Topography of the Papillary Muscles in the Mitral Valve Complex and Their Relevance for Mitral Valve Function
Abstract
1. Introduction
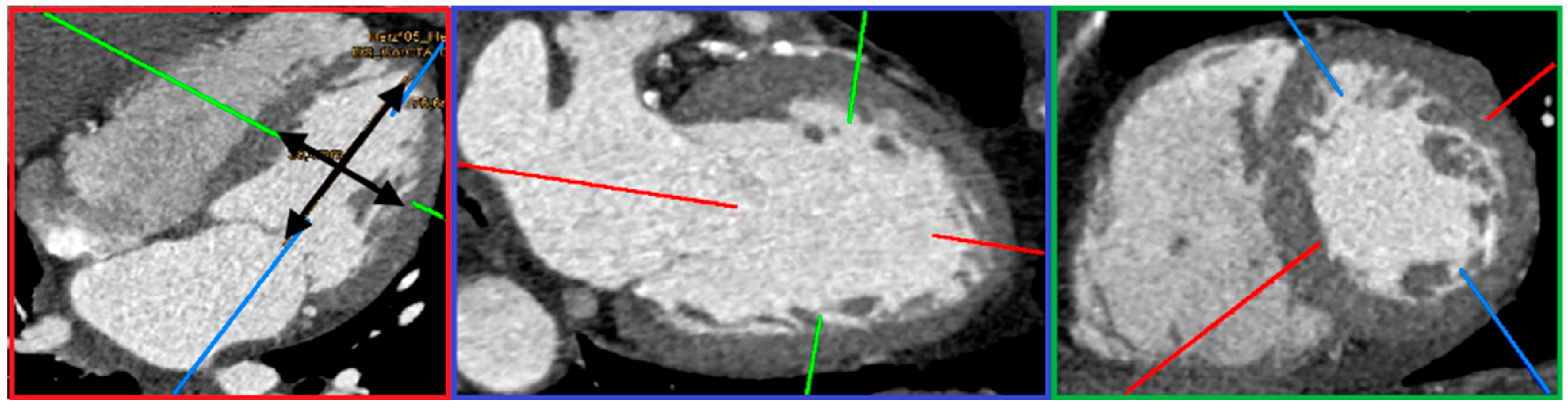
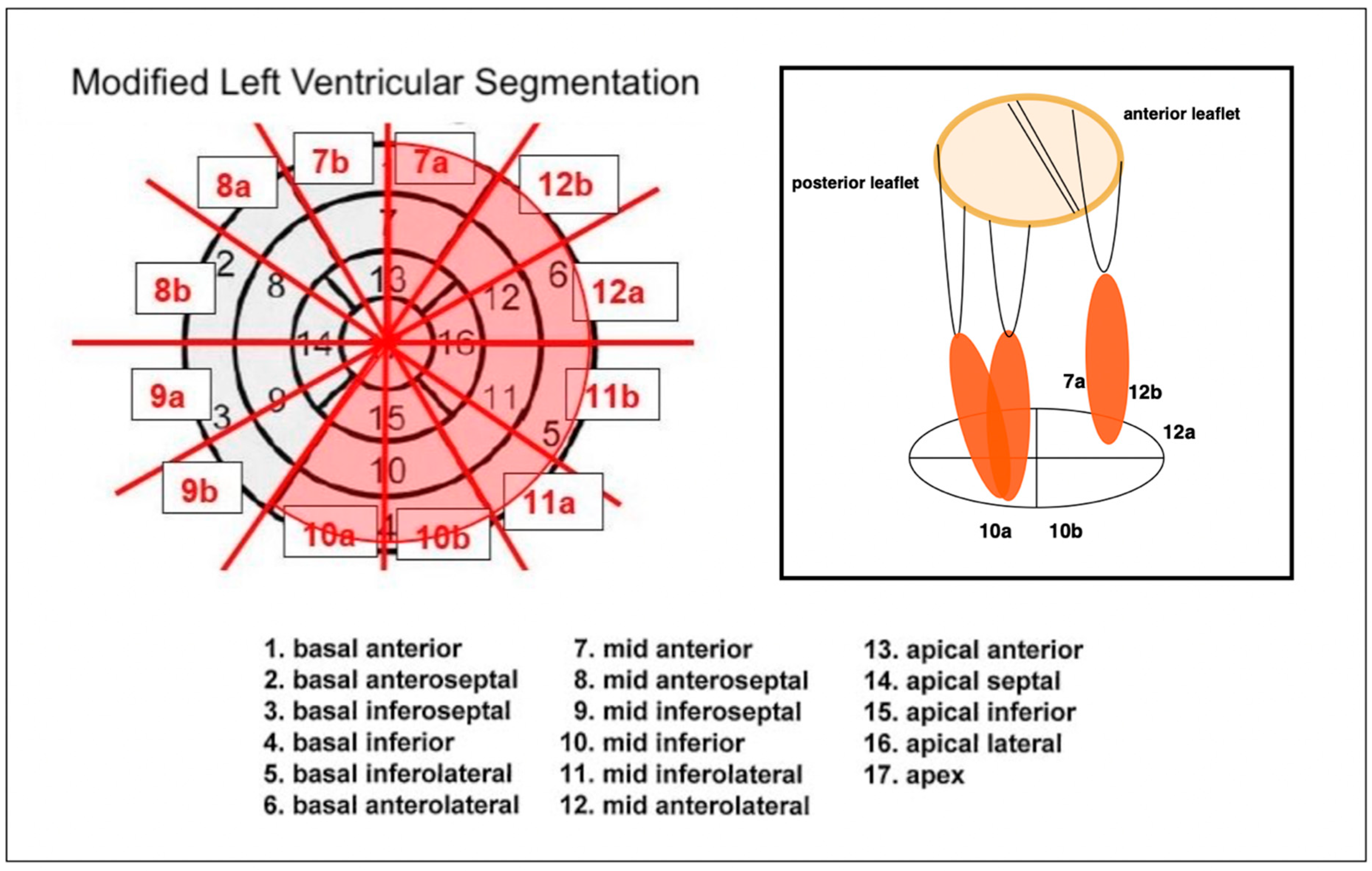
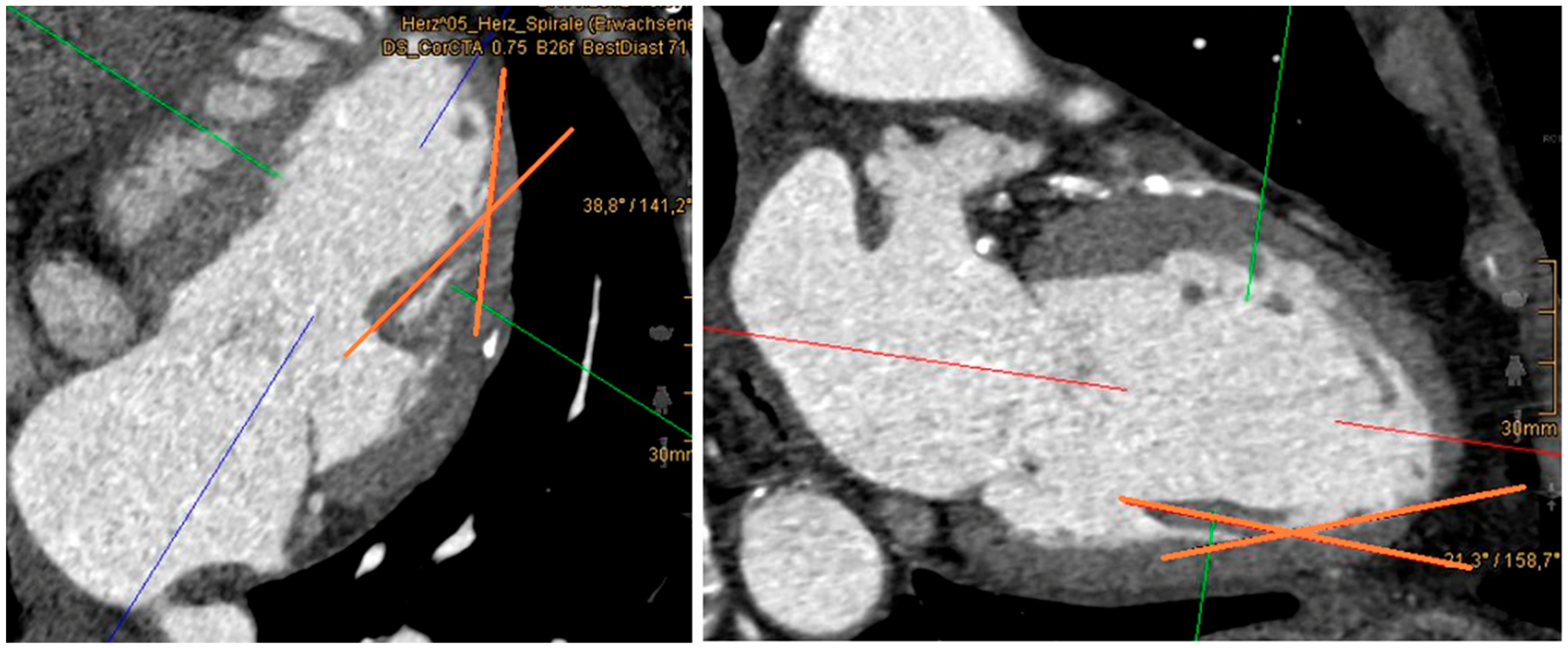
2. Materials and Methods
- (1)
- Control group (31 patients): This subgroup included all patients without mitral regurgitation (MR) or coronary heart disease (CHD). All individuals in this group had a normal ejection fraction (EF).
- (2)
- MR-only subgroup (37 patients): Patients in this group had isolated MR without coexisting CHD. A reduced EF was observed in 5 of these patients.
- (3)
- CHD-only subgroup (25 patients): Patients in this group had isolated CHD without MR. EF was reduced in 2 of these individuals.
- (4)
- Combined CHD and MR subgroup (55 patients): These patients had both CHD and MR. EF was reduced in 18 of them.
3. Statistical Data Analysis
4. Results
5. Discussion
6. Clinical Impact
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHD | coronary heart disease |
| CT | cardiac computed tomography |
| EF | ejection fraction |
| LAD | left anterior descending artery |
| LCX | left circumflex artery |
| MPR | multiplanar reconstruction |
| MR | mitral regurgitation |
| PACS | picture archiving and communication system |
| PM | papillary muscle |
| w/o | without |
References
