Multimodal Imaging from Fetal to Adult Life: A Comprehensive Approach to Hypoplastic Left Heart Syndrome (HLHS)
Abstract
1. Introduction
2. Multimodal Assessment During Fetal Life in Hypoplastic Left Heart Syndrome
3. Multimodal Assessment During Neonatal and Pediatric Life Until TCPC Completion
4. Multimodal Assessment During Pediatric and Adult Life Post-TCPC Completion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3VTV | Three-Vessel and Trachea View |
| ASD | Atrial Septal Defect |
| AV | Atrioventricular |
| APC | Aorto-Pulmonary Collaterals |
| S/D | Atrioventricular Ratio of Systolic to Diastolic Duration |
| BDCPC | Bidirectional Cavo-Pulmonary Connection |
| BNP | Brain Natriuretic Peptide |
| CHD | Congenital Heart Disease |
| CMR | Cardiac Magnetic Resonance |
| CCTA | Cardiac Computed Tomography Angiography |
| EF | Ejection Fraction |
| FAC | Fractional Area Change |
| FO | Foramen Ovale |
| FT | Feature Tracking |
| GCSs | Global Circumferential Strains |
| GLSs | Global Longitudinal Strains |
| HLHS | Hypoplastic Left Heart Syndrome |
| IAS | Intact Atrial Septum |
| ICV | Inferior Vena Cava |
| LGE | Late Gadolinium Enhancement |
| LPA | Left Pulmonary Artery |
| LV | Left Ventricle |
| NYHA | New York Heart Association |
| MPA | Main Pulmonary Artery |
| PAs | Pulmonary Arteries |
| PDA | Patent Ductus Arteriosus |
| PGE1 | Prostaglandin E1 |
| PV | Pulmonary Vein |
| PVR | Pulmonary Vascular Resistance |
| PWD | Pulsed-Wave Doppler |
| r-PFO | Restrictive Foramen Ovale |
| RV | Right Ventricle |
| RVEF | Right Ventricle Ejection Fraction |
| SV | Systemic Ventricle |
| SVC | Superior Vena Cava |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TCPC | Total Cavo-pulmonary Connection |
| TTE | Transthoracic Echocardiography |
| TOE | Transesophageal Echocardiography |
| TV | Tricuspid Valve |
References
- Gillum, R.F. Epidemiology of Congenital Heart Disease in the United States. Am. Heart J. 1994, 127, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.; Li, N.; Keavney, B.D. Global Birth Prevalence of Congenital Heart Defects 1970–2017: Updated Systematic Review and Meta-Analysis of 260 Studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Tchervenkov, C.I.; Jacobs, J.P.; Weinberg, P.M.; Aiello, V.D.; Béland, M.J.; Colan, S.D.; Elliott, M.J.; Franklin, R.C.G.; Gaynor, J.W.; Krogmann, O.N.; et al. The Nomenclature, Definition and Classification of Hypoplastic Left Heart Syndrome. Cardiol. Young 2006, 16, 339. [Google Scholar] [CrossRef]
- Franklin, R.C.G.; Béland, M.J.; Colan, S.D.; Walters, H.L.; Aiello, V.D.; Anderson, R.H.; Bailliard, F.; Boris, J.R.; Cohen, M.S.; Gaynor, J.W.; et al. Nomenclature for Congenital and Paediatric Cardiac Disease: The International Paediatric and Congenital Cardiac Code (IPCCC) and the Eleventh Iteration of the International Classification of Diseases (ICD-11). Cardiol. Young 2017, 27, 1872–1938. [Google Scholar] [CrossRef]
- Alphonso, N.; Angelini, A.; Barron, D.J.; Bellsham-Revell, H.; Blom, N.A.; Brown, K.; Davis, D.; Duncan, D.; Fedrigo, M.; Galletti, L.; et al. Guidelines for the Management of Neonates and Infants with Hypoplastic Left Heart Syndrome: The European Association for Cardio-Thoracic Surgery (EACTS) and the Association for European Paediatric and Congenital Cardiology (AEPC) Hypoplastic Left Heart Syndrome Guidelines Task Force. Eur. J. Cardiothorac. Surg. 2020, 58, 416–499. [Google Scholar] [CrossRef]
- Rosenthal, A. Physiology, Diagnosis and Clinical Profile of the Hypoplastic Left Heart Syndrome. Prog. Pediatr. Cardiol. 1996, 5, 19–22. [Google Scholar] [CrossRef]
- Roeleveld, P.P.; Axelrod, D.M.; Klugman, D.; Jones, M.B.; Chanani, N.K.; Rossano, J.W.; Costello, J.M. Hypoplastic Left Heart Syndrome: From Fetus to Fontan. Cardiol. Young 2018, 28, 1275–1288. [Google Scholar] [CrossRef]
- Ohye, R.G.; Schranz, D.; D’Udekem, Y. Current Therapy for Hypoplastic Left Heart Syndrome and Related Single Ventricle Lesions. Circulation 2016, 134, 1265–1279. [Google Scholar] [CrossRef]
- Norwood, W.I.; Kirklin, J.K.; Sanders, S.P. Hypoplastic Left Heart Syndrome: Experience with Palliative Surgery. Am. J. Cardiol. 1980, 45, 87–91. [Google Scholar] [CrossRef]
- Norwood, W.I.; Lang, P.; Casteneda, A.R.; Campbell, D.N. Experience with Operations for Hypoplastic Left Heart Syndrome. J. Thorac. Cardiovasc. Surg. 1981, 82, 511–519. [Google Scholar] [CrossRef]
- Yabrodi, M.; Mastropietro, C.W. Hypoplastic Left Heart Syndrome: From Comfort Care to Long-Term Survival. Pediatr. Res. 2017, 81, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Karamlou, T.; Peyvandi, S. Hybrid versus Norwood: “Fifty Shades of Grey”. J. Thorac. Cardiovasc. Surg. 2015, 150, 456–457. [Google Scholar] [CrossRef]
