Hemodynamic Analysis Shows High Wall Shear Stress Is Associated with Intraoperatively Observed Thin Wall Regions of Intracranial Aneurysms
Abstract
1. Introduction
2. Methods
2.1. Patient Population
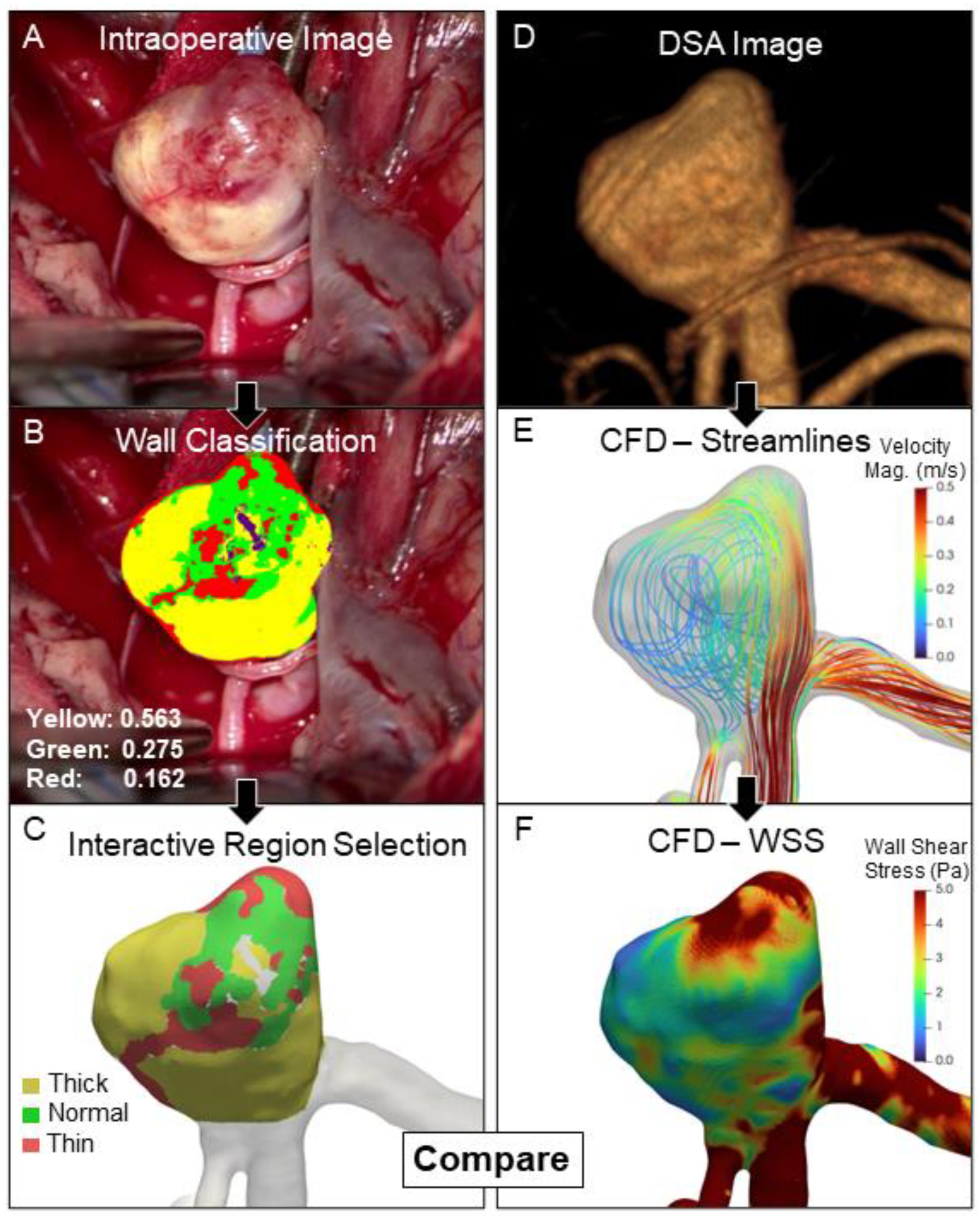
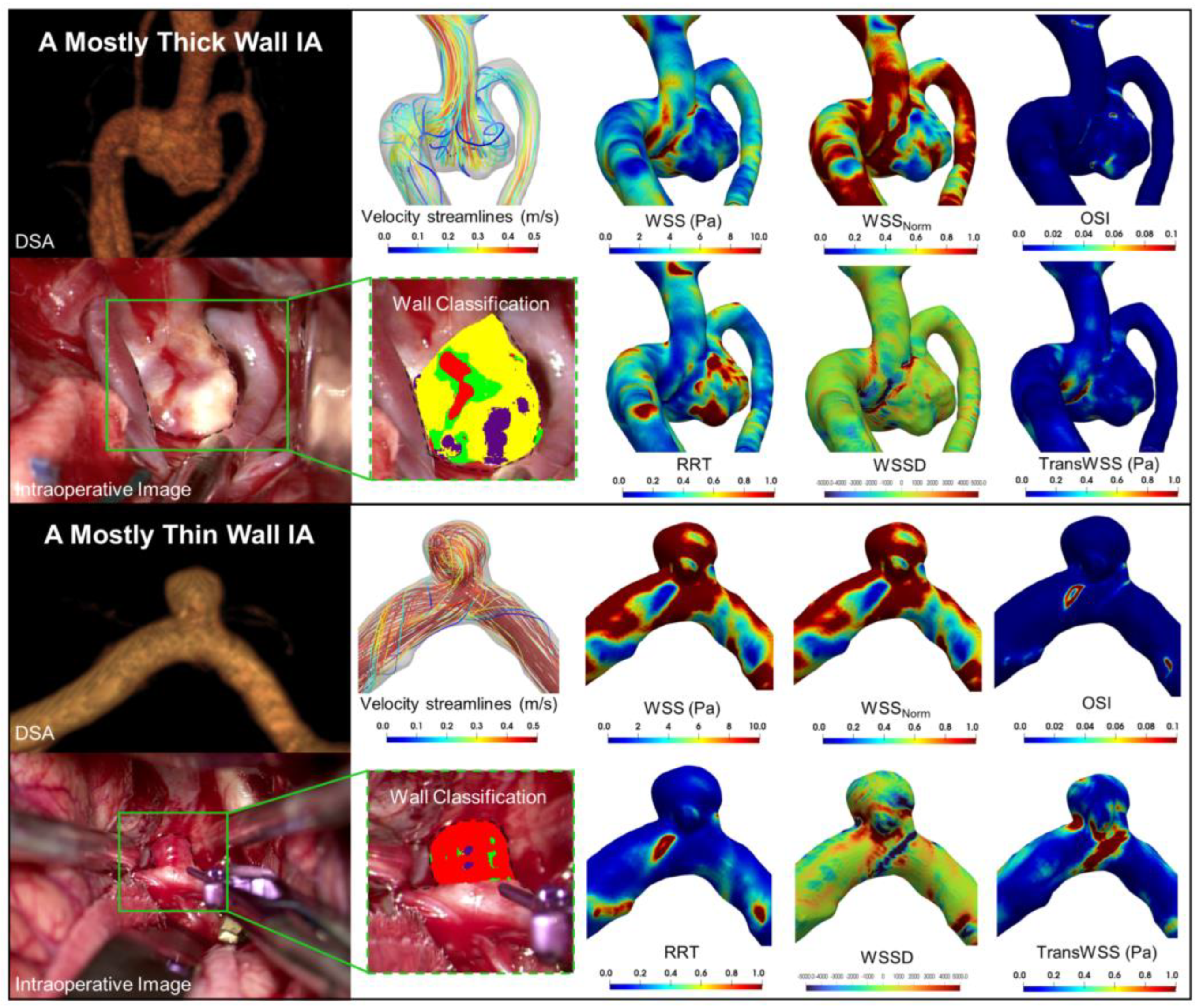
2.2. Delineation of Different IA Wall Types
2.3. Patient-Specific Hemodynamics
2.4. Image Co-Registration
2.5. Statistical Analysis
3. Results
3.1. Higher WSS in Thin and High RRT in Thick IA Wall Regions
3.2. Correlation between Hemodynamic Variables and Percentage Wall Type
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vlak, M.H.M.; Algra, A.; Brandenburg, R.; Rinkel, G.J.E. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Wiebers, D.O. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Etminan, N.; Rinkel, G.J. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat. Rev. Neurol. 2016, 12, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S.; Poussa, K.; Lehto, H.; Porras, M. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. Stroke J. Cereb. Circ. 2013, 44, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Metaxa, E.; Tremmel, M.; Natarajan, S.K.; Xiang, J.; Paluch, R.A.; Mandelbaum, M.; Siddiqui, A.H.; Kolega, J.; Mocco, J.; Meng, H. Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke J. Cereb. Circ. 2010, 41, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Tutino, V.M.; Xiang, J.; Siddiqui, A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. Am. J. Neuroradiol. 2014, 35, 1254–1262. [Google Scholar] [CrossRef]