- Watanabe, N. The Mitral Valve Complex: Divine Perfection. Circ. Cardiovasc. Imaging 2016, 9, e004353. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Fu, W.; Li, F.; Gu, H.; Cui, N.; Lin, Y.; Xie, M.; Yang, Y. Left Ventricular Papillary Muscle: Anatomy, Pathophysiology, and Multimodal Evaluation. Diagnostics 2024, 14, 1270. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y. Anatomy of the mitral valve. Heart 2002, 88, iv5–iv10. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kaji, S.; An, Y.; Nishino, T.; Tani, T.; Kitai, T.; Furukawa, Y. Interpapillary muscle distance independently affects severity of functional mitral regurgitation in patients with systolic left ventricular dysfunction. J. Thorac. Cardiovasc. Surg. 2014, 148, 434–440.e1. [Google Scholar] [CrossRef]
- Dudzinski, D.M.; Hung, J. Echocardiographic assessment of ischemic mitral regurgitation. Cardiovasc. Ultrasound 2014, 12, 46. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Chello, M.; Mihos, C.G. Papillary muscle approximation in mitral valve repair for secondary MR. J. Thorac. Dis. 2017, 9 (Suppl. S7), S635–S639. [Google Scholar] [CrossRef]
- Harmel, E.K.; Reichenspurner, H.; Girdauskas, E. Subannular reconstruction in secondary mitral regurgitation: A meta-analysis. Heart 2018, 104, 1783–1790. [Google Scholar] [CrossRef]
- Delgado, V.; Tops, L.F.; Schuijf, J.D.; de Roos, A.; Brugada, J.; Schalij, M.J.; Thomas, J.D.; Bax, J.J. Assessment of Mitral Valve Anatomy and Geometry With Multislice Computed Tomography. JACC Cardiovasc. Imaging 2009, 2, 556–565. [Google Scholar] [CrossRef]
- Stolzmann, P.; Scheffel, H.; Leschka, S.; Schertler, T.; Frauenfelder, T.; Kaufmann, P.A.; Marincek, B.; Alkadhi, H. Reference values for quantitative left ventricular and left atrial measurements in cardiac computed tomography. Eur. Radiol. 2008, 18, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef]
