Pulsed-Field Ablation Using a Novel Ablation-Mapping Integrated System for Pulmonary Vein Isolation—A Preliminary Animal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Procedure
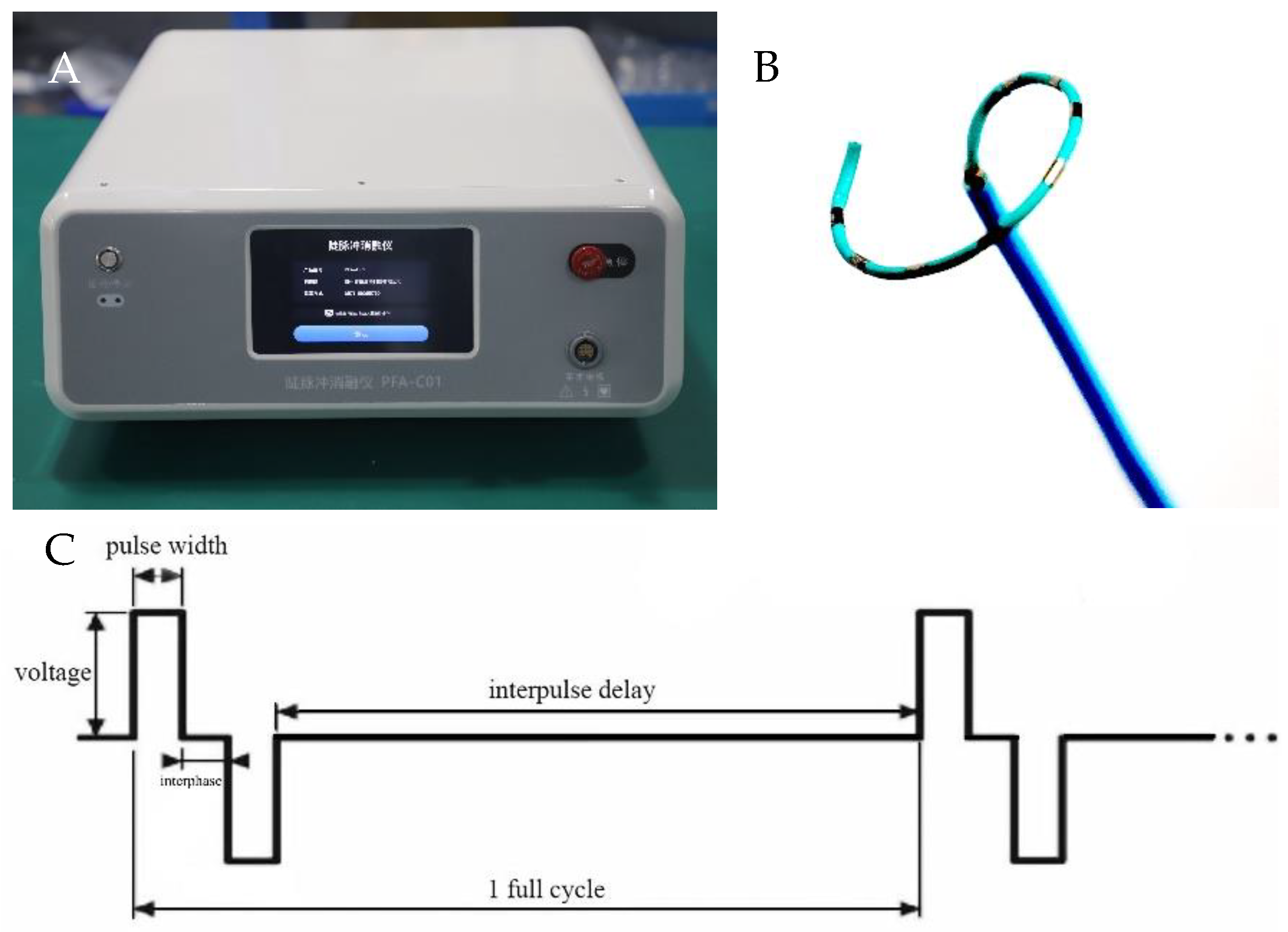
2.2. PFA System and Catheter
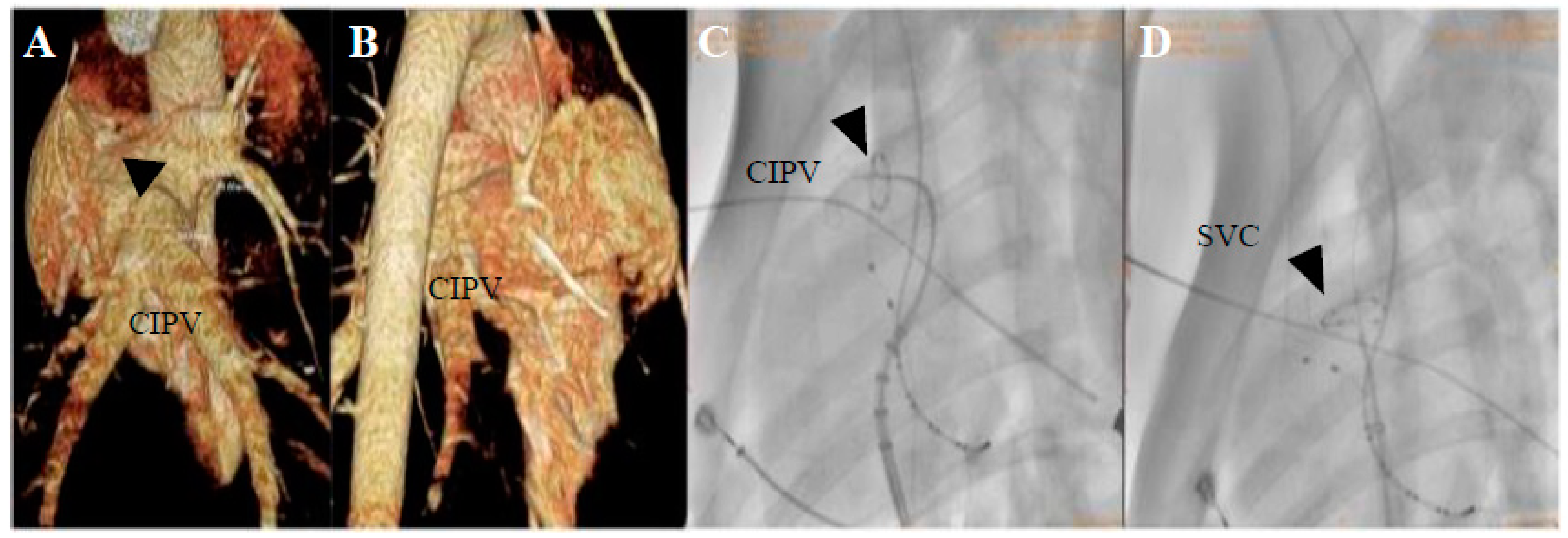
2.3. Mapping and Ablation
2.4. Gross Pathology and Pathological Analysis
2.5. Statistical Analysis
3. Results
3.1. PFA Applications and Dosages
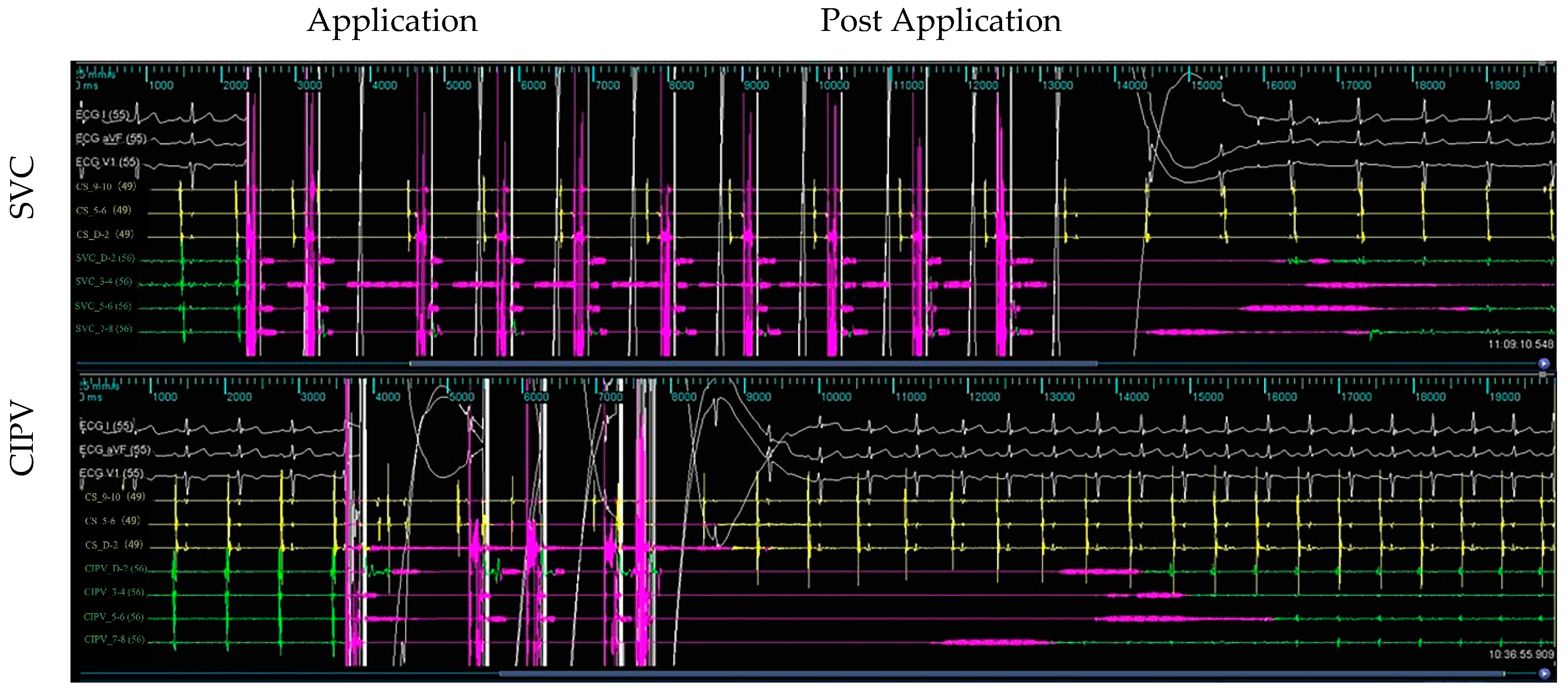
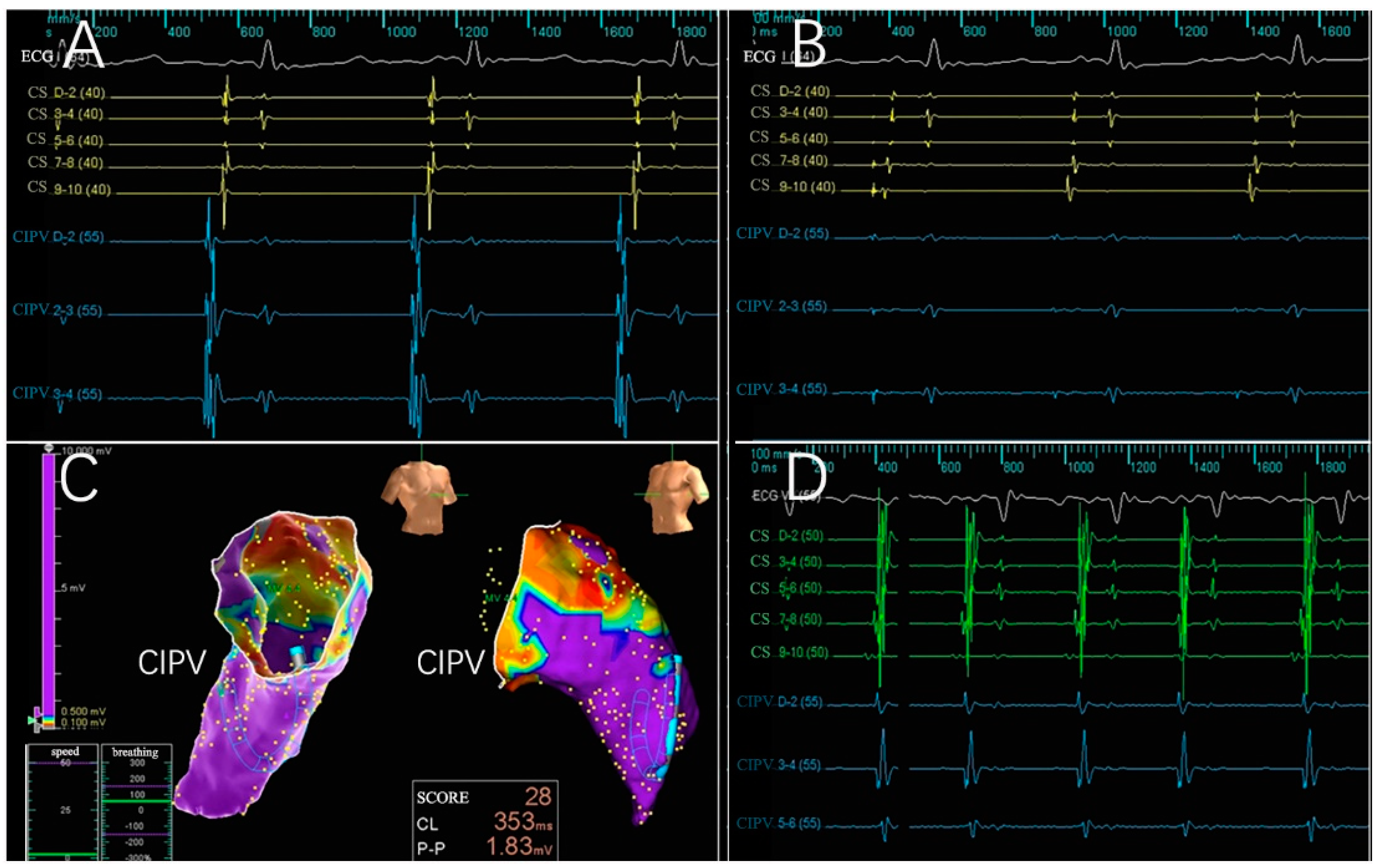
3.2. Electrogram, Voltage Mapping, and Complications
3.3. Gross Pathology
4. Discussion
4.1. Features of PFA Systems
4.2. Ablation Effectiveness of the PFA System
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef]
- Chao, T.F.; Joung, B.; Takahashi, Y.; Lim, T.W.; Choi, E.K.; Chan, Y.H.; Guo, Y.; Sriratanasathavorn, C.; Oh, S.; Okumura, K.; et al. 2021 Focused Update