Physical Activity, Sedentary Behavior and Sleep Time: Association with Cardiovascular Hemodynamic Parameters, Blood Pressure and Structural and Functional Arterial Properties in Childhood
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Anthropometric Evaluation
2.3. Cardiovascular Evaluation
2.4. Peripheral and Central Pressure and Aortic Wave-Derived Parameters
2.5. Regional Arterial Stiffness: PWV (cfPWV and crPWV) and PWV Ratio
2.6. Arterial Diameter, Intima-Media Thickness and Local Arterial Stiffness
2.7. Carotid, Femoral and Brachial Doppler-Derived Blood Velocity
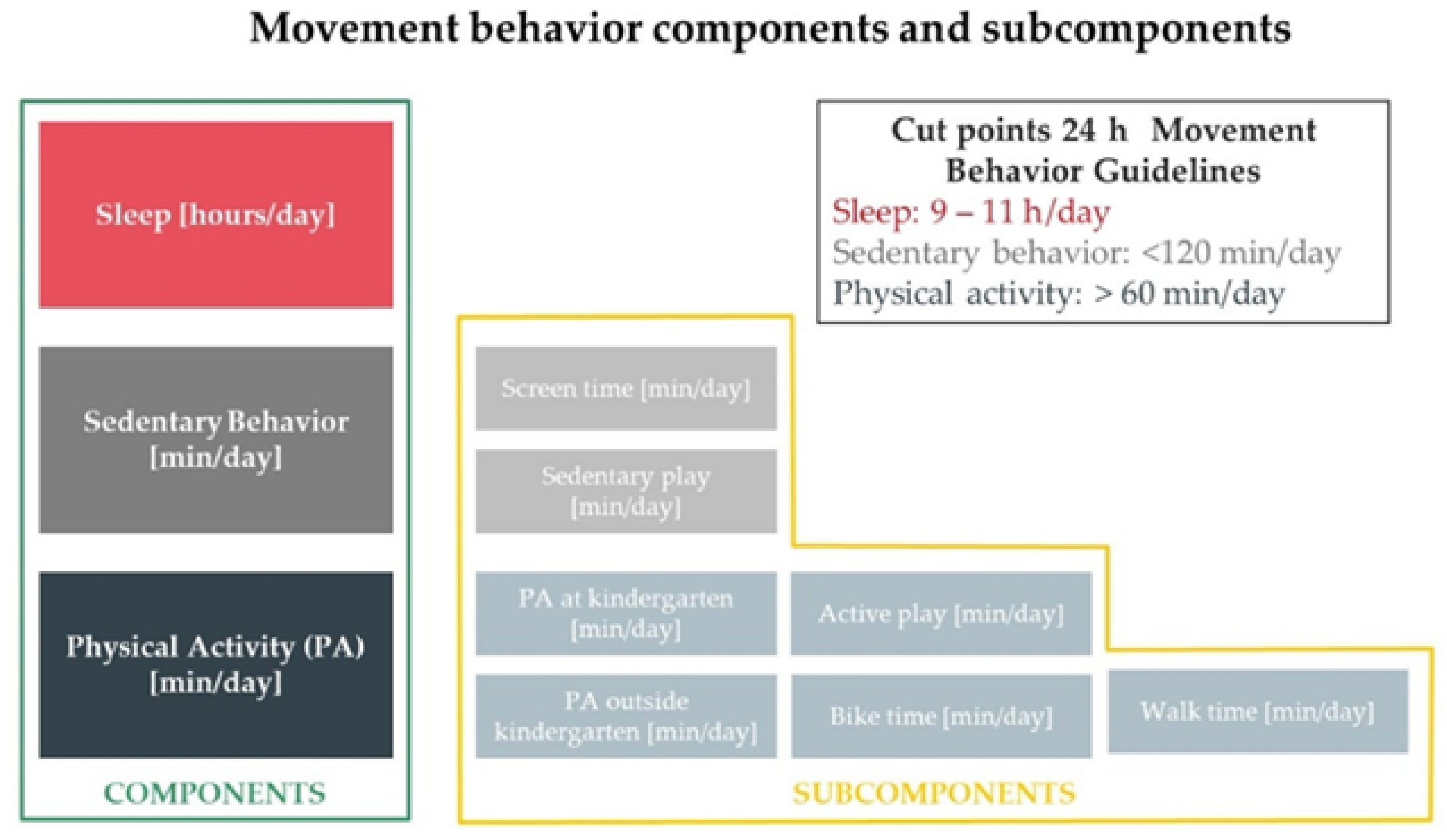
2.8. Movement Behavior Evaluation
2.9. Data Analysis
3. Results
3.1. Movement Behavior, Blood Pressure and Arterial Characteristics’ Associations
3.2. Sedentary Behavior and Cardiovascular Properties: Independent Associations
3.3. Sleep Time and Cardiovascular Properties: Independent Associations
3.4. Physical Activity and Cardiovascular Properties: Independent Associations
4. Discussion
4.1. Sedentary Behavior and Cardiovascular Inter-Individual Variations: Independent Associations
4.1.1. SB and Central and Peripheral Blood Pressure
4.1.2. SB and Arterial Structural and Functional Parameters
4.2. Sleep Time and Cardiovascular Inter-Individual Variations
4.3. Physical Activity and Cardiovascular Inter-Individual Variations
4.3.1. PA and Central Pressure, Peripheral Pressure and Blood Flow Velocities
4.3.2. PA and Arterial Structural Parameters
4.3.3. PA and Arterial Functional Parameters
4.4. Movement Behavior and Cardiovascular Properties: Independence and Impact on Different Arteries
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI | Ankle Brachial index |
| AIx@75 | AIx adjusted to a 75 beats/min heart rate |
| aoBP | Central (aortic) blood pressure |
| aoDBP | Aortic diastolic blood pressure |
| aoPP | Aortic pulse pressure |
| aoSBP | Aortic systolic blood pressure |
| BA | Brachial artery |
| baBP | Brachial artery blood pressure |
| baDBP | Brachial artery (peripheral) diastolic blood pressure |
| baPP | Brachial artery pulse pressure |
| baSBP | Brachial artery (peripheral) systolic blood pressure |
| BH | Body height |
| BMI | Body mass index |
| BP | Blood pressure |
| BW | Body weight |
| CCA | Common carotid artery |
| CFA | Common femoral artery |
| cfPWV | Carotid-femoral pulse wave velocity |
| CRFs | Cardiovascular risk factors |
| crPWV | Carotid-radial pulse wave velocity |
| CV | Cardiovascular |
| DD | End-diastolic arterial diameter |
| EDV | End-diastolic blood flow velocity |
| EM | Pressure-strain or Petrson arterial elastic modulus |
| IMT | Intima-media thickness |
| MB | Movement behavior |
| MBP | Brachial artery mean blood pressure |
| MET | Metabolic equivalent |
| MOG | Mobil-O-Graph PWA-monitor device (MOG; I.E.M.-GmbH, Stolberg, Germany) |
| PA | Physical activity |
| Pb | Amplitude of the aortic pressure waveform backward component |
| Pf | Amplitude of the aortic pressure waveform forward component |
| PSV | Peak systolic blood flow velocity |
| raDBP | Radial artery diastolic blood pressure |
| raPP | Radial artery pulse pressure |
| raSBP | Radial artery systolic blood pressure |
| SB | Sedentary behavior |
| SCOR | SphygmoCor-CvMS device (SCOR; v.9, AtCor-Medical, Australia) |
| ST | Sleep time |
| SystD | Peak systolic arterial diameter |
| taDBP | Tibial artery diastolic blood pressure |
| taPP | Tibial artery pulse pressure |
| taSBP | Tibial artery systolic blood pressure |
| β | Beta or stiffness (local) index |
References
- Mora, S.; Cook, N.; Buring, J.E.; Ridker, P.M.; Lee, I.M. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation 2007, 116, 2110–2118. [Google Scholar] [CrossRef]
- Idris, N.S.; Evelein, A.M.; Geerts, C.C.; Sastroasmoro, S.; Grobbee, D.E.; Uiterwaal, C.S. Effect of physical activity on vascular characteristics in young children. Eur. J. Prev. Cardiol. 2015, 22, 656–664. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Carson, V.; Chaput, J.P.; Connor Gorber, S.; Dinh, T.; Duggan, M.; Faulkner, G.; Gray, C.E.; Gruber, R.; Janson, K.; et al. Canadian 24-Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. Appl. Physiol. Nutr. Metab. 2016, 41, S311–S327. [Google Scholar] [CrossRef] [PubMed]
- Rollo, S.; Antsygina, O.; Tremblay, M.S. The whole day matters: Understanding 24-hour movement-guideline adherence and relationships with health indicators across the lifespan. J. Sport Health Sci. 2020, 9, 493–510. [Google Scholar] [CrossRef] [PubMed]
- AGDH. Australian 24-Hour Movement Guidelines for Children and Young People (5–17 years): An Integration of Physical Activity, Sedentary Behaviour, and Sleep; Australian Goverment Department of Health: Canberra, Australia, 2019.