- Molina, D.K.; DiMaio, V.J.M. Normal Organ Weights in Men: Part I—The Heart. Am. J. Forensic Med. Pathol. 2012, 33, 362–367. [Google Scholar] [CrossRef]
- Molina, D.K.; DiMaio, V.J.M. Normal Organ Weights in Women: Part I—The Heart. Am. J. Forensic Med. Pathol. 2015, 36, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Fulton, N.L.; Bolen, M. Magnetic resonance imaging of the papillary muscles of the left ventricle: Normal anatomy, variants, and abnormalities. Insights Imaging 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Berdajs, D.; Lajos, P.; Turina, M.I. A new classification of the mitral papillary muscle. Med. Sci. Monit. 2005, 11, Br18–Br21. [Google Scholar] [PubMed]
- Saha, A.; Roy, S. Papillary muscles of left ventricle—Morphological variations and it’s clinical relevance. Indian Heart J. 2018, 70, 894–900. [Google Scholar] [CrossRef]
- Kavitha, S.; Shastri, D.; Shanthi, K. Morphometric Analysis of Papillary Muscles in Both Ventricles of Hearts. IOSR J. Dent. Med. Sci. 2018, 17, 26–33. [Google Scholar]
- Yiu, S.F.; Enriquez-Sarano, M.; Tribouilloy, C.; Seward, J.B.; Tajik, A.J. Determinants of the Degree of Functional Mitral Regurgitation in Patients with Systolic Left Ventricular Dysfunction. Circulation 2000, 102, 1400–1406. [Google Scholar] [CrossRef]
- Otsuji, Y.; Levine, R.A.; Takeuchi, M.; Sakata, R.; Tei, C. Mechanism of ischemic mitral regurgitation. J. Cardiol. 2008, 51, 145–156. [Google Scholar] [CrossRef]
