [CrossRef]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2018, 20, 33–42. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Cho, M.S.; Park, H.S.; Cha, M.J.; Lee, S.R.; Park, J.K.; Kim, T.H.; Lee, J.M.; Park, J.; Park, H.W.; Kang, K.W.; et al. Clinical impact of left atrial enlargement in Korean patients with atrial fibrillation. Sci. Rep. 2021, 11, 23808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Z.; Ferrari, M.W.; Ferrari-Kuehne, K.; Bulter, J.; Xu, X.; Zhou, Q.; Zhang, Y.; Zhang, J. Anticoagulation in cardiomyopathy: Unravelling the hidden threat and challenging the threat individually. ESC Heart Fail. 2021, 8, 4737–4750. [Google Scholar] [CrossRef] [PubMed]
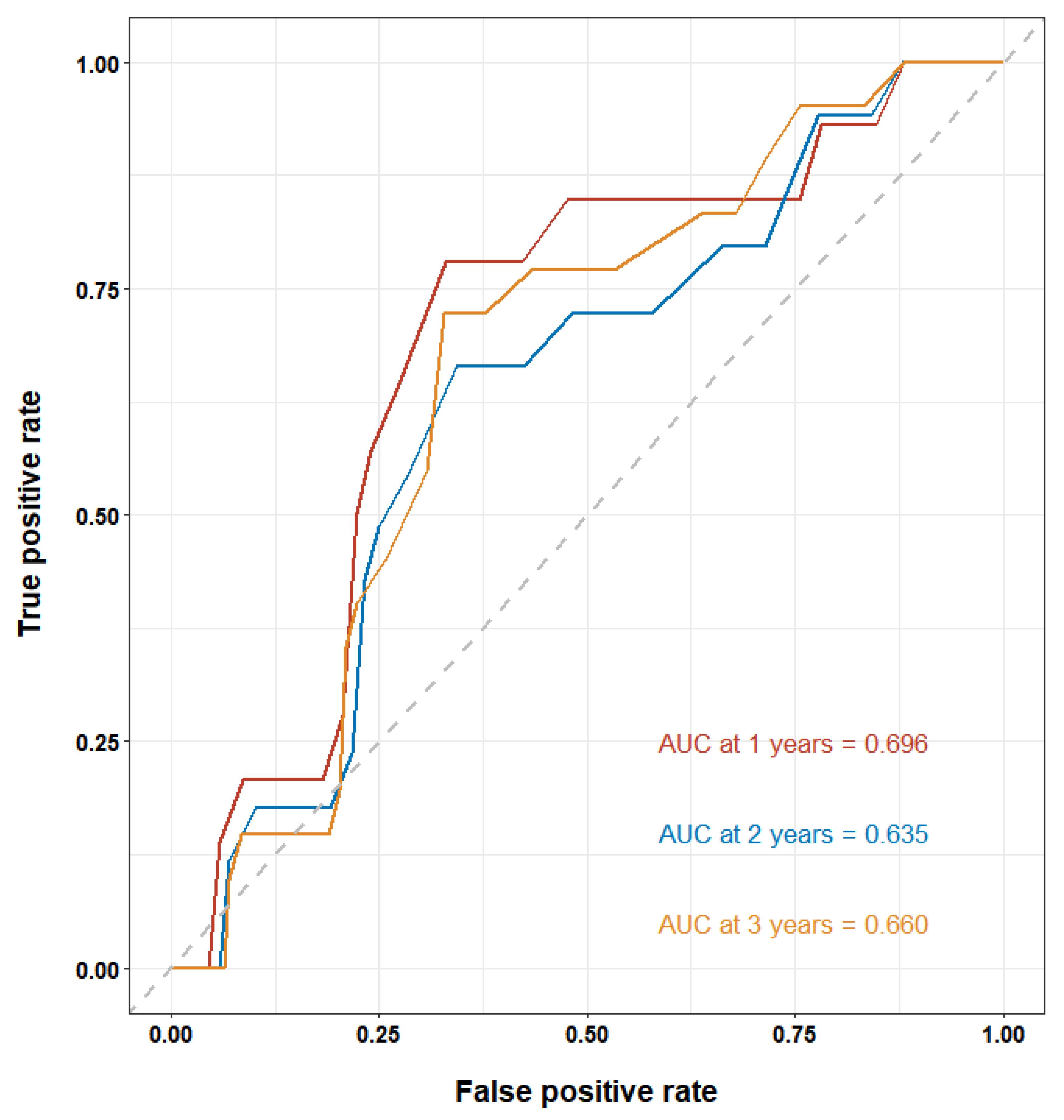
| Total | LAD1 (LAD < 43 mm) | LAD2 (LAD ≥ 43 mm) | p-Value | |
|---|---|---|---|---|
| n | 320 | 157 | 163 | |
| Male [n (%)] | 211 (65.9%) | 83 (52.9%) | 128 (78.5%) | <0.001 |
| Age (y) | 45 (30–57) | 45 (29–57) | 45 (30–57) | 0.689 |
| Height (cm) | 168.8 ± 8.4 | 167.3 ± 8.2 | 170.4 ± 8.3 | 0.001 |
| BMI (kg/m2) | 23.56 ± 4.16 | 22.86 ± 4.00 | 24.22 ± 4.20 | 0.004 |
| SBP (mmHg) | 115 (104–128) | 120 (105–130) | 110 (102–123) | 0.012 |
| DBP (mmHg) | 72 (65–80) | 71 (67–80) | 72 (65–80) | 0.522 |
| HR (beats/min) | 75 (66–88) | 72 (65–85) | 78 (68–89) | 0.023 |
| Cardiomyopathy family history [n (%)] | 19 (5.9%) | 8 (5.1%) | 11 (6.7%) | 0.453 |
| Smoking [n (%)] | 115 (35.9%) | 45 (28.7%) | 70 (42.9%) | 0.007 |
| Drinking [n (%)] | 115 (35.9%) | 47 (29.9%) | 68 (41.7%) | 0.020 |
| Syncope [n (%)] | 56 (17.5%) | 34 (21.7%) | 22 (13.5%) | 0.055 |
| Cardiogenic shock [n (%)] | 7 (2.2%) | 5 (3.2%) | 2 (1.2%) | 0.231 |
| CPR [n (%)] | 12 (3.8%) | 4 (2.5%) | 8 (4.9%) | 0.267 |
| Comorbidities | ||||
| Heart failure [n (%)] | 267 (83.4%) | 114 (72.6%) | 153 (93.9%) | <0.001 |
| NYHA class [n (%)] | <0.001 | |||
| I | 25 (7.8%) | 18 (11.5%) | 7 (4.3%) | |