- Saunders, T.J.; Gray, C.E.; Poitras, V.J.; Chaput, J.P.; Janssen, I.; Katzmarzyk, P.T.; Olds, T.; Connor Gorber, S.; Kho, M.E.; Sampson, M.; et al. Combinations of physical activity, sedentary behaviour and sleep: Relationships with health indicators in school-aged children and youth. Appl. Physiol. Nutr. Metab. 2016, 41, S283–S293. [Google Scholar] [CrossRef] [PubMed]
- Carson, V.; Chaput, J.P.; Janssen, I.; Tremblay, M. Health associations with meeting new 24-hour movement guidelines for Canadian children and youth. Prev. Med. 2017, 95, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Roman-Viñas, B.; Chaput, J.-P.; Katzmarzyk, P.T.; Fogelholm, M.; Lambert, E.V.; Maher, C.; Maia, J.; Olds, T.; Onywera, V.; Sarmiento, O.L. Proportion of children meeting recommendations for 24-hour movement guidelines and associations with adiposity in a 12-country study. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 123. [Google Scholar] [CrossRef]
- Chaput, J.P.; Willumsen, J.; Bull, F.; Chou, R.; Ekelund, U.; Firth, J.; Jago, R.; Ortega, F.B.; Katzmarzyk, P.T. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5–17 years: Summary of the evidence. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Castro, J.M.; Garcia-Espinosa, V.; Zinoveev, A.; Marin, M.; Severi, C.; Chiesa, P.; Bia, D.; Zocalo, Y. Arterial structural and functional characteristics at end of early childhood and beginning of adulthood: Impact of body size gain during early, intermediate, late and global growth. J. Cardiovasc. Dev. Dis. 2019, 6, 33. [Google Scholar] [CrossRef]
- Garcia-Espinosa, V.; Bia, D.; Castro, J.; Zinoveev, A.; Marin, M.; Giachetto, G.; Chiesa, P.; Zócalo, Y. Peripheral and Central Aortic Pressure, Wave-Derived Reflection Parameters, Local and Regional Arterial Stiffness and Structural Parameters in Children and Adolescents: Impact of Body Mass Index Variations. High Blood Press. Cardiovasc. Prev. 2018, 25, 267–280. [Google Scholar] [CrossRef]
- Zócalo, Y.; Marotta, M.; García-Espinosa, V.; Curcio, S.; Chiesa, P.; Giachetto, G.; Bia, D. Children and Adolescents Macrovascular Reactivity Level and Dynamics, But Not the Microvascular Response, is Associated with Body Mass Index and Arterial Stiffness Levels. High Blood Press. Cardiovasc. Prev. 2017, 24, 371–386. [Google Scholar] [CrossRef] [PubMed]
- García-Espinosa, V.; Curcio, S.; Castro, J.M.; Arana, M.; Giachetto, G.; Chiesa, P.; Zócalo, Y.; Bia, D. Children and Adolescent Obesity Associates with Pressure-Dependent and Age-Related Increase in Carotid and Femoral Arteries’ Stiffness and Not in Brachial Artery, Indicative of Nonintrinsic Arterial Wall Alteration. Int. J. Vasc. Med. 2016, 4916246. [Google Scholar] [CrossRef]
- Castro, J.M.; García-Espinosa, V.; Curcio, S.; Arana, M.; Chiesa, P.; Giachetto, G.; Zócalo, Y.; Bia, D. Childhood Obesity Associates Haemodynamic and Vascular Changes That Result in Increased Central Aortic Pressure with Augmented Incident and Reflected Wave Components, without Changes in Peripheral Amplification. Int. J. Vasc. Med. 2016, 3129304. [Google Scholar] [CrossRef]
- Pahkala, K.; Laitinen, T.T.; Heinonen, O.J.; Viikari, J.S.A.; Ronnemaa, T.; Niinikoski, H.; Helajarvi, H.; Juonala, M.; Simell, O.; Raitakari, O.T. Association of Fitness With Vascular Intima-Media Thickness and Elasticity in Adolescence. Pediatrics 2013, 132, e77–e84. [Google Scholar] [CrossRef]
- Nettlefold, L.; McKay, H.A.; Naylor, P.-J.; Bredin, S.S.D.; Warburton, D.E.R. The Relationship Between Objectively Measured Physical Activity, Sedentary Time, and Vascular Health in Children. Am. J. Hypertens. 2012, 25, 914–919. [Google Scholar] [CrossRef]
- Ried-Larsen, M.; Grontved, A.; Moller, N.C.; Larsen, K.T.; Froberg, K.; Andersen, L.B. Associations between objectively measured physical activity intensity in childhood and measures of subclinical cardiovascular disease in adolescence: Prospective observations from the European Youth Heart Study. Br. J. Sports Med. 2014, 48, 1502–1507. [Google Scholar] [CrossRef]
- Ried-Larsen, M.; Grontved, A.; Ostergaard, L.; Cooper, A.R.; Froberg, K.; Andersen, L.B.; Moller, N.C. Associations between bicycling and carotid arterial stiffness in adolescents: The European Youth Hearts Study. Scand. J. Med. Sci. Sports 2015, 25, 661–669. [Google Scholar] [CrossRef]
- Santana, D.B.; Zócalo, Y.A.; Armentano, R. Integrated e-Health approach based on vascular ultrasound and pulse wave analysis for asymptomatic atherosclerosis detection and cardiovascular risk stratification in the community. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 287–294. [Google Scholar] [CrossRef]
- Santana, D.B.; Zócalo, Y.A.; Ventura, I.F.; Arrosa, J.F.T.; Florio, L.; Lluberas, R.; Armentano, R. Health informatics design for assisted diagnosis of subclinical atherosclerosis, structural, and functional arterial age calculus and patient-specific cardiovascular risk evaluation. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Bia, D.; Zócalo, Y. Physiological Age- and Sex-Related Profiles for Local (Aortic) and Regional (Carotid-Femoral, Carotid-Radial) Pulse Wave Velocity and Center-to-Periphery Stiffness Gradient, with and without Blood Pressure Adjustments: Reference Intervals and Agreement between Methods in Healthy Subjects (3–84 Years). J. Cardiovasc. Dev. Dis. 2021, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Zócalo, Y.; Bia, D. Ultrasonografía carotídea para detección de placas de ateroma y medición del espesor íntima-media; índice tobillo-brazo: Evaluación no invasiva en la práctica clínica: Importancia clínica y análisis de las bases metodológicas para su evaluación. Rev. Urug. Cardiol. 2016, 31, 47–60. [Google Scholar]