- Cebral, J.R.; Detmer, F.; Chung, B.J.; Choque-Velasquez, J.; Rezai, B.; Lehto, H.; Tulamo, R.; Hernesniemi, J.; Niemela, M.; Yu, A.; et al. Local Hemodynamic Conditions Associated with Focal Changes in the Intracranial Aneurysm Wall. Am. J. Neuroradiol. 2019, 40, 510–516. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Q.; Wu, J.; Chen, X.; Li, M.; Li, Z.; Yang, S.; Guo, R.; Gao, B.; Cao, Y.; et al. Hemodynamic characteristics associated with thinner regions of intracranial aneurysm wall. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2019, 67, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.M.; Rajabzadeh-Oghaz, H.; Veeturi, S.S.; Poppenberg, K.E.; Waqas, M.; Mandelbaum, M.; Liaw, N.; Siddiqui, A.H.; Meng, H.; Kolega, J. Endogenous animal models of intracranial aneurysm development: A review. Neurosurg. Rev. 2021, 44, 2545–2570. [Google Scholar] [CrossRef]
- Furukawa, K.; Ishida, F.; Tsuji, M.; Miura, Y.; Kishimoto, T.; Shiba, M.; Tanemura, H.; Umeda, Y.; Sano, T.; Yasuda, R.; et al. Hemodynamic characteristics of hyperplastic remodeling lesions in cerebral aneurysms. PLoS ONE 2018, 13, e0191287. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.I.; Endo, H.; Niizuma, K.; Endo, T.; Funamoto, K.; Ohta, M.; Tominaga, T. Computational Hemodynamic Analysis for the Diagnosis of Atherosclerotic Changes in Intracranial Aneurysms: A Proof-of-Concept Study Using 3 Cases Harboring Atherosclerotic and Nonatherosclerotic Aneurysms Simultaneously. Comput. Math. Methods Med. 2016, 2016, 2386031. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Funamoto, K.; Sugiyama, S.; Nakayama, T.; Hayase, T.; Tominaga, T. Investigation of characteristic hemodynamic parameters indicating thinning and thickening sites of cerebral aneurysms. J. Biomech. Sci. Eng. 2015, 10, 14–00265. [Google Scholar] [CrossRef]