| II | 73 (22.8%) | 39 (24.8%) | 34 (20.9%) | |
| III | 108 (33.8%) | 45 (28.7%) | 63 (38.7%) | |
| IV | 61 (19.1%) | 12 (7.6%) | 49 (30.1%) | |
| Coronary artery disease [n (%)] | 59 (18.4%) | 33 (21.0%) | 26 (16.0%) | 0.243 |
| Pulmonary arterial hypertension [n (%)] | 90 (28.1%) | 17 (10.8%) | 73 (44.8%) | <0.001 |
| Valvular heart disease [n (%)] | 147 (45.9%) | 45 (28.7%) | 102 (62.6%) | <0.001 |
| Hypertension [n (%)] | 84 (26.3%) | 47 (29.9%) | 37 (22.7%) | 0.141 |
| Diabetes mellitus [n (%)] | 40 (12.5%) | 17 (10.8%) | 23 (14.1%) | 0.375 |
| Atrial fibrillation [n (%)] | 62 (19.4%) | 18 (11.5%) | 44 (27.0%) | <0.001 |
| Prior stroke/TIA [n (%)] | 28 (8.8%) | 15 (9.6%) | 13 (8.0%) | 0.617 |
| CHA2DS2VASc score [n (%)] | 0.103 | |||
| ≤1 | 112 (35.0%) | 48 (30.6%) | 64 (39.3%) | |
| ≥2 | 208 (65.0%) | 109 (69.4%) | 99 (60.7%) | |
| Echocardiography | ||||
| LAD (mm) | 43(37–49) | 37 (23–40) | 49 (45–53) | <0.001 |
| LVEDD (mm) | 63 (56–70) | 59 (53–65) | 67 (59–73) | <0.001 |
| LVEF (%) | 35 (27–52) | 43 (30–57) | 30 (25–40) | <0.001 |
| LVEF < 40% [n (%)] | 190 (59.4%) | 69 (44.0%) | 121 (74.2%) | <0.001 |
| Extent of noncompaction | ||||
| Apex-only involvement [n (%)] | 52 (17.5%) | 20 (12.7%) | 32 (19.6%) | 0.095 |
| Mid or basal involvement [n (%)] | 245 (82.5%) | 117 (74.5%) | 128 (78.5%) | 0.040 |
| Medications in hospital | ||||
| Antiplatelets [n (%)] | 133 (41.6%) | 74 (47.1%) | 59 (36.2%) | 0.024 |
| Oral anticoagulants [n (%)] | 91 (28.4%) | 37 (23.6%) | 54 (33.1%) | 0.079 |
| Digoxin [n (%)] | 141 (44.1%) | 49 (31.2%) | 92 (56.4%) | <0.001 |
| ACEI/ARB [n (%)] | 201 (62.8%) | 95 (60.5%) | 106 (65.0%) | 0.403 |
| ARNI [n (%)] | 29 (9.1%) | 12 (7.6%) | 17 (10.4%) | 0.385 |
| Beta blockers [n (%)] | 253 (79.1%) | 127 (80.9%) | 126 (77.3%) | 0.430 |
| CCBs [n (%)] | 43 (13.4%) | 18 (11.5%) | 25 (15.3%) | 0.310 |
| Diuretics [n (%)] | 266 (83.1%) | 115 (73.2%) | 151 (92.6%) | <0.001 |