- Zinoveev, A.; Castro, J.M.; Garcia-Espinosa, V.; Marin, M.; Chiesa, P.; Bia, D.; Zocalo, Y. Aortic pressure and forward and backward wave components in children, adolescents and young-adults: Agreement between brachial oscillometry, radial and carotid tonometry data and analysis of factors associated with their differences. PLoS ONE 2019, 14, e0226709. [Google Scholar] [CrossRef] [PubMed]
- Zocalo, Y.; Garcia-Espinosa, V.; Castro, J.M.; Zinoveev, A.; Marin, M.; Chiesa, P.; Diaz, A.; Bia, D. Stroke volume and cardiac output non-invasive monitoring based on brachial oscillometry-derived pulse contour analysis: Explanatory variables and reference intervals throughout life (3–88 years). Cardiol. J. 2020. [Google Scholar] [CrossRef]
- Bia, D.; Zócalo, Y. Rigidez arterial: Evaluación no invasiva en la práctica clínica Importancia clínica y análisis de las bases metodológicas de los equipos disponibles para su evaluación. Rev. Urug. Cardiol. 2014, 29, 39–59. [Google Scholar]
- Fortier, C.; Sidibé, A.; Desjardins, M.P.; Marquis, K.; De Serres, S.A.; Mac-Way, F.; Agharazii, M. Aortic-Brachial Pulse Wave Velocity Ratio: A Blood Pressure-Independent Index of Vascular Aging. Hypertension 2017, 69, 96–101. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, J.; Norris, S.; Cameron, N.; Pettifor, J. Criterion validity and test-retest reliability of a physical activity questionnaire in south African primary school aged children. J. Sports Med. 2012, 24, 43–48. [Google Scholar]
- McVeigh, J.; Meiring, R. Physical activity and sedentary behavior in an ethnically diverse group of South african school children. J. Sports Sci. Med. 2014, 13, 371–378. [Google Scholar]
- Hanson, S.K.; Munthali, R.J.; Micklesfield, L.K.; Lobelo, F.; Cunningham, S.A.; Hartman, T.J.; Norris, S.A.; Stein, A.D. Longitudinal patterns of physical activity, sedentary behavior and sleep in urban South African adolescents, Birth-To-Twenty Plus cohort. BMC Pediatr. 2019, 19, 241. [Google Scholar] [CrossRef]
- Butte, N.F.; Watson, K.B.; Ridley, K.; Zakeri, I.F.; McMurray, R.G.; Pfeiffer, K.A.; Crouter, S.E.; Herrmann, S.D.; Bassett, D.R.; Long, A.; et al. A Youth Compendium of Physical Activities: Activity Codes and Metabolic Intensities. Med. Sci. Sports Exerc. 2018, 50, 246–256. [Google Scholar] [CrossRef]
- Ridley, K.; Ainsworth, B.E.; Olds, T.S. Development of a compendium of energy expenditures for youth. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 45. [Google Scholar] [CrossRef]
- Sasaki, J.E.; Howe, C.; John, D.; Hickey, A.; Steeves, J.; Conger, S.; Lyden, K.; Kozey-Keadle, S.; Burkart, S.; Alhassan, S.; et al. Energy Expenditure for 70 Activities in Children and Adolescents. J. Phys. Act. Health 2016, 13, S24–S28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saint-Maurice, P.F.; Kim, Y.; Welk, G.J.; Gaesser, G.A. Kids are not little adults: What MET threshold captures sedentary behavior in children? Eur. J. Appl. Physiol. 2016, 116, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lumley, T.; Diehr, P.; Emerson, S.; Chen, L. The importance of the normality assumption in large public health data sets. Annu. Rev. Public Health 2002, 23, 151–169. [Google Scholar] [CrossRef]
- Martinez-Gomez, D.; Tucker, J.; Heelan, K.A.; Welk, G.J.; Eisenmann, J.C. Associations between sedentary behavior and blood pressure in young children. Arch. Pediatr. Adolesc. Med. 2009, 163, 724–730. [Google Scholar] [CrossRef]
- Guillaume, M.; Lapidus, L.; Bjorntorp, P.; Lambert, A. Physical activity, obesity, and cardiovascular risk factors in children. The Belgian Luxembourg Child Study II. Obes. Res. 1997, 5, 549–556. [Google Scholar] [CrossRef]
- Wells, J.C.; Hallal, P.C.; Reichert, F.F.; Menezes, A.M.; Araujo, C.L.; Victora, C.G. Sleep patterns and television viewing in relation to obesity and blood pressure: Evidence from an adolescent Brazilian birth cohort. Int. J. Obes. 2008, 32, 1042–1049. [Google Scholar] [CrossRef]
- Pardee, P.E.; Norman, G.J.; Lustig, R.H.; Preud’homme, D.; Schwimmer, J.B. Television viewing and hypertension in obese children. Am. J. Prev. Med. 2007, 33, 439–443. [Google Scholar] [CrossRef]
- Ekelund, U.; Brage, S.; Froberg, K.; Harro, M.; Anderssen, S.A.; Sardinha, L.B.; Riddoch, C.; Andersen, L.B. TV viewing and physical activity are independently associated with metabolic risk in children: The European Youth Heart Study. PLoS Med. 2006, 3, e488. [Google Scholar] [CrossRef] [PubMed]
- Chinapaw, M.J.; Altenburg, T.M.; van Eijsden, M.; Gemke, R.J.; Vrijkotte, T.G. Screen time and cardiometabolic function in Dutch 5–6 year olds: Cross-sectional analysis of the ABCD-study. BMC Public Health 2014, 14, 933. [Google Scholar] [CrossRef]
- Pedersen, J.; Rasmussen, M.G.; Neland, M.; Grontved, A. Screen-based media use and blood pressure in preschool-aged children: A prospective study in the Odense Child Cohort. Scand. J. Public Health 2020. [Google Scholar] [CrossRef]
- Martinez-GóMez, D.; Gomez-Martinez, S.; Ruiz, J.R.; Ortega, F.B.; Marcos, A.; Veiga, O.L. Video game playing time and cardiometabolic risk in adolescents: The AFINOS study. Med. Clin. 2012, 139, 290–292. [Google Scholar] [CrossRef]
- Gopinath, B.; Hardy, L.L.; Kifley, A.; Baur, L.A.; Mitchell, P. Activity behaviors in schoolchildren and subsequent 5-yr change in blood pressure. Med. Sci. Sports Exerc. 2014, 46, 724–729. [Google Scholar] [CrossRef]
- Gopinath, B.; Baur, L.A.; Hardy, L.L.; Kifley, A.; Rose, K.A.; Wong, T.Y.; Mitchell, P. Relationship between a range of sedentary behaviours and blood pressure during early adolescence. J. Hum. Hypertens. 2012, 26, 350–356. [Google Scholar] [CrossRef]