- Voci, P.; Bilotta, F.; Caretta, Q.; Mercanti, C.; Marino, B. Papillary Muscle Perfusion Pattern. Circulation 1995, 91, 1714–1718. [Google Scholar] [CrossRef]
- Massimi, G.; Matteucci, M.; Kowalewski, M.; Ronco, D.; Jiritano, F.; Beghi, C.; Severgnini, P.; Lorusso, R. Surgical treatment of post-infarction papillary muscle rupture: Systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2022, 11, 252–260. [Google Scholar] [CrossRef]
- Estes, E.H.; Dalton, F.M.; Entman, M.L.; Dixon, H.B.; Hackel, D.B. The anatomy and blood supply of the papillary muscles of the left ventricle. Am. Heart J. 1966, 71, 356–362. [Google Scholar] [CrossRef]
- Wendell, D.; Jenista, E.; Kim, H.W.; Chen, E.-L.; Azevedo, C.F.; Kaolawanich, Y.; Alenezi, F.; Rehwald, W.; Darty, S.; Parker, M.; et al. Assessment of Papillary Muscle Infarction with Dark-Blood Delayed Enhancement Cardiac MRI in Canines and Humans. Radiology 2022, 305, 329–338. [Google Scholar] [CrossRef]
- Agricola, E.; Oppizzi, M.; Pisani, M.; Meris, A.; Maisano, F.; Margonato, A. Ischemic mitral regurgitation: Mechanisms and echocardiographic classification. Eur. J. Echocardiogr. 2007, 9, 207–221. [Google Scholar] [CrossRef][Green Version]
- Uemura, T.; Otsuji, Y.; Nakashiki, K.; Yoshifuku, S.; Maki, Y.; Yu, B.; Mizukami, N.; Kuwahara, E.; Hamasaki, S.; Biro, S.; et al. Papillary Muscle Dysfunction Attenuates Ischemic Mitral Regurgitation in Patients with Localized Basal Inferior Left Ventricular Remodeling: Insights from Tissue Doppler Strain Imaging. J. Am. Coll. Cardiol. 2005, 46, 113–119. [Google Scholar] [CrossRef]