Consensus Guidelines of the Asia Pacific Heart Rhythm Society on Stroke Prevention in Atrial Fibrillation: Executive Summary. Thromb. Haemost. 2022, 122, 20–47. [Google Scholar] [CrossRef] [PubMed]
- Brundel, B.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial fibrillation. Nat. Rev. Dis. Primers 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Guo, L.; Xia, S.; Du, J.; Anderson, C.; Arima, H.; Huffman, M.; Yuan, Y.; Zheng, Y.; Wu, S.; et al. Atrial fibrillation prevalence, awareness and management in a nationwide survey of adults in China. Heart 2021, 107, 535–541. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- Kotalczyk, A.; Lip, G.Y.; Calkins, H. The 2020 ESC Guidelines on the Diagnosis and Management of Atrial Fibrillation. Arrhythm. Electrophysiol. Rev. 2021, 10, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Gibson, D.; Banker, R.; Doshi, S.K.; Gidney, B.; Gomez, T.; Berman, D.; Datta, K.; Govari, A.; Natale, A. In vivo porcine characterization of atrial lesion safety and efficacy utilizing a circular pulsed-field ablation catheter including assessment of collateral damage to adjacent tissue in supratherapeutic ablation applications. J. Cardiovasc. Electrophysiol. 2022, 33, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Bordignon, S.; Tohoku, S.; Chen, S.; Bologna, F.; Urbanek, L.; Pansera, F.; Ernst, M.; Chun, K.R.J. 5S Study: Safe and Simple Single Shot Pulmonary Vein Isolation With Pulsed Field Ablation Using Sedation. Circ. Arrhythm. Electrophysiol. 2022, 15, e010817. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.T.; Haines, D.E.; Miklavcic, D.; Kos, B.; Kirchhof, N.; Barka, N.; Mattison, L.; Martien, M.; Onal, B.; Howard, B.; et al. Safety and chronic lesion characterization of pulsed field ablation in a Porcine model. J. Cardiovasc. Electrophysiol. 