- Poitras, V.J.; Gray, C.E.; Janssen, X.; Aubert, S.; Carson, V.; Faulkner, G.; Goldfield, G.S.; Reilly, J.J.; Sampson, M.; Tremblay, M.S. Systematic review of the relationships between sedentary behaviour and health indicators in the early years (0–4 years). BMC Public Health 2017, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Wong, J.E.; Shanita, S.N.; Ismail, M.N.; Deurenberg, P.; Poh, B.K. Daily physical activity and screen time, but not other sedentary activities, are associated with measures of obesity during childhood. Int. J. Environ. Res. Public Health 2014, 12, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Haapala, E.A.; Vaisto, J.; Veijalainen, A.; Lintu, N.; Wiklund, P.; Westgate, K.; Ekelund, U.; Lindi, V.; Brage, S.; Lakka, T.A. Associations of objectively measured physical activity and sedentary time with arterial stiffness in pre-pubertal children. Pediatr. Exerc. Sci. 2017, 29, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Veijalainen, A.; Tompuri, T.; Haapala, E.A.; Viitasalo, A.; Lintu, N.; Vaisto, J.; Laitinen, T.; Lindi, V.; Lakka, T.A. Associations of cardiorespiratory fitness, physical activity, and adiposity with arterial stiffness in children. Scand. J. Med. Sci. Sports 2016, 26, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Kochli, S.; Endes, K.; Steiner, R.; Engler, L.; Infanger, D.; Schmidt-Trucksass, A.; Zahner, L.; Hanssen, H. Obesity, High Blood Pressure, and Physical Activity Determine Vascular Phenotype in Young Children. Hypertension 2019, 73, 153–161. [Google Scholar] [CrossRef]
- Sparano, S.; Lauria, F.; Ahrens, W.; Fraterman, A.; Thumann, B.; Iacoviello, L.; Marild, S.; Michels, N.; Molnar, D.; Moreno, L.A.; et al. Sleep duration and blood pressure in children: Analysis of the pan-European IDEFICS cohort. J. Clin. Hypertens. 2019, 21, 572–578. [Google Scholar] [CrossRef]
- Fobian, A.D.; Elliott, L.; Louie, T. A Systematic Review of Sleep, Hypertension, and Cardiovascular Risk in Children and Adolescents. Curr. Hypertens. Rep. 2018, 20, 42. [Google Scholar] [CrossRef]
- Matthews, K.A.; Pantesco, E.J. Sleep characteristics and cardiovascular risk in children and adolescents: An enumerative review. Sleep Med. 2016, 18, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, J.; Fu, J.; Wu, H.; Wang, Y.; Li, L.; Zhao, Y.; Li, M.; Gao, S. Association between sleep duration and cardiac structure in youths at risk for metabolic syndrome. Sci. Rep. 2016, 6, 39017. [Google Scholar] [CrossRef][Green Version]
- Leary, S.D.; Ness, A.R.; Smith, G.D.; Mattocks, C.; Deere, K.; Blair, S.N.; Riddoch, C. Physical activity and blood pressure in childhood: Findings from a population-based study. Hypertension 2008, 51, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Brage, S.; Wedderkopp, N.; Ekelund, U.; Franks, P.W.; Wareham, N.J.; Andersen, L.B.; Froberg, K.; European Youth Heart Study (EYHS). Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: The European Youth Heart Study (EYHS). Diabetes Care 2004, 27, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Hardy, L.L.; Teber, E.; Mitchell, P. Association between physical activity and blood pressure in prepubertal children. Hypertens. Res. 2011, 34, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Melo, X.; Santa-Clara, H.; Pimenta, N.M.; Martins, S.S.; Minderico, C.S.; Fernhall, B.; Sardinha, L.B. Intima-media thickness in 11-to 13-year-old children: Variation attributed to sedentary behavior, physical activity, cardiorespiratory fitness, and waist circumference. J. Phys. Act. Health 2015, 12, 610–617. [Google Scholar] [CrossRef]
- Walker, D.J.; MacIntosh, A.; Kozyrskyj, A.; Becker, A.; McGavock, J. The associations between cardiovascular risk factors, physical activity, and arterial stiffness in youth. J. Phys. Act. Health 2013, 10, 198–204. [Google Scholar] [CrossRef]
| MV | SD | Min | p25th | p50th | p75th | Max | |
|---|---|---|---|---|---|---|---|
| Demographic, anthropometric and factors related with increased cardiovascular risk | |||||||
| Age (years) | 5.83 | 0.35 | 4.62 | 5.58 | 5.84 | 6.10 | 6.60 |
| Body weight (Kg) | 22.14 | 4.35 | 13.95 | 19.20 | 21.10 | 24.10 | 43.40 |
| Body height (cm) | 113.9 | 5.4 | 99.0 | 110.2 | 113.9 | 117.2 | 140.0 |
| z-Body weight for height at birth (SD) | 0.39 | 1.14 | −4.06 | −0.27 | 0.45 | 1.07 | 4.64 |
| z-Body height for age at birth (SD) | −0.47 | 1.33 | −6.12 | −1.15 | −0.35 | 0.46 | 4.75 |
| z-Body weight for age at birth (SD) | −0.22 | 1.24 | −5.98 | −0.77 | −0.07 | 0.61 | 2.56 |
| z-Body mas index for age at birth (SD) | 0.13 | 1.21 | −7.00 | −0.55 | 0.24 | 0.85 | 3.65 |
| Current z-Body mass index (SD) | 0.90 | 1.20 | −3.03 | 0.09 | 0.70 | 1.59 | 5.00 |
| baSBP percentile | 68 | 19 | 3 | 57 | 72 | 84 | 99 |
| baDBP percentile | 64 | 16 | 17 | 53 | 64 | 75 | 99 |
| High blood pressure state (%) | 14.3 | ||||||
| Family history of cardiovascular disease (%) | 36.9 | ||||||
| Diabetes (%) | 0.0 | ||||||
| Dyslipidemia (%) | 0.0 | ||||||
| Hypertension (%) | 0.0 | ||||||
| Obesity (%) | 16.6 | ||||||
| Physical activity, sedentary behavior and sleep time | |||||||
| Sedentary play (min/day) | 108 | 80 | 0 | 60 | 90 | 140 | 480 |
| Screen time (min/day) | 151 | 105 | 0 | 80 | 120 | 180 | 600 |
| Sedentary behavior (min/day) | 255 | 133 | 0 | 150 | 240 | 330 | 690 |
| Sleep time (hours/day) | 10 | 1 | 7 | 9 | 10 | 11 | 17 |
| PA at kindergarten (min/week) | 40 | 34 | 0 | 0 | 40 | 70 | 90 |
| PA out of kindergarten (min/week) | 30 | 66 | 0 | 0 | 0 | 0 | 260 |
| PA: active play (min/week) | 238 | 293 | 0 | 0 | 120 | 350 | 1200 |
| PA: biking (min/week) | 19 | 49 | 0 | 0 | 0 | 0 | 250 |
| PA: walking (min/week) | 104 | 109 | 0 | 30 | 75 | 150 | 500 |
| Total PA (min/week) | 448 | 365 | 0 | 190 | 350 | 600 | 1800 |