- Caldarone, C.A.; Benson, L.; Holtby, H.; Li, J.; Redington, A.N.; Van Arsdell, G.S. Initial Experience with Hybrid Palliation for Neonates with Single-Ventricle Physiology. Ann. Thorac. Surg. 2007, 84, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Pridjian, A.K.; Mendelsohn, A.M.; Lupinetti, F.M.; Beekman, R.H.; Dick, M.; Serwer, G.; Bove, E.L. Usefulness of the Bidirectional Glenn Procedure as Staged Reconstruction for the Functional Single Ventricle. Am. J. Cardiol. 1993, 71, 959–962. [Google Scholar] [CrossRef]
- Seliem, M.A.; Baffa, J.M.; Vetter, J.M.; Chen, S.-L.; Chin, A.J.; Norwood, W.I. Changes in Right Ventricular Geometry and Heart Rate Early After Hemi-Fontan Procedure. Ann. Thorac. Surg. 1993, 55, 1508–1512. [Google Scholar] [CrossRef]
- Scheurer, M.A.; Hill, E.G.; Vasuki, N.; Maurer, S.; Graham, E.M.; Bandisode, V.; Shirali, G.S.; Atz, A.M.; Bradley, S.M. Survival after Bidirectional Cavopulmonary Anastomosis: Analysis of Preoperative Risk Factors. J. Thorac. Cardiovasc. Surg. 2007, 134, 82–89. [Google Scholar] [CrossRef]
- Fontan, F.; Baudet, E. Surgical Repair of Tricuspid Atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef]
- Gersony, W.M. Fontan Operation After 3 Decades: What We Have Learned. Circulation 2008, 117, 13–15. [Google Scholar] [CrossRef]
- Lemler, M.S.; Scott, W.A.; Leonard, S.R.; Stromberg, D.; Ramaciotti, C. Fenestration Improves Clinical Outcome of the Fontan Procedure: A Prospective, Randomized Study. Circulation 2002, 105, 207–212. [Google Scholar] [CrossRef]
- Bouhout, I.; Ben-Ali, W.; Khalaf, D.; Raboisson, M.J.; Poirier, N. Effect of Fenestration on Fontan Procedure Outcomes: A Meta-Analysis and Review. Ann. Thorac. Surg. 2020, 109, 1467–1474. [Google Scholar] [CrossRef]
- Rychik, J. Forty Years of The Fontan Operation: A Failed Strategy. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2010, 13, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Almond, C.S.; Gauvreau, K.; Canter, C.E.; Rajagopal, S.K.; Piercey, G.E.; Singh, T.P. A Risk-Prediction Model for In-Hospital Mortality After Heart Transplantation in US Children. Am. J. Transplant. 2012, 12, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Neugebauer, R.; Lo, Y.; Gao, Q.; Lamour, J.M.; Weinstein, S.; Hsu, D.T. Outcomes and Risk Factors for Listing for Heart Transplantation after the Norwood Procedure: An Analysis of the Single Ventricle Reconstruction Trial. J. Heart Lung Transplant. 2016, 35, 306–311. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J.; Pahl, E.; Webber, S.A.; Rossano, J.W. Recent Advances in Heart Transplant Immunology: The Role of Antibodies. Prog. Pediatr. Cardiol. 2016, 43, 81–85. [Google Scholar] [CrossRef]
- Rossano, J.W.; Morales, D.L.S.; Zafar, F.; Denfield, S.W.; Kim, J.J.; Jefferies, J.L.; Dreyer, W.J. Impact of Antibodies against Human Leukocyte Antigens on Long-Term Outcome in Pediatric Heart Transplant Patients: An Analysis of the United Network for Organ Sharing Database. J. Thorac. Cardiovasc. Surg. 2010, 140, 694–699.e2. [Google Scholar] [CrossRef]
- Simpson, K.E.; Pruitt, E.; Kirklin, J.K.; Naftel, D.C.; Singh, R.K.; Edens, R.E.; Barnes, A.P.; Canter, C.E. Fontan Patient Survival After Pediatric Heart Transplantation Has Improved in the Current Era. Ann. Thorac. Surg. 2017, 103, 1315–1320. [Google Scholar] [CrossRef]
- Miller, J.R.; Simpson, K.E.; Epstein, D.J.; Lancaster, T.S.; Henn, M.C.; Schuessler, R.B.; Balzer, D.T.; Shahanavaz, S.; Murphy, J.J.; Canter, C.E.; et al. Improved Survival after Heart Transplant for Failed Fontan Patients with Preserved Ventricular Function. J. Heart Lung Transplant. 2016, 35, 877–883. [Google Scholar] [CrossRef]
- Pedra, S.R.F.F. Imaging for Hypoplastic Left Heart Syndrome. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 571–575. [Google Scholar] [CrossRef]
- Moscatelli, S.; Pozza, A.; Leo, I.; Ielapi, J.; Scatteia, A.; Piana, S.; Cavaliere, A.; Reffo, E.; Di Salvo, G. Importance of Cardiovascular Magnetic Resonance Applied to Congenital Heart Diseases in Pediatric Age: A Narrative Review. Children 2024, 11, 878. [Google Scholar] [CrossRef]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for Quantification Methods During the Performance of a Pediatric Echocardiogram: A Report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef]
- Plymale, J.M.; Frommelt, P.C.; Nugent, M.; Simpson, P.; Tweddell, J.S.; Shillingford, A.J. The Infant with Aortic Arch Hypoplasia and Small Left Heart Structures: Echocardiographic Indices of Mitral and Aortic Hypoplasia Predicting Successful Biventricular Repair. Pediatr. Cardiol. 2017, 38, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Kanngiesser, L.M.; Freitag-Wolf, S.; Boroni Grazioli, S.; Gabbert, D.D.; Hansen, J.H.; Uebing, A.S.; Voges, I. Serial Assessment of Right Ventricular Deformation in Patients with Hypoplastic Left Heart Syndrome: A Cardiovascular Magnetic Resonance Feature Tracking Study. J. Am. Heart Assoc. 2022, 11, e025332. [Google Scholar] [CrossRef] [PubMed]