2021, 32, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.T.; Haines, D.E.; Verma, A.; Kirchhof, N.; Barka, N.; Grassl, E.; Howard, B. Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model. Heart Rhythm. 2019, 16, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Koruth, J.; Kuroki, K.; Iwasawa, J.; Enomoto, Y.; Viswanathan, R.; Brose, R.; Buck, E.D.; Speltz, M.; Dukkipati, S.R.; Reddy, V.Y. Preclinical Evaluation of Pulsed Field Ablation: Electrophysiological and Histological Assessment of Thoracic Vein Isolation. Circ. Arrhythm. Electrophysiol. 2019, 12, e007781. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.; Haines, D.E.; Verma, A.; Packer, D.; Kirchhof, N.; Barka, N.; Onal, B.; Fraasch, S.; Miklavcic, D.; Stewart, M.T. Reduction in Pulmonary Vein Stenosis and Collateral Damage With Pulsed Field Ablation Compared With Radiofrequency Ablation in a Canine Model. Circ. Arrhythm. Electrophysiol. 2020, 13, e008337. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yao, C.; Rubinsky, B. A Conceivable Mechanism Responsible for the Synergy of High and Low Voltage Irreversible Electroporation Pulses. Ann. Biomed Eng. 2019, 47, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Chen, Y.G.; Ning, Z.P.; Chen, X.H. Advances in the application of pulsed field ablation for pulmonary vein isolation in patients with atrial fibrillation. Chin. J. Cardiol. 2020, 48, 990–992. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Wu, B.; Qiu, G.; Hong, L.; Chen, X.; Zhang, X. Study of necrotic apoptosis by pulsed electric field ablation in rabbit left ventricular myocardium. Front. Cardiovasc. Med. 2022, 9, 1012020. [Google Scholar] [CrossRef] [PubMed]