| Total PA (min/day) | 64 | 52 | 0 | 27 | 50 | 86 | 257 |
| International recommendations compliance | |||||||
| Sedentary behavior (%) | 18.00 | ||||||
| Sleep time (%) | 75.60 | ||||||
| Total weekly PA (%) | 41.60 | ||||||
| MV | SD | Min | p25th | p50th | p75th | Max | |
|---|---|---|---|---|---|---|---|
| Central and peripheral blood pressure, heart rate and hemodynamic parameters | |||||||
| aoSBP (mmHg) | 86 | 6 | 69 | 82 | 86 | 90 | 103 |
| aoDBP (mmHg) | 60 | 6 | 45 | 57 | 60 | 64 | 82 |
| aoPP (mmHg) | 26 | 5 | 14 | 22 | 26 | 29 | 51 |
| baSBP (mmHg) | 99 | 6 | 80 | 95 | 99 | 104 | 121 |
| baDBP (mmHg) | 59 | 5 | 45 | 56 | 59 | 62 | 81 |
| baPP (mmHg) | 40 | 5 | 24 | 36 | 40 | 44 | 60 |
| taSBP (mmHg) | 115 | 8 | 92 | 110 | 115 | 120 | 140 |
| taDBP (mmHg) | 60 | 6 | 44 | 56 | 60 | 64 | 81 |
| taPP (mmHg) | 55 | 7 | 29 | 50 | 55 | 60 | 76 |
| raSBP (mmHg) | 94 | 9 | 71 | 88 | 93 | 100 | 121 |
| raDBP (mmHg) | 59 | 6 | 45 | 55 | 59 | 62 | 81 |
| raPP (mmHg) | 35 | 8 | 19 | 29 | 34 | 40 | 69 |
| Heart rate (beats/minute) | 92 | 11 | 58 | 84 | 91 | 99 | 134 |
| Ankle Brachial Index | 1.16 | 0.08 | 0.96 | 1.1 | 1.15 | 1.2 | 1.45 |
| Cardiac output (L/min) | 4.24 | 0.47 | 3.1 | 3.9 | 4.22 | 4.5 | 6.17 |
| Systemic vascular resistances (mmHg/L/min) | 1.15 | 0.11 | 0.82 | 1.08 | 1.15 | 1.22 | 1.45 |
| Cardiac Index (L/min/m2) | 5.15 | 0.67 | 3.4 | 4.67 | 5.1 | 5.58 | 7.85 |
| Common carotid artery (CCA) | |||||||
| CCA SystD (mm) | 5.96 | 0.45 | 4.84 | 5.64 | 5.97 | 6.24 | 7.45 |
| CCA DD (mm) | 5.29 | 0.42 | 4.28 | 4.97 | 5.29 | 5.57 | 6.95 |
| CCA IMT (mm) | 0.42 | 0.02 | 0.36 | 0.41 | 0.42 | 0.43 | 0.52 |
| CCA EM (mmHg) | 208 | 51 | 101 | 177 | 205 | 237 | 447 |
| CCA β | 2.88 | 0.71 | 1.31 | 2.39 | 2.8 | 3.31 | 5.87 |
| CCA PSV (cm/s) | 129 | 21 | 82 | 116 | 127 | 141 | 209 |
| CCA EDV (cm/s) | 34 | 6 | 17 | 30 | 34 | 38 | 57 |
| CCA RI | 0.74 | 0.04 | 0.59 | 0.71 | 0.74 | 0.76 | 0.86 |
| CCA PI | 1.73 | 0.27 | 1.09 | 1.54 | 1.7 | 1.88 | 2.71 |
| CCA S-D Index | 3.91 | 0.69 | 2.44 | 3.44 | 3.83 | 4.27 | 7.17 |
| Common femoral artery (CFA) | |||||||
| CFA SystD (mm) | 4.72 | 0.49 | 3.6 | 4.39 | 4.72 | 5.01 | 6.37 |
| CFA DD (mm) | 4.40 | 0.49 | 3.26 | 4.08 | 4.38 | 4.68 | 5.92 |
| CFA IMT (mm) | 0.33 | 0.03 | 0.27 | 0.32 | 0.33 | 0.35 | 0.41 |
| CFA EM (mmHg) | 606 | 222 | 291 | 446 | 572 | 697 | 1978 |
| CFA β | 7.78 | 2.81 | 3.46 | 5.96 | 7.28 | 8.9 | 23.57 |
| CFA PSV (cm/s) | 125 | 25 | 71 | 108 | 123 | 142 | 231 |
| CFA EDV (cm/s) | −17.88 | 15.8 | −55.3 | −26.4 | −20.2 | −13 | 41.85 |
| CFA RI | 0.98 | 0.11 | 0.65 | 0.91 | 0.94 | 1.05 | 1.8 |
| CFA PI | 5.49 | 3.49 | 1.71 | 3.35 | 4.52 | 6.35 | 29.15 |
| CFA S-D Index | 29.81 | 42.21 | −0.34 | 9.03 | 11.2 | 15.63 | 192.3 |
| Brachial artery (BA) | |||||||
| BA SystD (mm) | 2.5 | 0.31 | 1.49 | 2.31 | 2.48 | 2.69 | 3.52 |
| BA DD (mm) | 2.33 | 0.31 | 1.39 | 2.1 | 2.32 | 2.5 | 3.32 |
| BA EM (mm) | 572 | 214 | 210 | 420 | 521 | 687 | 1583 |
| BA β | 7.33 | 2.61 | 3.21 | 5.59 | 6.72 | 8.74 | 19.58 |
| BA PSV (cm/s) | 100.13 | 23.69 | 49 | 82.2 | 98.1 | 116.6 | 191.1 |
| BA EDV (cm/s) | 9.76 | 8.67 | −20.9 | 7.21 | 10.75 | 14.4 | 33 |
| BA RI | 0.91 | 0.09 | 0.76 | 0.86 | 0.89 | 0.92 | 1.27 |
| Regional arterial stiffness | |||||||
| cfPWV (m/s) | 5.04 | 0.69 | 3.1 | 4.5 | 5 | 5.4 | 7.7 |
| β cfPWV | 0.67 | 0.18 | 0.26 | 0.54 | 0.65 | 0.77 | 1.45 |
| crPWV (m/s) | 7.45 | 1.48 | 4.4 | 6.6 | 7.5 | 8.2 | 12.3 |
| β crPWV | 1.48 | 0.59 | 0.52 | 1.12 | 1.44 | 1.71 | 3.74 |
| PWV Ratio | 0.68 | 0.13 | 0.42 | 0.61 | 0.68 | 0.72 | 1.05 |
| β PWV Ratio | 0.47 | 0.18 | 0.17 | 0.37 | 0.46 | 0.53 | 1.11 |
| Aortic pressure levels, wave components, reflection and wave-derived parameters | |||||||
| aoSBP (RT, SCOR) (mmHg) | 83 | 6 | 64 | 78 | 83 | 87 | 100 |
| aoSBP (CT, SCOR) (mmHg) | 91 | 11 | 69 | 84 | 90 | 97 | 152 |
| aoSBP (MOG) (mmHg) | 86 | 6 | 71 | 82 | 86 | 90 | 101 |
| AIx@75 (RT, SCOR) (%) | 17.5 | 9.3 | −10.0 | 11.0 | 18.0 | 24.0 | 43.0 |
| AIx@75 (CT, SCOR) (%) | −10.4 | 12.3 | −50.0 | −18.0 | −11.0 | −4.0 | 28.0 |
| AIx@75 (MOG) (%) | 28.5 | 9.6 | 3.0 | 22.3 | 28.1 | 34.0 | 65.0 |
| SEVR (RT, SCOR) (%) | 116 | 19 | 59 | 101 | 116 | 128 | 179 |
| SEVR (CT, SCOR) (%) | 110 | 18 | 57 | 96 | 110 | 120 | 156 |
| aoBPPf (RT, SCOR) (mmHg) | 19.9 | 4.6 | 7.0 | 17.0 | 19.0 | 23.0 | 43.0 |
| aoBPPf (CT, SCOR) (mmHg) | 31.7 | 9.7 | 17.0 | 25.0 | 30.0 | 37.0 | 76.0 |
| aoBPPf (MOG) (mmHg) | 17.6 | 3.6 | 10.3 | 15.0 | 17.2 | 19.6 | 33.6 |
| aoBP Pb (RT, SCOR) (mmHg) | 10.0 | 2.1 | 3.0 | 9.0 | 10.0 | 11.0 | 17.0 |
| aoBP Pb (CT, SCOR) (mmHg) | 11.2 | 2.7 | 7.0 | 9.0 | 11.0 | 13.0 | 24.0 |
| aoBP Pb (MOG) (mmHg) | 9.3 | 2.1 | 4.2 | 7.9 | 9.2 | 10.7 | 17.3 |
| Dependent Variable | Independent Variables | Bu | SE | C.I. LL | C.I. UL | Bs | p | VIF | R | R2 | Adj R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Central (aortic) and peripheral blood pressure and hemodynamic parameters | |||||||||||
| z-aoDBP (SD) | Constant | −0.172 | 0.093 | −0.355 | 0.011 | 0.066 | 0.416 | 0.173 | 0.165 | ||
| HBP (1:Yes, 0:No) | 1.101 | 0.154 | 0.798 | 1.404 | 0.362 | <0.001 | 1.007 | ||||
| Current z-BW (SD) | 0.095 | 0.039 | 0.018 | 0.173 | 0.122 | 0.016 | 1.007 | ||||
| Screen time (min/day) | 0.001 | 0.000 | 0.000 | 0.002 | 0.121 | 0.017 | 1.006 | ||||
| z-baSBP (SD) | Constant | −0.060 | 0.082 | −0.221 | 0.101 | 0.463 | 0.636 | 0.404 | 0.399 | ||
| HBP (1:Yes, 0:No) | 1.938 | 0.129 | 1.684 | 2.192 | 0.545 | <0.001 | 1.016 | ||||
| Current z-BW (SD) | 0.164 | 0.056 | 0.055 | 0.273 | 0.171 | 0.003 | 2.562 | ||||
| Current z-BH (SD) | 0.183 | 0.068 | 0.049 | 0.317 | 0.154 | 0.008 | 2.554 | ||||
| PA, Kinder (min/w) | −0.003 | 0.001 | −0.006 | −0.001 | −0.093 | 0.011 | 1.014 | ||||