- Khoo, N.S.; Smallhorn, J.F.; Kaneko, S.; Myers, K.; Kutty, S.; Tham, E.B. Novel Insights into RV Adaptation and Function in Hypoplastic Left Heart Syndrome Between the First 2 Stages of Surgical Palliation. JACC Cardiovasc. Imaging 2011, 4, 128–137. [Google Scholar] [CrossRef]
- Moscatelli, S.; Borrelli, N.; Sabatino, J.; Leo, I.; Avesani, M.; Montanaro, C.; Di Salvo, G. Role of Cardiovascular Imaging in the Follow-Up of Patients with Fontan Circulation. Children 2022, 9, 1875. [Google Scholar] [CrossRef] [PubMed]
- Fogel, M. Cardiac Magnetic Resonance of Single Ventricles. J. Cardiovasc. Magn. Reson. 2006, 8, 661–670. [Google Scholar] [CrossRef]
- Bellsham-Revell, H.R.; Tibby, S.M.; Bell, A.J.; Witter, T.; Simpson, J.; Beerbaum, P.; Anderson, D.; Austin, C.B.; Greil, G.F.; Razavi, R. Serial Magnetic Resonance Imaging in Hypoplastic Left Heart Syndrome Gives Valuable Insight into Ventricular and Vascular Adaptation. J. Am. Coll. Cardiol. 2013, 61, 561–570. [Google Scholar] [CrossRef]
- Erikssen, G.; Aboulhosn, J.; Lin, J.; Liestøl, K.; Estensen, M.E.; Gjesdal, O.; Skulstad, H.; Døhlen, G.; Lindberg, H.L. Survival in Patients with Univentricular Hearts: The Impact of Right versus Left Ventricular Morphology. Open Heart 2018, 5, e000902. [Google Scholar] [CrossRef]
- Angeli, E.; Pace Napoleone, C.; Balducci, A.; Formigari, R.; Lovato, L.; Candini, L.; Oppido, G.; Gargiulo, G. Natural and Modified History of Single-Ventricle Physiology in Adult Patients. Eur. J. Cardiothorac. Surg. 2012, 42, 996–1002. [Google Scholar] [CrossRef]
- Fratz, S.; Chung, T.; Greil, G.F.; Samyn, M.M.; Taylor, A.M.; Valsangiacomo Buechel, E.R.; Yoo, S.-J.; Powell, A.J. Guidelines and Protocols for Cardiovascular Magnetic Resonance in Children and Adults with Congenital Heart Disease: SCMR Expert Consensus Group on Congenital Heart Disease. J. Cardiovasc. Magn. Reson. 2013, 15, 51. [Google Scholar] [CrossRef]
- Moscatelli, S.; Avesani, M.; Borrelli, N.; Sabatino, J.; Pergola, V.; Leo, I.; Montanaro, C.; Contini, F.; Gaudieri, G.; Ielapi, J.; et al. Complete Transposition of the Great Arteries in the Pediatric Field: A Multimodality Imaging Approach. Children 2024, 11, 626. [Google Scholar] [CrossRef]
- Bellsham-Revell, H. Noninvasive Imaging in Interventional Cardiology: Hypoplastic Left Heart Syndrome. Front. Cardiovasc. Med. 2021, 8, 637838. [Google Scholar] [CrossRef] [PubMed]
- Secinaro, A.; Ait-Ali, L.; Curione, D.; Clemente, A.; Gaeta, A.; Giovagnoni, A.; Alaimo, A.; Esposito, A.; Tchana, B.; Sandrini, C.; et al. Recommendations for Cardiovascular Magnetic Resonance and Computed Tomography in Congenital Heart Disease: A Consensus Paper from the CMR/CCT Working Group of the Italian Society of Pediatric Cardiology (SICP) and the Italian College of Cardiac Radiology Endorsed by the Italian Society of Medical and Interventional Radiology (SIRM) Part I. Radiol. Med. (Torino) 2022, 127, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Szugye, N.A.; Zafar, F.; Villa, C.; Lorts, A.; Morales, D.L.S.; Moore, R.A. 3D Holographic Virtual Surgical Planning for a Single Right Ventricle Fontan Patient Needing Heartmate III Placement. ASAIO J. 2021, 67, e211–e215. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Cardis, B.; Mahle, W.; Lewis, R.; Huckaby, J.; Favaloro-Sabatier, J.; Fyfe, D. Pre- and Postoperative Quantitation of Right Ventricular Tissue Doppler Velocities in Infants with Hypoplastic Left Heart Syndrome. Echocardiography 2006, 23, 303–307. [Google Scholar] [CrossRef]
- Muhiudeen, I.A.; Roberson, D.A.; Silverman, N.H.; Haas, G.S.; Turley, K.; Cahalan, M.K. Intraoperative Echocardiography for Evaluation of Congenital Heart Defects in Infants and Children. Anesthesiology 1992, 76, 165–172. [Google Scholar] [CrossRef]
- McBrien, A.; Hornberger, L.K. Early Fetal Echocardiography. Birth Defects Res. 2019, 111, 370–379. [Google Scholar] [CrossRef]
- De Robertis, V.; Rembouskos, G.; Fanelli, T.; Volpe, G.; Muto, B.; Volpe, P. The Three-vessel and Trachea View (3VTV) in the First Trimester of Pregnancy: An Additional Tool in Screening for Congenital Heart Defects (CHD) in an Unselected Population. Prenat. Diagn. 2017, 37, 693–698. [Google Scholar] [CrossRef]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and Treatment of Fetal Cardiac Disease: A Scientific Statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef]