- Batta, A.; Hatwal, J.; Batta, A.; Verma, S.; Sharma, Y.P. Atrial fibrillation and coronary artery disease: An integrative review focusing on therapeutic implications of this relationship. World J. Cardiol. 2023, 15, 229–243. [Google Scholar] [CrossRef]
- Katsi, V.; Georgiopoulos, G.; Magkas, N.; Oikonomou, D.; Virdis, A.; Nihoyannopoulos, P.; Toutouzas, K.; Tousoulis, D. The Role of Arterial Hypertension in Mitral Valve Regurgitation. Curr. Hypertens. Rep. 2019, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.C., Jr.; Gillinov, A.M.; Blackstone, E.H.; Rajeswaran, J.; Cohen, G.; Najam, F.; Shiota, T.; Sabik, J.F.; Lytle, B.W.; McCarthy, P.M.; et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J. Thorac. Cardiovasc. Surg. 2004, 128, 916–924. [Google Scholar] [CrossRef]
- Bouma, W.; van der Horst, I.C.C.; Wijdh-den Hamer, I.J.; Erasmus, M.E.; Zijlstra, F.; Mariani, M.A.; Ebels, T. Chronic ischaemic mitral regurgitation. Current treatment results and new mechanism-based surgical approaches. Eur. J. Cardio-Thorac. Surg. 2010, 37, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; van de Veire, N.R.; Klautz, R.J.M.; Versteegh, M.I.M.; Holman, E.R.; Westenberg, J.J.M.; Boersma, E.; van der Wall, E.E.; Bax, J.J.; Dion, R.A.E. Restrictive Mitral Annuloplasty Cures Ischemic Mitral Regurgitation and Heart Failure. Ann. Thorac. Surg. 2008, 85, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Acker, M.A.; Parides, M.K.; Perrault, L.P.; Moskowitz, A.J.; Gelijns, A.C.; Voisine, P.; Smith, P.K.; Hung, J.W.; Blackstone, E.H.; Puskas, J.D.; et al. Mitral-Valve Repair versus Replacement for Severe Ischemic Mitral Regurgitation. N. Engl. J. Med. 2014, 370, 23–32. [Google Scholar] [CrossRef]