| z-baDBP (SD) | Constant | −0.393 | 0.091 | −0.572 | −0.214 | <0.001 | 0.512 | 0.262 | 0.258 | ||
| HBP (1:Yes, 0:No) | 1.621 | 0.141 | 1.343 | 1.899 | 0.461 | <0.001 | 1.005 | ||||
| Current z-BW (SD) | 0.153 | 0.038 | 0.078 | 0.228 | 0.161 | <0.001 | 1.007 | ||||
| Screen time (min/day) | 0.001 | 0.000 | 0.000 | 0.002 | 0.100 | 0.014 | 1.004 | ||||
| z-taSBP (SD) | Constant | 0.069 | 0.118 | −0.163 | 0.301 | 0.560 | 0.504 | 0.254 | 0.242 | ||
| HBP (1:Yes, 0:No) | 0.931 | 0.152 | 0.632 | 1.229 | 0.311 | <0.001 | 1.012 | ||||
| Current z-BW (SD) | 0.252 | 0.041 | 0.171 | 0.332 | 0.314 | <0.001 | 1.024 | ||||
| Sedentary play (min/day) | −0.002 | 0.001 | −0.003 | −0.001 | −0.146 | 0.004 | 1.016 | ||||
| PA, Kinder (min/w) | −0.004 | 0.002 | −0.007 | −0.001 | −0.148 | 0.004 | 1.029 | ||||
| PA (min/w) | 0.000 | 0.000 | 0.000 | 0.001 | 0.118 | 0.021 | 1.023 | ||||
| z-taDBP (SD) | Constant | 0.080 | 0.111 | −0.139 | 0.299 | 0.473 | 0.470 | 0.221 | 0.208 | ||
| HBP (1:Yes, 0:No) | 1.004 | 0.169 | 0.672 | 1.336 | 0.311 | <0.001 | 1.029 | ||||
| Obesity (1:Yes, 0:No) | 0.657 | 0.148 | 0.365 | 0.948 | 0.231 | <0.001 | 1.029 | ||||
| Sex (1:Female, 0:Male) | 0.409 | 0.115 | 0.182 | 0.635 | 0.186 | <0.001 | 1.035 | ||||
| Sedentary play (min/day) | −0.002 | 0.001 | −0.003 | 0.000 | −0.124 | 0.018 | 1.032 | ||||
| PA: bike time (min/w) | 0.002 | 0.001 | 0.000 | 0.005 | 0.109 | 0.037 | 1.021 | ||||
| z-taPP (SD) | Constant | −0.021 | 0.119 | −0.255 | 0.213 | 0.862 | 0.310 | 0.096 | 0.084 | ||
| Sex (1:Female, 0:Male) | −0.393 | 0.117 | −0.625 | −0.162 | −0.187 | 0.001 | 1.020 | ||||
| Current z-BW (SD) | 0.127 | 0.046 | 0.037 | 0.218 | 0.154 | 0.006 | 1.011 | ||||
| PA: active play (min/w) | 0.001 | 0.000 | 0.000 | 0.001 | 0.142 | 0.012 | 1.027 | ||||
| PA, Kinder (min/w) | −0.004 | 0.002 | −0.007 | 0.000 | −0.117 | 0.036 | 1.014 | ||||
| z-raDBP (SD) | Constant | −1.158 | 0.486 | −2.115 | −0.201 | 0.018 | 0.467 | 0.218 | 0.203 | ||
| HBP (1:Yes, 0:No) | 1.036 | 0.180 | 0.680 | 1.391 | 0.347 | <0.001 | 1.011 | ||||
| Current z-BW (SD) | 0.184 | 0.049 | 0.087 | 0.280 | 0.226 | <0.001 | 1.007 | ||||
| Sex (1:Female, 0:Male) | 0.249 | 0.116 | 0.020 | 0.477 | 0.129 | 0.033 | 1.001 | ||||
| Sleep (hours/day) | 0.099 | 0.047 | 0.007 | 0.191 | 0.129 | 0.034 | 1.011 | ||||
| z-Ankle-brachial Index (SD) | Constant | 0.220 | 0.112 | 0.000 | 0.441 | 0.051 | 0.234 | 0.055 | 0.045 | ||
| Sedent behavior (min/day) | −0.001 | 0.000 | −0.002 | 0.000 | −0.161 | 0.005 | 1.001 | ||||
| HBP (1:Yes, 0:No) | −0.302 | 0.137 | −0.573 | −0.032 | −0.124 | 0.029 | 1.001 | ||||
| PA: active play (min/w) | 0.000 | 0.000 | 0.000 | 0.001 | 0.111 | 0.050 | 1.000 | ||||
| z-Cardiac Output (SD) | Constant | 0.591 | 0.342 | −0.082 | 1.264 | 0.085 | 0.561 | 0.315 | 0.300 | ||
| Current z-BW (SD) | 0.355 | 0.071 | 0.216 | 0.494 | 0.538 | <0.001 | 4.501 | ||||
| Sex (1:Female, 0:Male) | −0.588 | 0.088 | −0.761 | −0.415 | −0.340 | <0.001 | 1.012 | ||||
| HBP (1:Yes, 0:No) | 0.575 | 0.127 | 0.326 | 0.825 | 0.232 | <0.001 | 1.027 | ||||
| Current z-BMI (SD) | −0.335 | 0.080 | −0.494 | −0.177 | −0.500 | <0.001 | 5.631 | ||||
| Obesity (1:Yes, 0:No) | 0.637 | 0.180 | 0.283 | 0.991 | 0.288 | <0.001 | 2.598 | ||||
| Sleep (hours/day) | −0.068 | 0.032 | −0.132 | −0.005 | −0.108 | 0.035 | 1.012 | ||||
| Arterial structural parameters | |||||||||||
| z-CCA SystD (SD) | Constant | −0.252 | 0.097 | −0.443 | −0.060 | 0.010 | 0.479 | 0.230 | 0.218 | ||
| Current z-BW (SD) | 0.265 | 0.044 | 0.179 | 0.351 | 0.374 | <0.001 | 1.008 | ||||
| Sex (1:Female, 0:Male) | −0.370 | 0.116 | −0.598 | −0.141 | −0.200 | 0.002 | 1.052 | ||||
| PA, out kinder (min/w) | 0.002 | 0.001 | 0.001 | 0.004 | 0.199 | 0.002 | 1.057 | ||||
| z-CCA DD (SD) | Constant | −0.252 | 0.102 | −0.453 | −0.051 | 0.014 | 0.485 | 0.235 | 0.224 | ||
| Current z-BW (SD) | 0.273 | 0.046 | 0.183 | 0.363 | 0.365 | <0.001 | 1.008 | ||||
| Sex (1:Female, 0:Male) | −0.423 | 0.122 | −0.663 | −0.183 | −0.218 | 0.001 | 1.052 | ||||
| PA, out kinder (min/w) | 0.003 | 0.001 | 0.001 | 0.004 | 0.205 | 0.001 | 1.057 | ||||
| z-CCA IMT (SD) | Constant | −0.720 | 0.158 | −1.031 | −0.409 | <0.001 | 0.400 | 0.160 | 0.148 | ||
| Current z-BMI (SD) | 0.234 | 0.061 | 0.113 | 0.355 | 0.245 | <0.001 | 1.009 | ||||
| Sex (1:Female, 0:Male) | −0.602 | 0.161 | −0.920 | −0.285 | −0.240 | <0.001 | 1.003 | ||||
| PA, Kinder (min/w) | 0.006 | 0.002 | 0.001 | 0.011 | 0.167 | 0.010 | 1.007 | ||||
| z-CFA SystD (SD) | Constant | −0.668 | 0.156 | −0.975 | −0.361 | <0.001 | 0.521 | 0.271 | 0.260 | ||
| Current z-BH (SD) | 0.446 | 0.074 | 0.300 | 0.591 | 0.369 | <0.001 | 1.004 | ||||
| Sex (1:Female, 0:Male) | −0.640 | 0.152 | −0.939 | −0.341 | −0.261 | <0.001 | 1.034 | ||||
| PA: typical week (min/w) | 0.001 | 0.000 | 0.000 | 0.001 | 0.202 | 0.001 | 1.037 | ||||
| z-CFA DD (SD) | Constant | −0.547 | 0.159 | −0.861 | −0.232 | 0.001 | 0.502 | 0.252 | 0.241 | ||
| Current z-BH (SD) | 0.450 | 0.075 | 0.301 | 0.598 | 0.368 | <0.001 | 1.004 | ||||
| Sex (1:Female, 0:Male) | −0.607 | 0.155 | −0.912 | −0.301 | −0.245 | <0.001 | 1.034 | ||||
| PA: typical week (min/w) | 0.001 | 0.000 | 0.000 | 0.001 | 0.183 | 0.004 | 1.037 | ||||
| z-BA SystD (SD) | Constant | −0.759 | 0.098 | −0.954 | −0.565 | <0.001 | 0.485 | 0.235 | 0.217 | ||
| Current z-BW (SD) | 0.406 | 0.078 | 0.252 | 0.560 | 0.627 | <0.001 | 2.459 | ||||
| PA: walk time (min/w) | 0.002 | 0.001 | 0.001 | 0.003 | 0.246 | 0.002 | 1.008 | ||||
| Obesity (1:Yes, 0:No) | −0.690 | 0.256 | −1.196 | −0.185 | −0.325 | 0.008 | 2.465 | ||||
| z-BA DD (SD) | Constant | −0.715 | 0.104 | −0.920 | −0.510 | <0.001 | 0.477 | 0.227 | 0.209 | ||