- Beroukhim, R.S.; Gauvreau, K.; Benavidez, O.J.; Baird, C.W.; LaFranchi, T.; Tworetzky, W. Perinatal Outcome after Prenatal Diagnosis of Single-Ventricle Cardiac Defects. Ultrasound Obstet. Gynecol. 2015, 45, 657–663. [Google Scholar] [CrossRef]
- Sathanandam, S.K.; Philip, R.; Gamboa, D.; Van Bergen, A.; Ilbawi, M.N.; Knott-Craig, C.; Waller, B.R.; Javois, A.J.; Cuneo, B.F. Management of Hypoplastic Left Heart Syndrome with Intact Atrial Septum: A Two-Centre Experience. Cardiol. Young 2016, 26, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Tworetzky, W.; Benson, C.B.; Wilkins-Haug, L.; Mizrahi-Arnaud, A.; McElhinney, D.B.; Lock, J.E.; Marshall, A.C. Technical Challenges of Atrial Septal Stent Placement in Fetuses with Hypoplastic Left Heart Syndrome and Intact Atrial Septum. Catheter. Cardiovasc. Interv. 2014, 84, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ryd, D.; Fricke, K.; Bhat, M.; Arheden, H.; Liuba, P.; Hedström, E. Utility of Fetal Cardiovascular Magnetic Resonance for Prenatal Diagnosis of Complex Congenital Heart Defects. JAMA Netw. Open 2021, 4, e213538. [Google Scholar] [CrossRef] [PubMed]
- Dargahpour Barough, M.; Tavares De Sousa, M.; Hergert, B.; Fischer, R.; Huber, L.; Seliger, J.M.; Kaul, M.G.; Adam, G.; Herrmann, J.; Bannas, P.; et al. Myocardial Strain Assessment in the Human Fetus by Cardiac MRI Using Doppler Ultrasound Gating and Feature Tracking. Eur. Radiol. 2024, 34, 4920–4927. [Google Scholar] [CrossRef]
- Vollbrecht, T.M.; Hart, C.; Katemann, C.; Isaak, A.; Pieper, C.C.; Kuetting, D.; Attenberger, U.; Geipel, A.; Strizek, B.; Luetkens, J.A. Fetal Cardiovascular Magnetic Resonance Feature Tracking Myocardial Strain Analysis in Congenital Heart Disease. J. Cardiovasc. Magn. Reson. 2024, 26, 101094. [Google Scholar] [CrossRef]
- Akintürk, H.; Michel-Behnke, I.; Valeske, K.; Mueller, M.; Thul, J.; Bauer, J.; Hagel, K.-J.; Schranz, D. Hybrid Transcatheter–Surgical Palliation: Basis for Univentricular or Biventricular Repair: The Giessen Experience. Pediatr. Cardiol. 2007, 28, 79–87. [Google Scholar] [CrossRef]
- Tchervenkov, C.I.; Jacobs, M.L.; Tahta, S.A. Congenital Heart Surgery Nomenclature and Database Project: Hypoplastic Left Heart Syndrome. Ann. Thorac. Surg. 2000, 69, 170–179. [Google Scholar] [CrossRef]
- Wilson, H.C.; Sood, V.; Romano, J.C.; Zampi, J.D.; Lu, J.C.; Yu, S.; Lowery, R.E.; Kleeman, K.; Balasubramanian, S. Hypoplastic Left Heart Syndrome with Mitral Stenosis and Aortic Atresia—Echocardiographic Findings and Early Outcomes. J. Am. Soc. Echocardiogr. 2024, 37, 603–612. [Google Scholar] [CrossRef]
- Atz, A.M.; Travison, T.G.; Williams, I.A.; Pearson, G.D.; Laussen, P.C.; Mahle, W.T.; Cook, A.L.; Kirsh, J.A.; Sklansky, M.; Khaikin, S.; et al. Prenatal Diagnosis and Risk Factors for Preoperative Death in Neonates with Single Right Ventricle and Systemic Outflow Obstruction: Screening Data from the Pediatric Heart Network Single Ventricle Reconstruction Trial∗. J. Thorac. Cardiovasc. Surg. 2010, 140, 1245–1250. [Google Scholar] [CrossRef]
- Bellsham-Revell, H.R.; Simpson, J.M.; Miller, O.I.; Bell, A.J. Subjective Evaluation of Right Ventricular Systolic Function in Hypoplastic Left Heart Syndrome: How Accurate Is It? J. Am. Soc. Echocardiogr. 2013, 26, 52–56. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Smith, S.N.; Srinivasan, P.; Tacy, T.A.; Hanley, F.L.; Chen, S.; Wright, G.E.; Peng, L.F.; Punn, R. Longitudinal Assessment of Right Ventricular Function in Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2021, 42, 1394–1404. [Google Scholar] [CrossRef]
- Borrelli, N.; Di Salvo, G.; Sabatino, J.; Ibrahim, A.; Avesani, M.; Sirico, D.; Josen, M.; Penco, M.; Fraisse, A.; Michielon, G. Serial Changes in Longitudinal Strain Are Associated with Outcome in Children with Hypoplastic Left Heart Syndrome. Int. J. Cardiol. 2020, 317, 56–62. [Google Scholar] [CrossRef]
- Avesani, M.; Sabatino, J.; Borrelli, N.; Cattapan, I.; Leo, I.; Pelaia, G.; Moscatelli, S.; Bianco, F.; Bassareo, P.; Martino, F.; et al. The Mechanics of Congenital Heart Disease: From a Morphological Trait to the Functional Echocardiographic Evaluation. Front. Cardiovasc. Med. 2024, 11, 1301116. [Google Scholar] [CrossRef]
- Sato, T.; Calderon, R.J.; Klas, B.; Pedrizzetti, G.; Banerjee, A. Simultaneous Volumetric and Functional Assessment of the Right Ventricle in Hypoplastic Left Heart Syndrome After Fontan Palliation, Utilizing 3-Dimensional Speckle-Tracking Echocardiography. Circ. J. 2020, 84, 235–244. [Google Scholar] [CrossRef]