- Caluori, G.; Odehnalova, E.; Jadczyk, T.; Pesl, M.; Pavlova, I.; Valikova, L.; Holzinger, S.; Novotna, V.; Rotrekl, V.; Hampl, A.; et al. AC Pulsed Field Ablation Is Feasible and Safe in Atrial and Ventricular Settings: A Proof-of-Concept Chronic Animal Study. Front. Bioeng. Biotechnol. 2020, 8, 552357. [Google Scholar] [CrossRef] [PubMed]
- Neven, K.; Futing, A.; Byrd, I.; Heil, R.W., Jr.; Fish, J.M.; Feeney, D.A.; Donskoy, E.; Jensen, J.A. Absence of (sub-)acute cerebral events or lesions after electroporation ablation in the left-sided canine heart. Heart Rhythm. 2021, 18, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Kotb, A.; Chin, S.H.; Ng, G.A. Recent advances in the tools available for atrial fibrillation ablation. Expert Rev. Med. Devices 2022, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
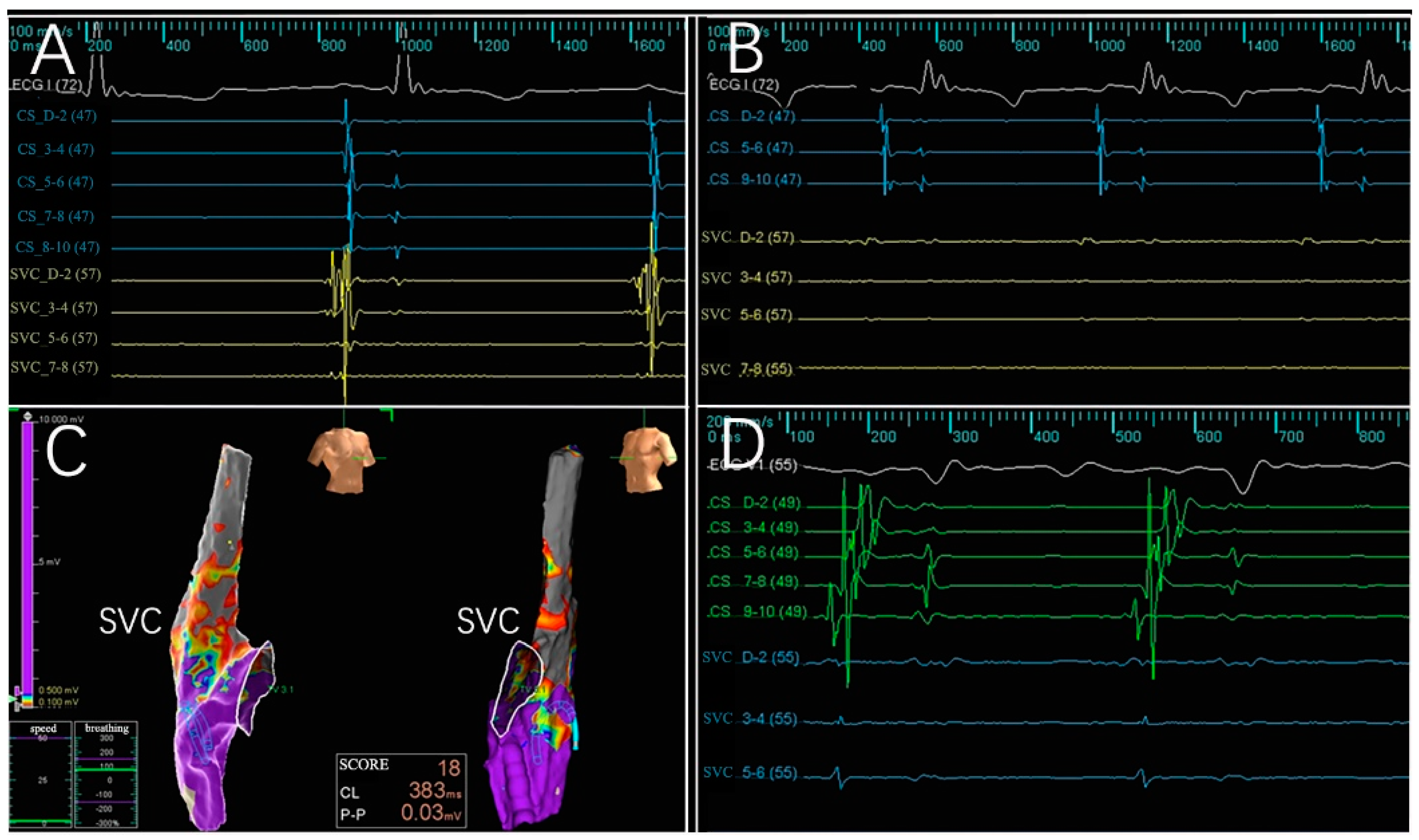
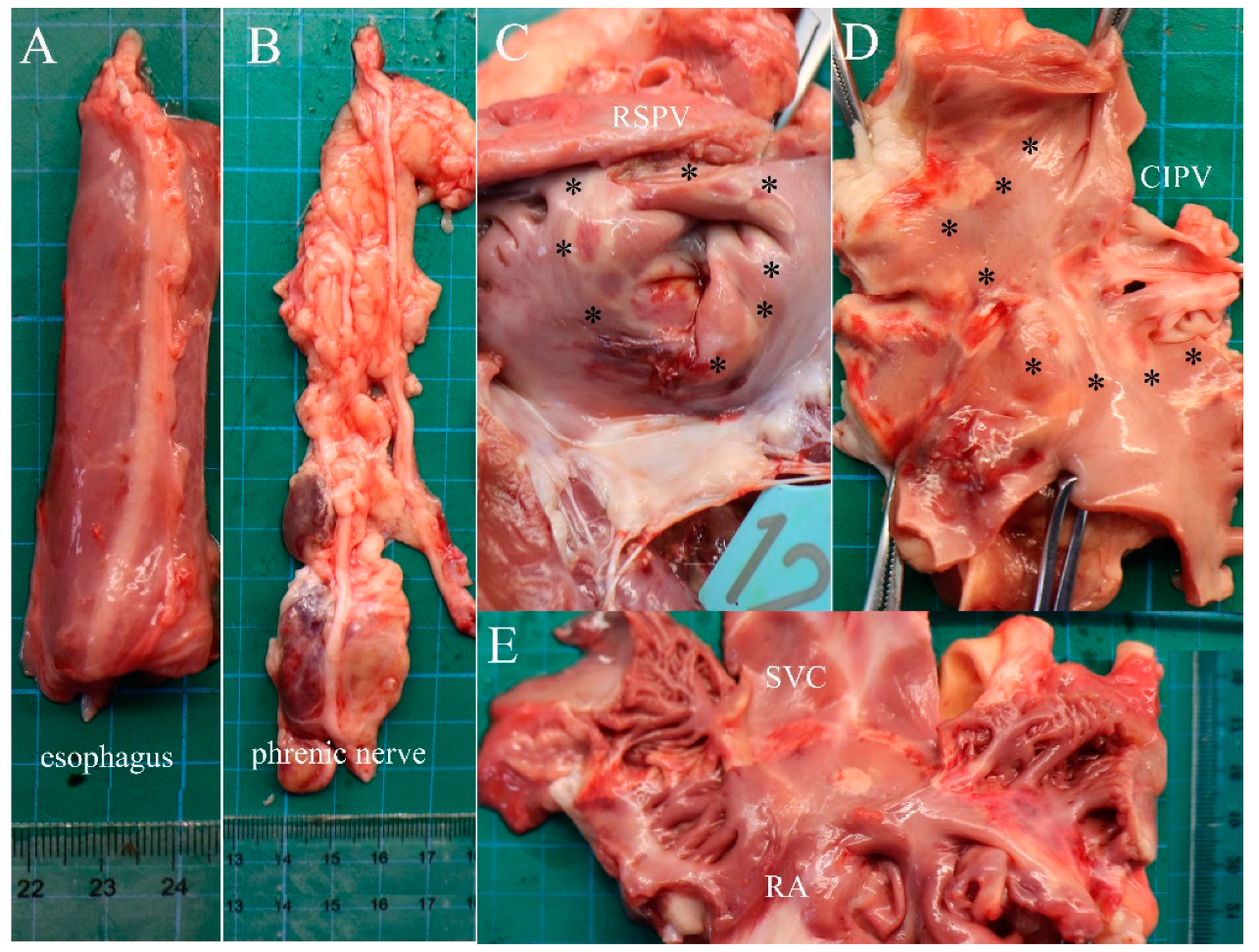
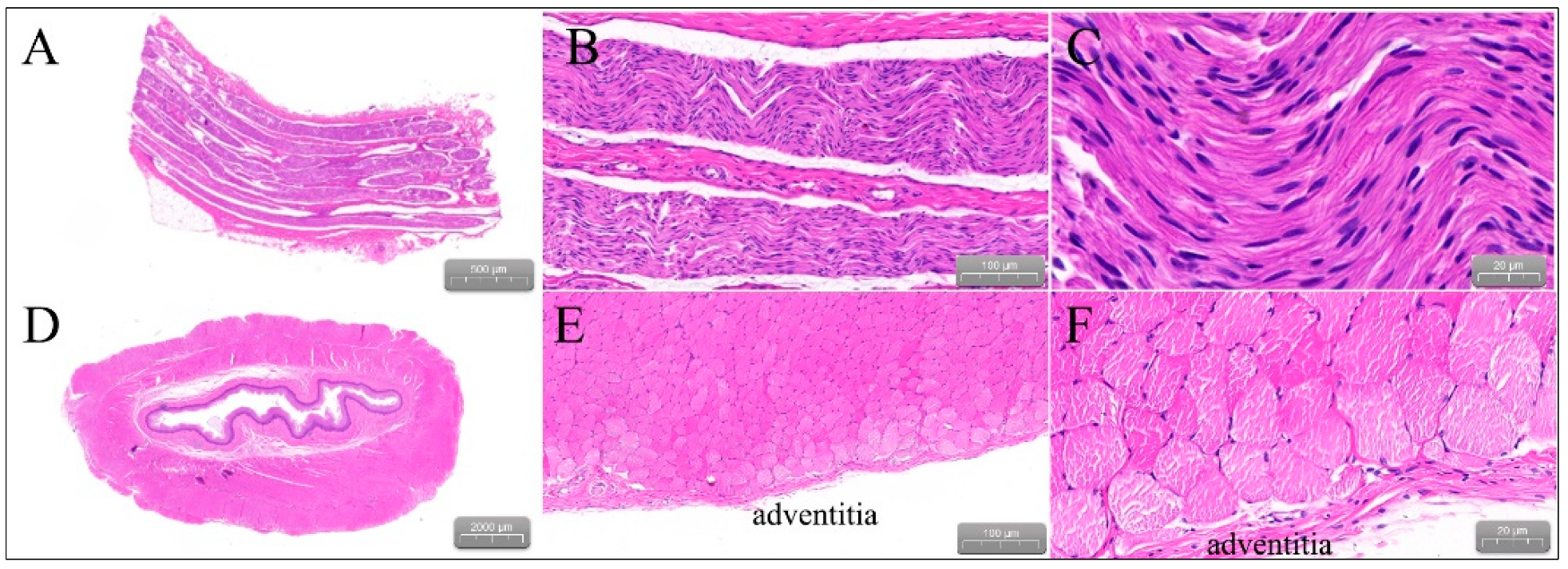