| Current z-BW (SD) | 0.419 | 0.082 | 0.256 | 0.581 | 0.617 | <0.001 | 2.459 | ||||
| PA: walk time (min/w) | 0.002 | 0.001 | 0.001 | 0.003 | 0.235 | 0.003 | 1.008 | ||||
| Obesity (1:Yes, 0:No) | −0.692 | 0.269 | −1.225 | −0.160 | −0.311 | 0.011 | 2.465 | ||||
| Blood flow velocity and Doppler-derived parameters | |||||||||||
| z-CCA EDV (SD) | Constant | 0.648 | 0.108 | 0.434 | 0.861 | <0.001 | 0.252 | 0.063 | 0.054 | ||
| Current z-BW (SD) | −0.153 | 0.053 | −0.258 | −0.048 | −0.197 | 0.004 | 1.005 | ||||
| PA, Kinder (min/w) | −0.004 | 0.002 | −0.008 | 0.000 | −0.145 | 0.035 | 1.005 | ||||
| z-CCA RI (SD) | Constant | −0.531 | 0.100 | −0.728 | −0.334 | <0.001 | 0.299 | 0.090 | 0.080 | ||
| Current z-BH (SD) | 0.187 | 0.057 | 0.074 | 0.300 | 0.220 | 0.001 | 1.004 | ||||
| PA: typical week (min/w) | 0.001 | 0.000 | 0.000 | 0.001 | 0.189 | 0.005 | 1.004 | ||||
| z-CCA PI (SD) | Constant | −0.784 | 0.088 | −0.957 | −0.611 | <0.001 | 0.380 | 0.144 | 0.132 | ||
| Current z-BW (SD) | 0.155 | 0.039 | 0.078 | 0.232 | 0.261 | <0.001 | 1.012 | ||||
| PA: typical week(min/w) | 0.001 | 0.000 | 0.000 | 0.001 | 0.270 | <0.001 | 1.060 | ||||
| PA: bike time (min/w) | −0.002 | 0.001 | −0.004 | 0.000 | −0.134 | 0.048 | 1.066 | ||||
| z-CCA S-D Index (SD) | Constant | −0.549 | 0.091 | −0.729 | −0.370 | <0.001 | 0.346 | 0.120 | 0.111 | ||
| Current z-BH (SD) | 0.195 | 0.052 | 0.093 | 0.298 | 0.248 | <0.001 | 1.004 | ||||
| PA: typical week (min/w) | 0.001 | 0.000 | 0.000 | 0.001 | 0.226 | 0.001 | 1.004 | ||||
| z-CFA PSV (SD) | Constant | −0.304 | 0.125 | −0.550 | −0.059 | 0.015 | 0.304 | 0.092 | 0.078 | ||
| PA: walk time (min/w) | 0.002 | 0.001 | 0.000 | 0.003 | 0.164 | 0.019 | 1.016 | ||||
| Sex (1:Female, 0:Male) | 0.365 | 0.143 | 0.082 | 0.648 | 0.176 | 0.012 | 1.003 | ||||
| HBP (1:Yes, 0:No) | 0.445 | 0.197 | 0.056 | 0.833 | 0.157 | 0.025 | 1.013 | ||||
| z-CFA RI (SD) | Constant | 0.046 | 0.140 | −0.229 | 0.322 | 0.741 | 0.380 | 0.145 | 0.131 | ||
| Obesity (1:Yes, 0:No) | −0.861 | 0.207 | −1.271 | −0.452 | −0.283 | <0.001 | 1.033 | ||||
| HBP (1:Yes, 0:No) | −0.567 | 0.227 | −1.014 | −0.120 | −0.170 | 0.013 | 1.031 | ||||
| Sedent play (min/day) | 0.002 | 0.001 | 0.000 | 0.004 | 0.148 | 0.029 | 1.006 | ||||
| z-CFA S-D Index (SD) | Constant | −3.661 | 1.571 | −6.760 | −0.563 | 0.021 | 0.239 | 0.057 | 0.047 | ||
| PA: bike time (min/w) | 0.005 | 0.002 | 0.002 | 0.009 | 0.203 | 0.005 | 1.016 | ||||
| Age (years) | 0.595 | 0.273 | 0.056 | 1.134 | 0.154 | 0.031 | 1.016 | ||||
| z-BA EDV (SD) | Constant | −0.081 | 0.098 | −0.275 | 0.112 | 0.409 | 0.384 | 0.148 | 0.122 | ||
| Screen time (min/day) | −0.001 | 0.000 | −0.002 | 0.000 | −0.234 | 0.005 | 1.026 | ||||
| PA, out kinder (min/w) | −0.002 | 0.001 | −0.003 | 0.000 | −0.206 | 0.012 | 1.012 | ||||
| Current z-BMI (SD) | 0.248 | 0.080 | 0.089 | 0.406 | 0.572 | 0.002 | 5.340 | ||||
| Current z-BW (SD) | −0.185 | 0.080 | −0.344 | −0.026 | −0.426 | 0.023 | 5.341 | ||||
| Aortic pressure levels, wave components, reflection and wave-derived parameters | |||||||||||
| z-aoSBP (RT) (SD) | Constant | −2.169 | 0.865 | −3.874 | −0.464 | 0.013 | 0.576 | 0.332 | 0.320 | ||
| HBP (1:Yes, 0:No) | 1.206 | 0.153 | 0.905 | 1.507 | 0.436 | <0.001 | 1.004 | ||||
| Current z-BW (SD) | 0.232 | 0.041 | 0.152 | 0.312 | 0.315 | <0.001 | 1.009 | ||||
| PA, Kinder (min/w) | −0.004 | 0.001 | −0.007 | −0.001 | −0.154 | 0.008 | 1.068 | ||||
| Age (years) | 0.395 | 0.153 | 0.094 | 0.695 | 0.147 | 0.010 | 1.062 | ||||
| z-AIx@75 (CT) (SD) | Constant | −0.509 | 0.149 | −0.804 | −0.214 | 0.001 | 0.440 | 0.194 | 0.176 | ||
| Sex (1:Female, 0:Male) | 0.744 | 0.157 | 0.434 | 1.055 | 0.321 | <0.001 | 1.021 | ||||
| Current z-BW (SD) | −0.240 | 0.067 | −0.372 | −0.107 | −0.240 | <0.001 | 1.002 | ||||
| Sedent play (min/day) | −0.002 | 0.001 | −0.004 | 0.000 | −0.171 | 0.013 | 1.023 | ||||
| z-BWH birth (SD) | 0.165 | 0.073 | 0.022 | 0.309 | 0.153 | 0.024 | 1.004 | ||||
| z-AIx@75 (MOG) (SD) | Constant | 0.209 | 0.134 | −0.054 | 0.472 | 0.119 | 0.477 | 0.227 | 0.216 | ||
| Sex (1:Female, 0:Male) | 0.810 | 0.127 | 0.560 | 1.060 | 0.343 | <0.001 | 1.002 | ||||
| Current z-BW (SD) | −0.248 | 0.049 | −0.344 | −0.152 | −0.275 | <0.001 | 1.007 | ||||
| HBP (1:Yes, 0:No) | 0.459 | 0.182 | 0.101 | 0.817 | 0.136 | 0.012 | 1.008 | ||||
| Screen time (min/day) | 0.001 | 0.001 | 0.000 | 0.003 | 0.136 | 0.012 | 1.009 | ||||
| z-SEVR (CT) (SD) | Constant | −0.931 | 0.152 | −1.231 | −0.631 | <0.001 | 0.400 | 0.160 | 0.142 | ||
| PA: typical week (min/w) | 0.001 | 0.000 | 0.000 | 0.001 | 0.218 | 0.002 | 1.022 | ||||
| HBP (1:Yes, 0:No) | −0.621 | 0.225 | −1.064 | −0.178 | −0.185 | 0.006 | 1.000 | ||||
| z-BWH birth (SD) | 0.189 | 0.071 | 0.050 | 0.328 | 0.180 | 0.008 | 1.005 | ||||
| Sex (1:Female, 0:Male) | −0.381 | 0.151 | −0.678 | −0.084 | −0.171 | 0.012 | 1.017 | ||||
| z-aoBPPf (CT) (SD) | Constant | −4.335 | 2.261 | −8.808 | 0.138 | 0.057 | 0.460 | 0.212 | 0.182 | ||
| Age (years) | 1.156 | 0.368 | 0.428 | 1.884 | 0.246 | 0.002 | 1.014 | ||||
| HBP (1:Yes, 0:No) | 1.282 | 0.395 | 0.501 | 2.063 | 0.257 | 0.001 | 1.030 | ||||
| PA: bike time (min/w) | 0.005 | 0.002 | 0.001 | 0.009 | 0.210 | 0.009 | 1.034 | ||||
| Sleep (hours/day) | −0.187 | 0.087 | −0.360 | −0.014 | −0.168 | 0.034 | 1.019 | ||||
| Current z-BH (SD) | 0.224 | 0.113 | 0.001 | 0.447 | 0.157 | 0.049 | 1.023 | ||||
| Dependent Variable | Cardiovascular Variations Related to Movement Behavior Parameters Variations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | MV (RG) | SD (RG) | Modeled Variation (Δ Minutes): | 60 | 120 | 180 | 240 | 300 | Min | Max | Δ | Δ% |
| Central (aortic) and peripheral blood pressure and cardiac output | ||||||||||||