- Kadasi, L.M.; Dent, W.C.; Malek, A.M. Cerebral aneurysm wall thickness analysis using intraoperative microscopy: Effect of size and gender on thin translucent regions. J. Neurointerv. Surg. 2013, 5, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kadasi, L.M.; Dent, W.C.; Malek, A.M. Colocalization of thin-walled dome regions with low hemodynamic wall shear stress in unruptured cerebral aneurysms. J. Neurosurg. 2013, 119, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Talari, S.; Kato, Y.; Shang, H.; Yamada, Y.; Yamashiro, K.; Suyama, D.; Kawase, T.; Balik, V.; Rile, W. Comparison of computational fluid dynamics findings with intraoperative microscopy findings in unruptured intracranial aneurysms—An initial analysis. Asian J. Neurosurg. 2016, 11, 356–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajabzadeh-Oghaz, H.; van Ooij, P.; Veeturi, S.S.; Tutino, V.M.; Zwanenburg, J.J.; Meng, H. Inter-patient variations in flow boundary conditions at middle cerebral artery from 7T PC-MRI and influence on Computational Fluid Dynamics of intracranial aneurysms. Comput. Biol. Med. 2020, 120, 103759. [Google Scholar] [CrossRef]
- Stritt, M.; Stalder, A.K.; Vezzali, E. Orbit Image Analysis: An open-source whole slide image analysis tool. PLoS Comput. Biol. 2020, 16, e1007313. [Google Scholar] [CrossRef]
- Veeturi, S.S.; Rajabzadeh-Oghaz, H.; Pintér, N.K.; Waqas, M.; Hasan, D.M.; Snyder, K.V.; Siddiqui, A.H.; Tutino, V.M. Aneurysm risk metrics and hemodynamics are associated with greater vessel wall enhancement in intracranial aneurysms. R. Soc. Open Sci. 2021, 8, 211119. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Cancelliere, N.M.; Brina, O.; Bouillot, P.; Vargas, M.I.; Delattre, B.M.; Pereira, V.M.; Steinman, D.A. How patient-specific do internal carotid artery inflow rates need to be for computational fluid dynamics of cerebral aneurysms? J. Neurointerv. Surg. 2021, 13, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.O.; Steinman, D.A.; Valen-Sendstad, K. Non-Newtonian versus numerical rheology: Practical impact of shear-thinning on the prediction of stable and unstable flows in intracranial aneurysms. Int. J. Numer. Methods Biomed. Eng. 2017, 33, e2836. [Google Scholar] [CrossRef] [PubMed]
- Chnafa, C.; Brina, O.; Pereira, V.M.; Steinman, D.A. Better Than Nothing: A Rational Approach for Minimizing the Impact of Outflow Strategy on Cerebrovascular Simulations. Am. J. Neuroradiol. 2018, 39, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing Co.: Monterey, CA, USA, 1996. [Google Scholar]
- Jiang, P.; Liu, Q.; Wu, J.; Chen, X.; Li, M.; Yang, F.; Li, Z.; Yang, S.; Guo, R.; Gao, B.; et al. Hemodynamic findings associated with intraoperative appearances of intracranial aneurysms. Neurosurg. Rev. 2020, 43, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Niizuma, K.; Nakayama, T.; Shimizu, H.; Endo, H.; Inoue, T.; Fujimura, M.; Ohta, M.; Takahashi, A.; Tominaga, T. Relative residence time prolongation in intracranial aneurysms: A possible association with atherosclerosis. Neurosurgery 2013, 73, 767–776. [Google Scholar] [CrossRef]
- Cho, K.C.; Choi, J.H.; Oh, J.H.; Kim, Y.B. Prediction of Thin-Walled Areas of Unruptured Cerebral Aneurysms through Comparison of Normalized Hemodynamic Parameters and Intraoperative Images. BioMed Res. Int. 2018, 2018, 3047181. [Google Scholar] [CrossRef] [PubMed]
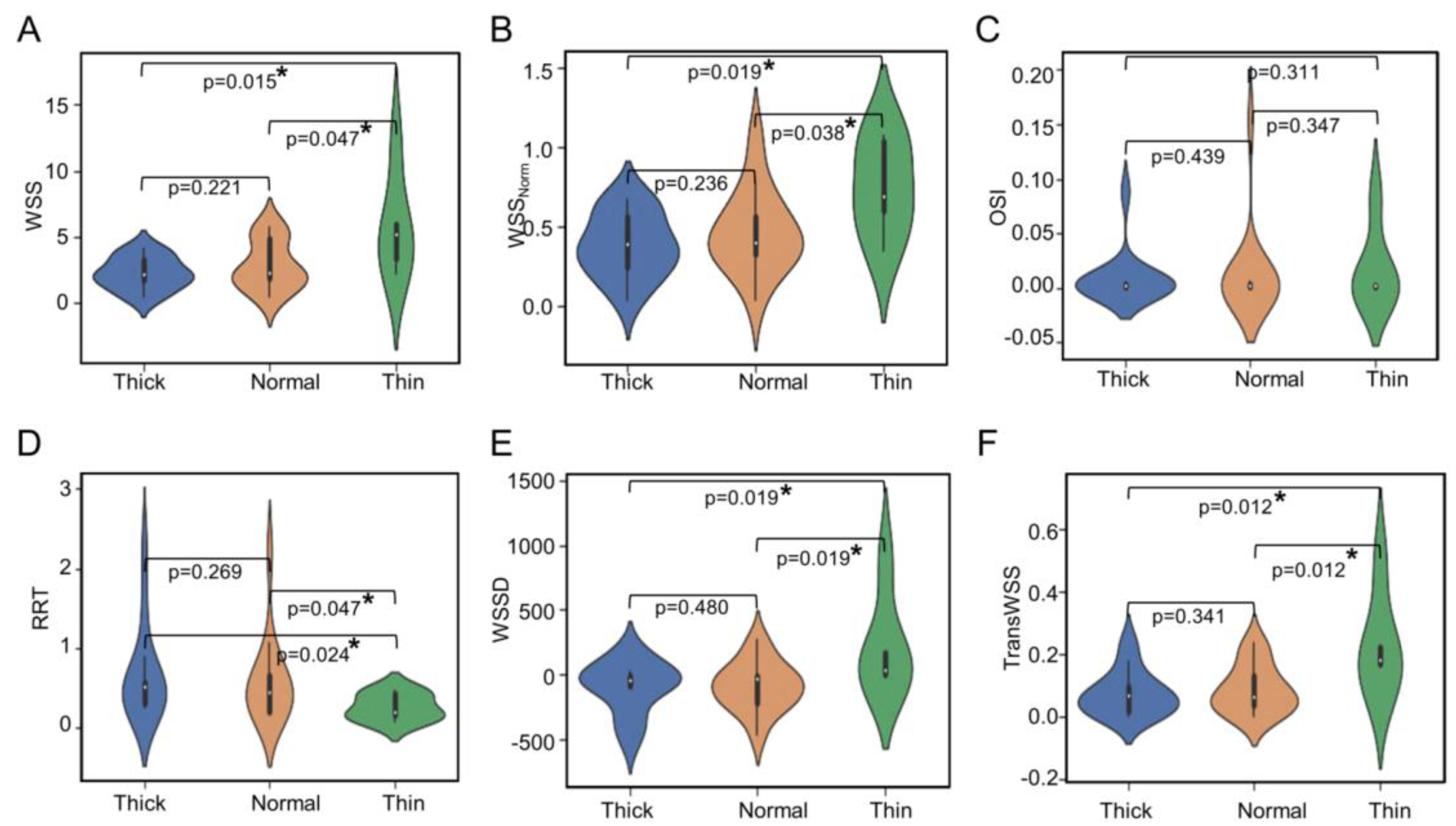
| Parameter | Thick (Median ± IQR) | Normal (Median ± IQR) | Thin (Median ± IQR) | p-Value |
|---|---|---|---|---|
| WSS (Pa) | 2.201 ± 1.507 | 2.268 ± 2.939 | 5.236 ± 2.625 | 0.074 |
| WSSNorm | 0.388 ± 0.317 | 0.400 ± 0.236 | 0.687 ± 0.435 | 0.029 * |
| OSI | 0.002 ± 0.003 | 0.002 ± 0.003 | 0.002 ± 0.002 | 0.853 |