- Michel, M.; Logoteta, J.; Entenmann, A.; Hansen, J.H.; Voges, I.; Kramer, H.-H.; Petko, C. Decline of Systolic and Diastolic 2D Strain Rate During Follow-Up of HLHS Patients After Fontan Palliation. Pediatr. Cardiol. 2016, 37, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, S.J.; Harrild, D.M.; Gauvreau, K.; Geva, T.; Rathod, R.H. Comparison Between Echocardiography and Cardiac Magnetic Resonance Imaging in Predicting Transplant-Free Survival After the Fontan Operation. Am. J. Cardiol. 2015, 116, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Sanchez Mejia, A.A.; Machefsky, A.; Fowler, S.; Holland, M.R.; Singh, G.K. Normal ranges of right ventricular systolic and diastolic strain measures in children: A systematic review and meta-analysis. J. Am. Soc. Echocardiogr. 2014, 27, 549–560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colquitt, J.L.; Loar, R.W.; Morris, S.A.; Feagin, D.K.; Sami, S.; Pignatelli, R.H. Serial Strain Analysis Identifies Hypoplastic Left Heart Syndrome Infants at Risk for Cardiac Morbidity and Mortality: A Pilot Study. J. Am. Soc. Echocardiogr. 2019, 32, 643–650. [Google Scholar] [CrossRef]
- Nguyen, T.; Miller, M.; Gonzalez, J.; Nardell, K.; Galas, J.; John, J.B.; Timofeev, S.; Lambou, R.; Douglas, K.; Marx, G. Echocardiography of Hypoplastic Left Heart Syndrome. Cardiol. Young 2011, 21, 28–37. [Google Scholar] [CrossRef]
- Goo, H.W. Serial Changes in Anatomy and Ventricular Function on Dual-Source Cardiac Computed Tomography after the Norwood Procedure for Hypoplastic Left Heart Syndrome. Pediatr. Radiol. 2017, 47, 1776–1786. [Google Scholar] [CrossRef]
- Cheasty, E.; Mahboobani, S.; Rubens, M.; Nicol, E. The Use of Cardiovascular CT for the Follow up of Paediatric Hypoplastic Left Heart Syndrome. J. Cardiovasc. Comput. Tomogr. 2020, 14, e18–e19. [Google Scholar] [CrossRef] [PubMed]
- Noel, C.V.; Kovalchin, J.P.; Adler, B.; Yates, A.R. Incidence of Tracheobronchial Anomalies Found with Hypoplastic Left Heart Syndrome. Congenit. Heart Dis. 2014, 9, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Sagray, E.; Cetta, F.; O’Leary, P.W.; Qureshi, M.Y. How Does Cross-Sectional Imaging Impact the Management of Patients with Single Ventricle After Bidirectional Cavopulmonary Connection? World J. Pediatr. Congenit. Heart Surg. 2023, 14, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Derby, C.D.; Baffa, J.M.; Murdison, K.A.; Radtke, W.A. Improving the Outcome of High-Risk Neonates with Hypoplastic Left Heart Syndrome: Hybrid Procedure or Conventional Surgical Palliation? Eur. J. Cardiothorac. Surg. 2008, 33, 613–618. [Google Scholar] [CrossRef]
- Oreto, L.; Guccione, P.; Gitto, P.; Bruno, L.; Zanai, R.; Grasso, N.; Iannace, E.; Zito, C.; Carerj, S.; Agati, S. Hybrid Palliation for Hypoplastic Left Heart Syndrome: Role of Echocardiography. Children 2023, 10, 1012. [Google Scholar] [CrossRef]
- Averin, K.; Seckeler, M.D.; Bauser-Heaton, H.; Schwartz, M.C.; Tannous, P.; Seaman, C.; Whiteside, W.; Nicholson, G.T.; Patel, P.M.; Gordon, B.M.; et al. Hybrid Versus Percutaneous Left Atrial Decompression in Infants with Hypoplastic Left Heart Variants and an Intact or Highly Restrictive Atrial Septum: A Multicenter PICES Study. Circ. Cardiovasc. Interv. 2025, 18, e014243. [Google Scholar] [CrossRef]
- Hussain, T.; Lossnitzer, D.; Bellsham-Revell, H.; Valverde, I.; Beerbaum, P.; Razavi, R.; Bell, A.J.; Schaeffter, T.; Botnar, R.M.; Uribe, S.A.; et al. Three-Dimensional Dual-Phase Whole-Heart MR Imaging: Clinical Implications for Congenital Heart Disease. Radiology 2012, 263, 547–554. [Google Scholar] [CrossRef]
- Grishin, S.A.; Svechkov, M.D.; Shorokhov, S.E.; Avramenko, A.A. False Aneurysm of the Proximal Sano Shunt in a Patient with Hypoplastic Left Heart Syndrome After Bidirectional Superior Cavopulmonary Connection. World J. Pediatr. Congenit. Heart Surg. 2023, 14, 395–396. [Google Scholar] [CrossRef]
- Meza, J.M.; Hickey, E.J.; Blackstone, E.H.; Jaquiss, R.D.B.; Anderson, B.R.; Williams, W.G.; Cai, S.; Van Arsdell, G.S.; Karamlou, T.; McCrindle, B.W. The Optimal Timing of Stage 2 Palliation for Hypoplastic Left Heart Syndrome: An Analysis of the Pediatric Heart Network Single Ventricle Reconstruction Trial Public Data Set. Circulation 2017, 136, 1737–1748. [Google Scholar] [CrossRef]
- Steele, J.M.; Moore, R.A.; Lang, S.M. Use of Advanced Cardiac Imaging in Congenital Heart Disease: Growth, Indications and Innovations. Curr. Opin. Pediatr. 2021, 33, 495–502. [Google Scholar] [CrossRef]
- Goldberg, D.J.; French, B.; Szwast, A.L.; McBride, M.G.; Paridon, S.M.; Rychik, J.; Mercer-Rosa, L. Tricuspid Annular Plane Systolic Excursion Correlates with Exercise Capacity in a Cohort of Patients with Hypoplastic Left Heart Syndrome after Fontan Operation. Echocardiography 2016, 33, 1897–1902. [Google Scholar] [CrossRef]
- Friedberg, M.K.; Silverman, N.H. The Systolic to Diastolic Duration Ratio in Children with Hypoplastic Left Heart Syndrome: A Novel Doppler Index of Right Ventricular Function. J. Am. Soc. Echocardiogr. 2007, 20, 749–755. [Google Scholar] [CrossRef]