| aoDBP (mmHg) | 59.2 | 5.4 | Screen time (m/d) | 0.4 | 0.7 | 1.1 | 1.5 | 1.8 | 0 | 3.6 | 3.6 | 6.1 |
| baSBP (mmHg) | 98.4 | 5.2 | PA, Kinder (m/w) | −1.1 | −2.1 | −3.2 | −4.2 | −5.3 | 0 | −1.59 | 1.59 | 1.62 |
| baDBP (mmHg) | 58.8 | 3.9 | Screen time (m/d) | 0.3 | 0.5 | 0.8 | 1.1 | 1.4 | 0 | 2.71 | 2.71 | 4.60 |
| taSBP (mmHg) | 113.6 | 7.5 | Sedent. play (m/d) | −0.8 | −1.6 | −2.3 | −3.1 | −3.9 | 0 | −6.25 | 6.25 | 5.50 |
| PA, Kinder (m/w) | −2.0 | −4.0 | −6.0 | −8.0 | −10.0 | 0 | −3.01 | 3.01 | 2.65 | |||
| PA, typical week (m/w) | 0.2 | 0.3 | 0.5 | 0.6 | 0.8 | 0 | 4.58 | 4.58 | 4.03 | |||
| taDBP (mmHg) | 58.1 | 5.3 | Sedent. play (m/d) | −0.5 | −1.0 | −1.5 | −2.0 | −2.5 | 0 | −4.05 | 4.05 | 6.97 |
| PA: bike time (m/w) | 0.8 | 1.6 | 2.4 | 3.1 | 3.9 | 0 | 3.27 | 3.27 | 5.63 | |||
| taPP (mmHg) | 55.5 | 7.0 | PA: active play (m/w) | 0.2 | 0.5 | 0.7 | 0.9 | 1.1 | 0 | 4.55 | 4.55 | 8.20 |
| PA, Kinder (m/w) | −1.5 | −3.1 | −4.6 | −6.1 | −7.7 | 0 | −2.30 | 2.30 | 4.14 | |||
| raDBP (mmHg) | 57.6 | 5.7 | Sleep (h/d) | 0.6 | 1.1 | 1.7 | 2.3 | 2.8 | 3.95 | 9.58 | 5.64 | 9.79 |
| Ankle Brachial Index | 1.15 | 0.09 | Sedent. behavior (m/d) | −0.01 | −0.01 | −0.02 | −0.02 | −0.03 | 0 | −0.06 | 0.06 | 5.43 |
| PA: active play (m/w) | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0 | 0.04 | 0.04 | 3.21 | |||
| CO (L/min) | 4.41 | 0.54 | Sleep (h/d) | −0.04 | −0.07 | −0.11 | −0.15 | −0.19 | −0.26 | −0.63 | 0.37 | 8.41 |
| Arterial structural parameters | ||||||||||||
| CCA SystD (mm) | 6.05 | 0.49 | PA, out Kinder (m/w) | 0.07 | 0.15 | 0.22 | 0.29 | 0.37 | 0 | 0.32 | 0.32 | 5.25 |
| CCA DD (mm) | 5.38 | 0.43 | PA, out Kinder (m/w) | 0.07 | 0.14 | 0.21 | 0.28 | 0.35 | 0 | 0.30 | 0.30 | 5.66 |
| CCA IMT (mm) | 0.43 | 0.02 | PA, Kinder (m/w) | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 | 0 | 0.01 | 0.01 | 2.52 |
| CFA SystD (mm) | 5.01 | 0.40 | PA: typical week (m/w) | 0.02 | 0.04 | 0.05 | 0.07 | 0.09 | 0 | 0.54 | 0.54 | 10.81 |
| CFA DD (mm) | 4.64 | 0.40 | PA: typical week (m/w) | 0.02 | 0.03 | 0.05 | 0.06 | 0.08 | 0 | 0.49 | 0.49 | 10.49 |
| BA SystD (mm) | 2.66 | 0.37 | PA: walk time (m/w) | 0.04 | 0.08 | 0.12 | 0.15 | 0.19 | 0 | 0.32 | 0.32 | 12.09 |
| BA DD (mm) | 2.45 | 0.35 | PA: walk time (m/w) | 0.04 | 0.07 | 0.11 | 0.15 | 0.19 | 0 | 0.31 | 0.31 | 12.59 |
| Blood flow velocity and Doppler-derived parameters | ||||||||||||
| CCA EDV (cm/s) | 31.68 | 6.28 | PA, Kinder (m/w) | −1.63 | −3.26 | −4.90 | −6.53 | −8.16 | 0 | −2.45 | 2.45 | 7.73 |
| CCA RI | 0.75 | 0.05 | PA: typical week (m/w) | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0 | 0.04 | 0.04 | 5.85 |
| CCA PI | 1.86 | 0.35 | PA: typical week (m/w) | 0.01 | 0.03 | 0.04 | 0.05 | 0.07 | 0 | 0.40 | 0.40 | 21.44 |
| PA: bike time (m/w) | −0.05 | −0.09 | −0.14 | −0.19 | −0.23 | 0 | −0.19 | 0.19 | 10.40 | |||
| CCA S-D Index | 4.11 | 0.81 | PA: typical week (m/w) | 0.03 | 0.05 | 0.08 | 0.11 | 0.14 | 0 | 0.82 | 0.82 | 19.97 |
| CFA PSV (cm/s) | 122.64 | 23.32 | PA: walk time (m/w) | 2.11 | 4.22 | 6.32 | 8.43 | 10.54 | 0 | 17.57 | 17.57 | 14.32 |
| CFA RI | 0.97 | 0.08 | Sedent. play (m/d) | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0 | 0.07 | 0.07 | 7.56 |
| CFA S-D Index | 34.04 | 33.03 | PA: bike time (m/w) | 10.59 | 21.17 | 31.76 | 42.35 | 52.93 | 0 | 44.11 | 44.11 | 129.57 |
| BA EDV (cm/s) | 13.21 | 14.07 | Screen time (m/d) | −1.11 | −2.21 | −3.32 | −4.42 | −5.53 | 0 | −11.05 | 11.05 | 83.64 |
| PA, out Kinder (m/w) | −1.44 | −2.88 | −4.32 | −5.76 | −7.20 | 0 | −6.24 | 6.24 | 47.22 | |||
| Aortic pressure levels, wave components, reflection and wave-derived parameters | ||||||||||||
| aoSBP (RT) (mmHg) | 81.00 | 7.06 | PA, Kinder (m/w) | −1.68 | −3.36 | −5.04 | −6.72 | −8.39 | 0 | −2.52 | 2.52 | 3.11 |
| AIx@75 (CT) (%) | −5.18 | 11.09 | Sedent. play (m/d) | −1.48 | −2.97 | −4.45 | −5.93 | −7.41 | 0 | −11.86 | 11.86 | 228.94 |
| AIx@75 (MOG) (%) | 21.81 | 8.28 | Screen time (m/d) | 0.73 | 1.45 | 2.18 | 2.91 | 3.63 | 0 | 7.27 | 7.27 | 33.32 |
| SEVR (CT) (%) | 122.18 | 15.70 | PA: typical week (m/w) | 0.67 | 1.33 | 2.00 | 2.66 | 3.33 | 0 | 19.98 | 19.98 | 16.36 |
| aoBPPf (CT) (mmHg) | 28.22 | 6.42 | PA: bike time (m/w) | 2.07 | 4.14 | 6.22 | 8.29 | 10.36 | 0 | 8.63 | 8.63 | 30.59 |
| Sleep (h/d) | −1.20 | −2.40 | −3.60 | −4.80 | −6.00 | −8.40 | −20.40 | 12.00 | 42.52 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-García, M.; Bia, D.; Zócalo, Y. Physical Activity, Sedentary Behavior and Sleep Time: Association with Cardiovascular Hemodynamic Parameters, Blood Pressure and Structural and Functional Arterial Properties in Childhood. J. Cardiovasc. Dev. Dis. 2021, 8, 62. https://doi.org/10.3390/jcdd8060062
Gómez-García M, Bia D, Zócalo Y. Physical Activity, Sedentary Behavior and Sleep Time: Association with Cardiovascular Hemodynamic Parameters, Blood Pressure and Structural and Functional Arterial Properties in Childhood. Journal of Cardiovascular Development and Disease. 2021; 8(6):62. https://doi.org/10.3390/jcdd8060062
Chicago/Turabian StyleGómez-García, Mariana, Daniel Bia, and Yanina Zócalo. 2021. "Physical Activity, Sedentary Behavior and Sleep Time: Association with Cardiovascular Hemodynamic Parameters, Blood Pressure and Structural and Functional Arterial Properties in Childhood" Journal of Cardiovascular Development and Disease 8, no. 6: 62. https://doi.org/10.3390/jcdd8060062
APA StyleGómez-García, M., Bia, D., & Zócalo, Y. (2021). Physical Activity, Sedentary Behavior and Sleep Time: Association with Cardiovascular Hemodynamic Parameters, Blood Pressure and Structural and Functional Arterial Properties in Childhood. Journal of Cardiovascular Development and Disease, 8(6), 62. https://doi.org/10.3390/jcdd8060062