| RRT (Pa−1) | 0.517 ± 0.269 | 0.444 ± 0.454 | 0.191 ± 0.264 | 0.104 |
| WSSD (Pa/m) | −41.158 ± 81.062 | −35.280 ± 213.057 | 31.912 ± 165.988 | 0.069 |
| TransWSS (Pa) | 0.068 ± 0.077 | 0.064 ± 0.092 | 0.182 ± 0.086 | 0.049 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veeturi, S.S.; Patel, T.R.; Baig, A.A.; Chien, A.; Monteiro, A.; Waqas, M.; Snyder, K.V.; Siddiqui, A.H.; Tutino, V.M. Hemodynamic Analysis Shows High Wall Shear Stress Is Associated with Intraoperatively Observed Thin Wall Regions of Intracranial Aneurysms. J. Cardiovasc. Dev. Dis. 2022, 9, 424. https://doi.org/10.3390/jcdd9120424
Veeturi SS, Patel TR, Baig AA, Chien A, Monteiro A, Waqas M, Snyder KV, Siddiqui AH, Tutino VM. Hemodynamic Analysis Shows High Wall Shear Stress Is Associated with Intraoperatively Observed Thin Wall Regions of Intracranial Aneurysms. Journal of Cardiovascular Development and Disease. 2022; 9(12):424. https://doi.org/10.3390/jcdd9120424
Chicago/Turabian StyleVeeturi, Sricharan S., Tatsat R. Patel, Ammad A. Baig, Aichi Chien, Andre Monteiro, Muhammad Waqas, Kenneth V. Snyder, Adnan H. Siddiqui, and Vincent M. Tutino. 2022. "Hemodynamic Analysis Shows High Wall Shear Stress Is Associated with Intraoperatively Observed Thin Wall Regions of Intracranial Aneurysms" Journal of Cardiovascular Development and Disease 9, no. 12: 424. https://doi.org/10.3390/jcdd9120424
APA StyleVeeturi, S. S., Patel, T. R., Baig, A. A., Chien, A., Monteiro, A., Waqas, M., Snyder, K. V., Siddiqui, A. H., & Tutino, V. M. (2022). Hemodynamic Analysis Shows High Wall Shear Stress Is Associated with Intraoperatively Observed Thin Wall Regions of Intracranial Aneurysms. Journal of Cardiovascular Development and Disease, 9(12), 424. https://doi.org/10.3390/jcdd9120424