| Animal | Rationale for Number of Active Electrodes | Target Anatomic Region and Frequency of Ablation | Electrogram Amplitude Reduction > 50% | Atrial Arrhythmias | ||
|---|---|---|---|---|---|---|
| RSPV (n = 22) | CIPV (n = 22) | SVC (n = 22) | ||||
| #1 | 6-8, Circumferential | 2 | 2 | 2 | 83.3% | 0 |
| #2 | 6-8, Circumferential | 4 | 4 | 4 | 100% | 0 |
| #3 | 6-8, Circumferential | 4 | 4 | 4 | 100% | 1 |
| #4 | 6-8, Circumferential | 4 | 4 | 4 | 100% | 0 |
| #5 | 6-8, Circumferential | 4 | 4 | 4 | 100% | 0 |
| #6 | 6-8, Circumferential | 4 | 4 | 4 | 100% | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Chen, Y.; Wu, B.; Qiu, G.; Hong, L.; Chen, X.; Zhang, X. Pulsed-Field Ablation Using a Novel Ablation-Mapping Integrated System for Pulmonary Vein Isolation—A Preliminary Animal Study. J. Cardiovasc. Dev. Dis. 2022, 9, 425. https://doi.org/10.3390/jcdd9120425
Zhao Z, Chen Y, Wu B, Qiu G, Hong L, Chen X, Zhang X. Pulsed-Field Ablation Using a Novel Ablation-Mapping Integrated System for Pulmonary Vein Isolation—A Preliminary Animal Study. Journal of Cardiovascular Development and Disease. 2022; 9(12):425. https://doi.org/10.3390/jcdd9120425
Chicago/Turabian StyleZhao, Zhihong, Yonggang Chen, Bin Wu, Gaodong Qiu, Liangjie Hong, Xinhua Chen, and Xingwei Zhang. 2022. "Pulsed-Field Ablation Using a Novel Ablation-Mapping Integrated System for Pulmonary Vein Isolation—A Preliminary Animal Study" Journal of Cardiovascular Development and Disease 9, no. 12: 425. https://doi.org/10.3390/jcdd9120425
APA StyleZhao, Z., Chen, Y., Wu, B., Qiu, G., Hong, L., Chen, X., & Zhang, X. (2022). Pulsed-Field Ablation Using a Novel Ablation-Mapping Integrated System for Pulmonary Vein Isolation—A Preliminary Animal Study. Journal of Cardiovascular Development and Disease, 9(12), 425. https://doi.org/10.3390/jcdd9120425