| Statins [n (%)] | 101 (31.6%) | 53 (33.8%) | 48 (29.4%) | 0.407 |
| Outcomes | Total (n = 320) | LAD < 43 mm (n = 157) | LAD ≥ 43 mm (n = 163) | p-Value |
|---|---|---|---|---|
| Thromboembolism events [n (%)] | 23 (7.2%) | 5 (3.2%) | 18 (11.0%) | 0.007 |
| Intracardiac thrombi [n (%)] | 14 (4.4%) | 3 (1.9%) | 11 (6.8%) | 0.034 |
| Stroke/TIA [n (%)] | 11 (3.4%) | 2 (1.3%) | 9 (5.5%) | 0.037 |
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Thromboembolism events | 3.983 | 1.474–10.763 | 0.006 | 4.435 | 1.605–12.256 | 0.004 | 4.168 | 1.344–12.927 | 0.013 |
| Intracardiac thrombi | 3.842 | 1.067–13.829 | 0.039 | 4.212 | 1.132–15.673 | 0.032 | 3.611 | 0.890–14.662 | 0.072 |
| Stroke/TIA | 5.017 | 1.082–23.274 | 0.039 | 5.323 | 1.117–25.368 | 0.036 | 8.654 | 1.335–56.104 | 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Yang, Y.; Zhu, J.; Tan, J.; Wang, J.; Wang, L. Left Atrial Diameter and the Risk of Thromboembolism in Patients with Left Ventricular Noncompaction. J. Cardiovasc. Dev. Dis. 2022, 9, 426. https://doi.org/10.3390/jcdd9120426
Xu W, Yang Y, Zhu J, Tan J, Wang J, Wang L. Left Atrial Diameter and the Risk of Thromboembolism in Patients with Left Ventricular Noncompaction. Journal of Cardiovascular Development and Disease. 2022; 9(12):426. https://doi.org/10.3390/jcdd9120426
Chicago/Turabian StyleXu, Wei, Yanmin Yang, Jun Zhu, Jiangshan Tan, Jingyang Wang, and Lulu Wang. 2022. "Left Atrial Diameter and the Risk of Thromboembolism in Patients with Left Ventricular Noncompaction" Journal of Cardiovascular Development and Disease 9, no. 12: 426. https://doi.org/10.3390/jcdd9120426
APA StyleXu, W., Yang, Y., Zhu, J., Tan, J., Wang, J., & Wang, L. (2022). Left Atrial Diameter and the Risk of Thromboembolism in Patients with Left Ventricular Noncompaction. Journal of Cardiovascular Development and Disease, 9(12), 426. https://doi.org/10.3390/jcdd9120426