- Cordina, R.; Ministeri, M.; Babu-Narayan, S.V.; Ladouceur, M.; Celermajer, D.S.; Gatzoulis, M.A.; Uebing, A.; Li, W. Evaluation of the Relationship between Ventricular End-Diastolic Pressure and Echocardiographic Measures of Diastolic Function in Adults with a Fontan Circulation. Int. J. Cardiol. 2018, 259, 71–75. [Google Scholar] [CrossRef]
- Cordina, R.; Von Klemperer, K.; Kempny, A.; West, C.; Senior, R.; Celermajer, D.S.; Gatzoulis, M.A.; Babu-Narayan, S.V.; Li, W. Echocardiographic Predictors of Mortality in Adults with a Fontan Circulation. JACC Cardiovasc. Imaging 2017, 10, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Peck, D.; Averin, K.; Khoury, P.; Veldhuis, G.; Alsaied, T.; Lubert, A.M.; Hirsch, R.; Whiteside, W.M.; Veldtman, G.; Goldstein, B.H. Occult Diastolic Dysfunction and Adverse Clinical Outcomes in Adolescents and Young Adults with Fontan Circulation. J. Am. Heart Assoc. 2023, 12, e026508. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Graham, E.M.; Taylor, C.L.; Savage, A.; McHugh, K.E.; Gaydos, S.; Nutting, A.C.; Zile, M.R.; Atz, A.M. Diastolic Dysfunction with Preserved Ejection Fraction After the Fontan Procedure. J. Am. Heart Assoc. 2022, 11, e024095. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jani, V.; Craft, M.; Olson, J.; Schuster, A.; Pedrizzetti, G.; Danford, D.; Kutty, S. Ventricular Flow Profile in Young Patients with Single Left Ventricle Fontan Using Echocardiographic Contrast Particle Imaging Velocimetry. J. Am. Soc. Echocardiogr. 2023, 36, 250–252. [Google Scholar] [CrossRef]
- Borrelli, N.; Avesani, M.; Sabatino, J.; Ibrahim, A.; Josen, M.; Paredes, J.; Di Salvo, G. Blood Speckle Imaging: A New Echocardiographic Approach to Study Fluid Dynamics in Congenital Heart Disease. Int. J. Cardiol. Congenit. Heart Dis. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.-Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult with Fontan Circulation: A Scientific Statement from the American Heart Association. Circulation 2019, 140, e234–e284. [Google Scholar] [CrossRef]
- Cohen, M.S.; Marino, B.S.; McElhinney, D.B.; Robbers-Visser, D.; Van Der Woerd, W.; Gaynor, J.W.; Spray, T.L.; Wernovsky, G. Neo-Aortic Root Dilation and Valve Regurgitation up to 21 Years after Staged Reconstruction for Hypoplastic Left Heart Syndrome. J. Am. Coll. Cardiol. 2003, 42, 533–540. [Google Scholar] [CrossRef]
- Devlin, P.J.; McCrindle, B.W.; Kirklin, J.K.; Blackstone, E.H.; DeCampli, W.M.; Caldarone, C.A.; Dodge-Khatami, A.; Eghtesady, P.; Meza, J.M.; Gruber, P.J.; et al. Intervention for Arch Obstruction after the Norwood Procedure: Prevalence, Associated Factors, and Practice Variability. J. Thorac. Cardiovasc. Surg. 2019, 157, 684–695.e8. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, T.; Sleeper, L.A.; Masci, M.; Ghelani, S.J.; Azcue, N.; Geva, T.; Powell, A.J.; Rathod, R.H. Maldistribution of Pulmonary Blood Flow in Patients after the Fontan Operation Is Associated with Worse Exercise Capacity. J. Cardiovasc. Magn. Reson. 2018, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Sundareswaran, K.S.; Kanter, K.R.; Kitajima, H.D.; Krishnankutty, R.; Sabatier, J.F.; Parks, W.J.; Sharma, S.; Yoganathan, A.P.; Fogel, M. Impaired Power Output and Cardiac Index with Hypoplastic Left Heart Syndrome: A Magnetic Resonance Imaging Study. Ann. Thorac. Surg. 2006, 82, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Voges, I.; Jerosch-Herold, M.; Hedderich, J.; Westphal, C.; Hart, C.; Helle, M.; Scheewe, J.; Pardun, E.; Kramer, H.-H.; Rickers, C. Maladaptive Aortic Properties in Children After Palliation of Hypoplastic Left Heart Syndrome Assessed by Cardiovascular Magnetic Resonance Imaging. Circulation 2010, 122, 1068–1076. [Google Scholar] [CrossRef]
- Voges, I.; Jerosch-Herold, M.; Wegner, P.; Hart, C.; Gabbert, D.; Al Bulushi, A.; Fischer, G.; Andrade, A.C.; Pham, H.M.; Kristo, I.; et al. Frequent Dilatation of the Descending Aorta in Children with Hypoplastic Left Heart Syndrome Relates to Decreased Aortic Arch Elasticity. J. Am. Heart Assoc. 2015, 4, e002107. [Google Scholar] [CrossRef]
- Geva, T. Is MRI the Preferred Method for Evaluating Right Ventricular Size and Function in Patients with Congenital Heart Disease? MRI Is the Preferred Method for Evaluating Right Ventricular Size and Function in Patients with Congenital Heart Disease. Circ. Cardiovasc. Imaging 2014, 7, 190–197. [Google Scholar] [CrossRef]
- Ballenberger, A.; Caliebe, A.; Krupickova, S.; Uebing, A.; Gabbert, D.D.; Voges, I. Cardiovascular Magnetic Resonance Reference Values of Right Ventricular Volumetric Variables in Patients with Hypoplastic Left Heart Syndrome. J. Cardiovasc. Magn. Reson. 2024, 26, 101038. [Google Scholar] [CrossRef]
- Sobh, M.; Freitag-Wolf, S.; Scheewe, J.; Kanngiesser, L.M.; Uebing, A.S.; Gabbert, D.D.; Voges, I. Serial Right Ventricular Assessment in Patients with Hypoplastic Left Heart Syndrome: A Multiparametric Cardiovascular Magnetic Resonance Study. Eur. J. Cardiothorac. Surg. 2021, 61, 36–42. [Google Scholar] [CrossRef]
- Ghelani, S.J.; Lu, M.; Sleeper, L.A.; Prakash, A.; Castellanos, D.A.; Clair, N.S.; Powell, A.J.; Rathod, R.H. Longitudinal Changes in Ventricular Size and Function Are Associated with Death and Transplantation Late after the Fontan Operation. J. Cardiovasc. Magn. Reson. 2022, 24, 56. [Google Scholar] [CrossRef]
- Ghelani, S.J.; Colan, S.D.; Azcue, N.; Keenan, E.M.; Harrild, D.M.; Powell, A.J.; Geva, T.; Rathod, R.H. Impact of Ventricular Morphology on Fiber Stress and Strain in Fontan Patients. Circ. Cardiovasc. Imaging 2018, 11, e006738. [Google Scholar] [CrossRef]
- Rathod, R.H.; Prakash, A.; Kim, Y.Y.; Germanakis, I.E.; Powell, A.J.; Gauvreau, K.; Geva, T. Cardiac Magnetic Resonance Parameters Predict Transplantation-Free Survival in Patients with Fontan Circulation. Circ. Cardiovasc. Imaging 2014, 7, 502–509. [Google Scholar] [CrossRef]
- Wong, J.; Pushparajah, K.; De Vecchi, A.; Ruijsink, B.; Greil, G.F.; Hussain, T.; Razavi, R. Pressure–Volume Loop-Derived Cardiac Indices during Dobutamine Stress: A Step towards Understanding Limitations in Cardiac Output in Children with Hypoplastic Left Heart Syndrome. Int. J. Cardiol. 2017, 230, 439–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, S.L.; Ridderbos, F.-J.S.; Wolff, D.; Eshuis, G.; Van Melle, J.P.; Ebels, T.; Berger, R.M.F.; Willems, T.P. Serial Cardiovascular Magnetic Resonance Feature Tracking Indicates Early Worsening of Cardiac Function in Fontan Patients. Int. J. Cardiol. 2020, 303, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Mitchell, M.B.; Frank, B.S.; Barker, A.J.; Stone, M.L.; Jaggers, J.; Von Alvensleben, J.C.; Hunter, K.S.; Friesen, R.M.; Ivy, D.D.; et al. Myocardial Strain-Curve Deformation Patterns after Fontan Operation. Sci. Rep. 2023, 13, 11912. [Google Scholar] [CrossRef] [PubMed]
- Gearhart, A.; Bassi, S.; Rathod, R.H.; Beroukhim, R.S.; Lipsitz, S.; Gold, M.P.; Harrild, D.M.; Dionne, A.; Ghelani, S.J. Ventricular Dyssynchrony Late after the Fontan Operation Is Associated with Decreased Survival. J. Cardiovasc. Magn. Reson. 2023, 25, 66. [Google Scholar] [CrossRef]
- Wang, A.P.; Kelle, A.M.; Hyun, M.; Reece, C.L.; Young, P.M.; O’Leary, P.W.; Qureshi, M.Y.; Wanek Family Program for Hypoplastic Left Heart Syndrome Imaging Pipeline. Negative Impact of the Left Ventricular Remnant Morphology on Systemic Right Ventricular Myocardial Deformation in Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2021, 42, 278–288. [Google Scholar] [CrossRef]
- Grosse-Wortmann, L.; Al-Otay, A.; Yoo, S.-J. Aortopulmonary Collaterals After Bidirectional Cavopulmonary Connection or Fontan Completion: Quantification With MRI. Circ. Cardiovasc. Imaging 2009, 2, 219–225. [Google Scholar] [CrossRef]
- Ait Ali, L.; Cadoni, A.; Rossi, G.; Keilberg, P.; Passino, C.; Festa, P. Effective Cardiac Index and Systemic-Pulmonary Collaterals Evaluated by Cardiac Magnetic Resonance Late After Fontan Palliation. Am. J. Cardiol. 2017, 119, 2069–2072. [Google Scholar] [CrossRef]
- Kodama, Y.; Ishikawa, Y.; Kuraoka, A.; Nakamura, M.; Oda, S.; Nakano, T.; Kado, H.; Sakamoto, I.; Ohtani, K.; Ide, T.; et al. Systemic-to-Pulmonary Collateral Flow Correlates with Clinical Condition Late After the Fontan Procedure. Pediatr. Cardiol. 2020, 41, 1800–1806. [Google Scholar] [CrossRef]
- Whitehead, K.K.; Harris, M.A.; Glatz, A.C.; Gillespie, M.J.; DiMaria, M.V.; Harrison, N.E.; Dori, Y.; Keller, M.S.; Rome, J.J.; Fogel, M.A. Status of Systemic to Pulmonary Arterial Collateral Flow After the Fontan Procedure. Am. J. Cardiol. 2015, 115, 1739–1745. [Google Scholar] [CrossRef]
- Latus, H.; Kruppa, P.; Hofmann, L.; Reich, B.; Jux, C.; Apitz, C.; Schranz, D.; Voges, I.; Khalil, M.; Gummel, K. Impact of Aortopulmonary Collateral Flow and Single Ventricle Morphology on Longitudinal Hemodynamics in Fontan Patients: A Serial CMR Study. Int. J. Cardiol. 2020, 311, 28–34. [Google Scholar] [CrossRef]
- Rijnberg, F.M.; Westenberg, J.J.M.; Van Assen, H.C.; Juffermans, J.F.; Kroft, L.J.M.; Van Den Boogaard, P.J.; Terol Espinosa De Los Monteros, C.; Warmerdam, E.G.; Leiner, T.; Grotenhuis, H.B.; et al. 4D Flow Cardiovascular Magnetic Resonance Derived Energetics in the Fontan Circulation Correlate with Exercise Capacity and CMR-Derived Liver Fibrosis/Congestion. J. Cardiovasc. Magn. Reson. 2022, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, V.P.; Elbaz, M.S.M.; Van Den Boogaard, P.J.; Kroft, L.J.M.; Lamb, H.J.; Hazekamp, M.G.; Jongbloed, M.R.M.; Blom, N.A.; Helbing, W.A.; Roest, A.A.W.; et al. Stress Increases Intracardiac 4D Flow Cardiovascular Magnetic Resonance -Derived Energetics and Vorticity and Relates to VO2max in Fontan Patients. J. Cardiovasc. Magn. Reson. 2019, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.; Tang, E.; Haggerty, C.M.; Khiabani, R.H.; Mirabella, L.; Bethel, J.; Valente, A.M.; Whitehead, K.K.; McElhinney, D.B.; Fogel, M.A.; et al. Energetic Implications of Vessel Growth and Flow Changes Over Time in Fontan Patients. Ann. Thorac. Surg. 2015, 99, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hut, T.; Roest, A.; Gaillard, D.; Hazekamp, M.; Van Den Boogaard, P.; Lamb, H.; Kroft, L.; Jongbloed, M.; Westenberg, J.; Wentzel, J.; et al. Virtual Surgery to Predict Optimized Conduit Size for Adult Fontan Patients with 16-Mm Conduits. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 37, ivad126. [Google Scholar] [CrossRef]
- Sathananthan, G.; Johal, N.; Verma, T.; Sandhu, S.; Chakrabarti, S.; Riahi, M.; Human, D.; Leipsic, J.; Grewal, J. Clinical Importance of Fontan Circuit Thrombus in the Adult Population: Significant Association with Increased Risk of Cardiovascular Events. Can. J. Cardiol. 2019, 35, 1807–1814. [Google Scholar] [CrossRef]
- Vlahos, A.P.; Lock, J.E.; McElhinney, D.B.; van der Velde, M.E. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: Outcome after neonatal transcatheter atrial septostomy. Circulation 2004, 109, 2326–2330. [Google Scholar] [CrossRef]
- Chin, C.W.; Nicholson, G.T.; Bichell, D.P. Severe aortopulmonary collaterals are associated with lower transplant-free survival in patients undergoing staged single ventricle palliation. JTCVS Open 2023, 16, 844–854. [Google Scholar] [CrossRef]

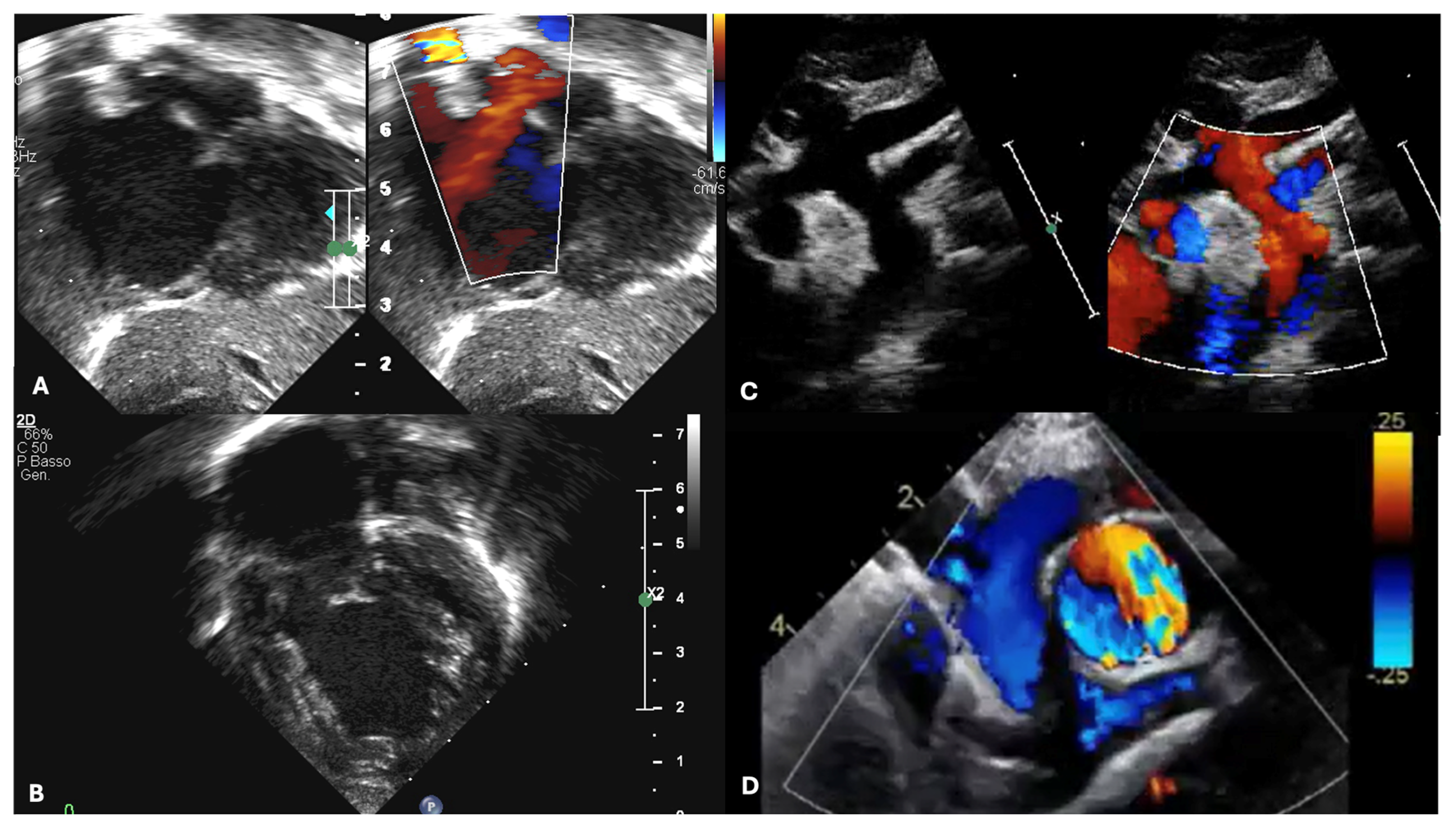


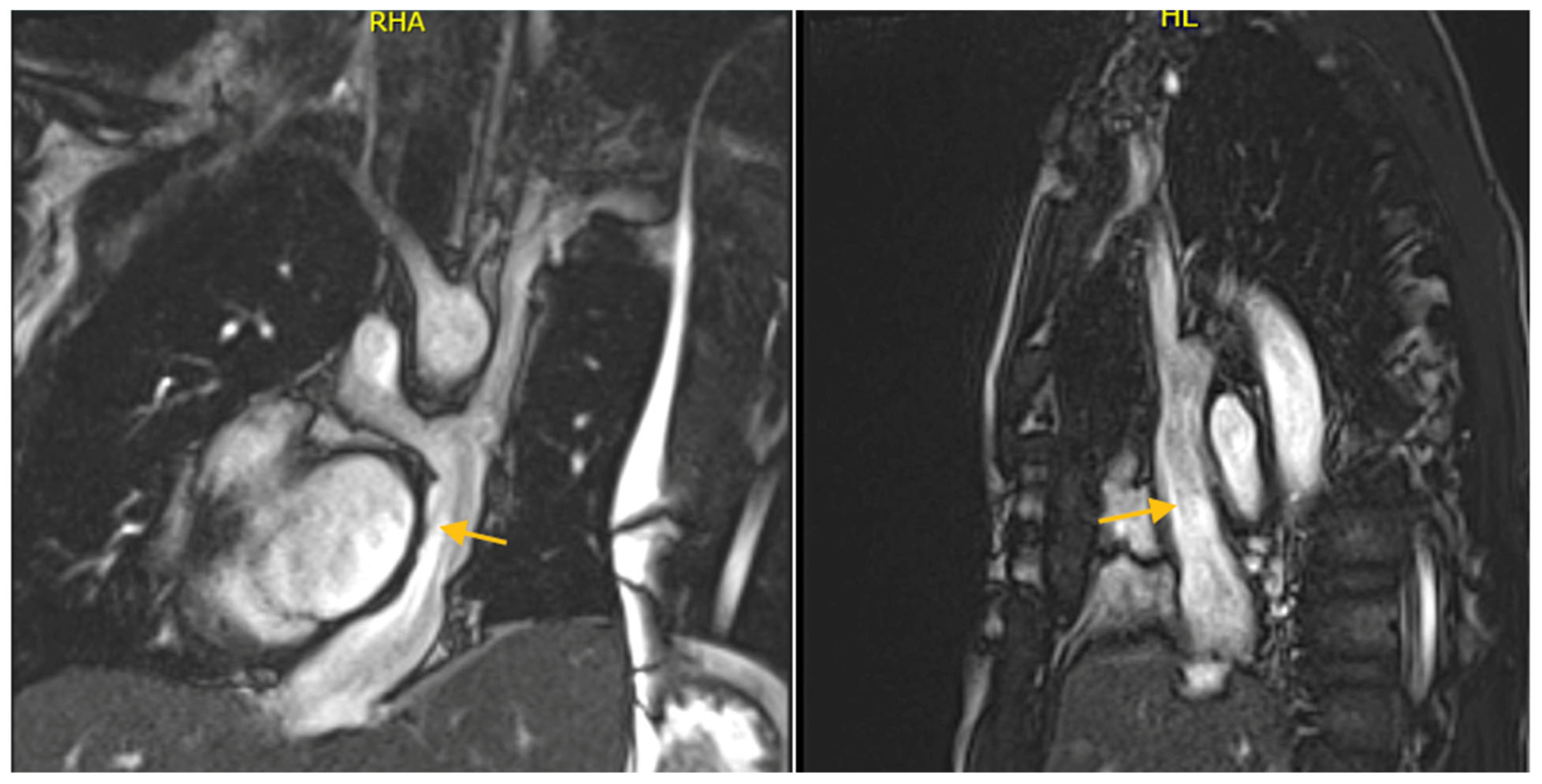
| Echocardiography | CMR | CCTA |
|---|---|---|
| Advantages | Advantages | Advantages |
|
|
|
| Disadvantages | Disadvantages | Disadvantages |
|
|
|
| Life Phases | Stage of Palliation | Echocardiography | Magnetic Resonance Imaging | Cardiac Computed Tomography Angiography |
|---|---|---|---|---|
| Fetal life | Prenatal diagnosis |
|
| / |
| Neonatal and Pediatric life | Initial assessment pre-Norwood/Sano |
| For selected cases (e.g., arch or pulmonary vein anomalies):
| For selected cases (e.g., arch or pulmonary vein anomalies):
|
| Post-Norwood/Sano |
| If concerns not addressed by echocardiography (e.g., shunt, PAs, arch, Ao-PA connection):
| If concerns not addressed by echocardiography (e.g., shunt, PAs, arch, Ao-PA connection):
| |
| Post-Glenn |
| If concerns not addressed by echocardiography (e.g., shunt, PAs, arch, Ao-PA connection):
| If concerns not addressed by echocardiography (e.g., shunt, PAs, arch, Ao-PA connection):
| |
| Post-TCPC |
| If concerns not addressed by echocardiography:
| If concerns not addressed by echocardiography:
| |
| Adult life | Follow-up post-TCPC |
| If concerns not addressed by echocardiography:
| If concerns not addressed by echocardiography:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscatelli, S.; Sabatino, J.; Leo, I.; Borrelli, N.; Avesani, M.; Di Salvo, G.; Montanaro, C.; Pergola, V.; Motta, R.; Ielapi, J.; et al. Multimodal Imaging from Fetal to Adult Life: A Comprehensive Approach to Hypoplastic Left Heart Syndrome (HLHS). J. Cardiovasc. Dev. Dis. 2025, 12, 349. https://doi.org/10.3390/jcdd12090349
Moscatelli S, Sabatino J, Leo I, Borrelli N, Avesani M, Di Salvo G, Montanaro C, Pergola V, Motta R, Ielapi J, et al. Multimodal Imaging from Fetal to Adult Life: A Comprehensive Approach to Hypoplastic Left Heart Syndrome (HLHS). Journal of Cardiovascular Development and Disease. 2025; 12(9):349. https://doi.org/10.3390/jcdd12090349
Chicago/Turabian StyleMoscatelli, Sara, Jolanda Sabatino, Isabella Leo, Nunzia Borrelli, Martina Avesani, Giovanni Di Salvo, Claudia Montanaro, Valeria Pergola, Raffaella Motta, Jessica Ielapi, and et al. 2025. "Multimodal Imaging from Fetal to Adult Life: A Comprehensive Approach to Hypoplastic Left Heart Syndrome (HLHS)" Journal of Cardiovascular Development and Disease 12, no. 9: 349. https://doi.org/10.3390/jcdd12090349
APA StyleMoscatelli, S., Sabatino, J., Leo, I., Borrelli, N., Avesani, M., Di Salvo, G., Montanaro, C., Pergola, V., Motta, R., Ielapi, J., Di Costanzo, A., De Sarro, R., Guglielmi, G., Cattapan, I., Gaudieri, G., Luedke, L., & Perrone, M. A. (2025). Multimodal Imaging from Fetal to Adult Life: A Comprehensive Approach to Hypoplastic Left Heart Syndrome (HLHS). Journal of Cardiovascular Development and Disease, 12(9), 349. https://doi.org/10.3390/jcdd12090349