- Carpentier, A. Cardiac valve surgery—The “French correction”. J. Thorac. Cardiovasc. Surg. 1983, 86, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Irqsusi, M.; Mansouri, A.L.; Ramaswamy, A.; Rexin, P.; Salman, M.; Mahmood, S.; Mirow, N.; Ghazi, T.; Ramzan, R.; Rastan, A.J.; et al. Role of matrix metalloproteinases in mitral valve regurgitation: Association between the of MMP-1, MMP-9, TIMP-1, and TIMP-2 expression, degree of mitral valve insufficiency, and pathologic etiology. J. Card. Surg. 2022, 37, 1613–1622. [Google Scholar] [CrossRef] [PubMed]

| Men | Women | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | ||
| body surface area (m2) | 1.54 | 2.74 | 2.03 | 0.22 | 1.35 | 2.14 | 1.85 | 0.18 | <0.001 |
| midventricular diameter (mm) | 29.00 | 86.70 | 54.32 | 9.13 | 34.9 | 86.70 | 50.15 | 8.02 | <0.001 |
| ventricular length (mm) | 60.50 | 131.00 | 84.36 | 11.21 | 58.40 | 90.20 | 75.14 | 7.22 | <0.001 |
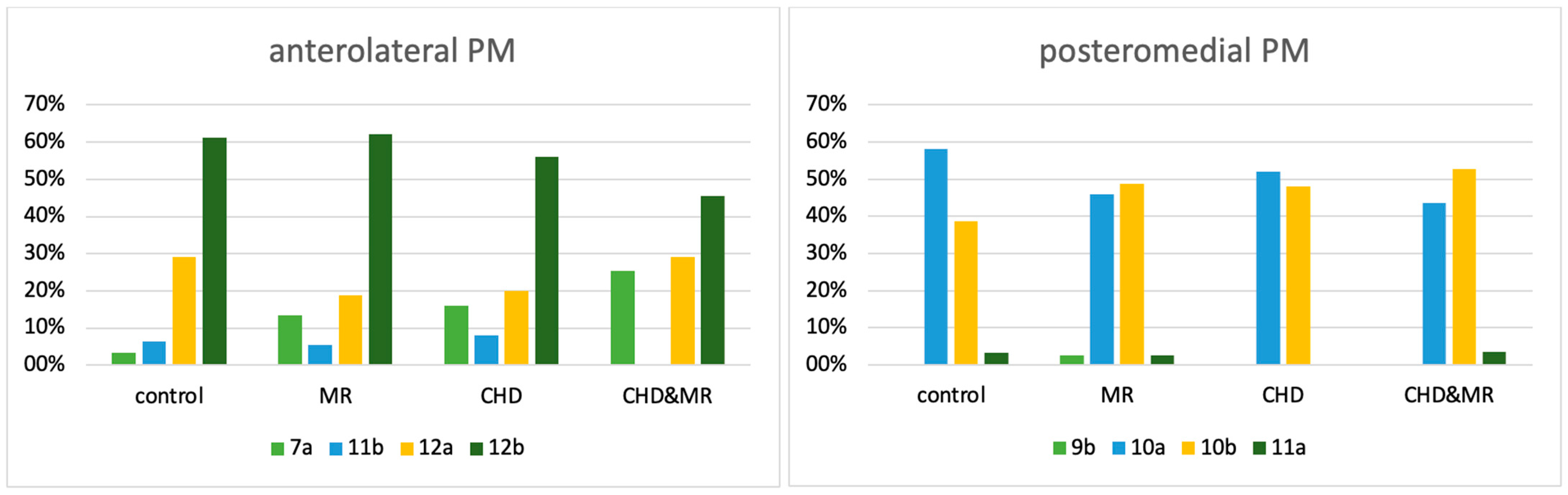
| Control | MR | CHD | CHD&MR | Total | |
|---|---|---|---|---|---|
| 7a | 1 | 5 | 4 | 14 | 24 |
| 11b | 2 | 2 | 2 | 0 | 6 |
| 12a | 9 | 7 | 5 | 16 | 37 |
| 12b | 19 | 23 | 14 | 25 | 81 |
| total | 31 | 37 | 25 | 55 | 148 |
| Control | MR | CHD | CHD&MR | Total | |
| 9b | 0 | 1 | 0 | 0 | 1 |
| 10a | 18 | 17 | 13 | 24 | 72 |
| 10b | 12 | 18 | 12 | 29 | 71 |
| 11a | 1 | 1 | 0 | 2 | 4 |
| total | 31 | 37 | 25 | 55 | 148 |
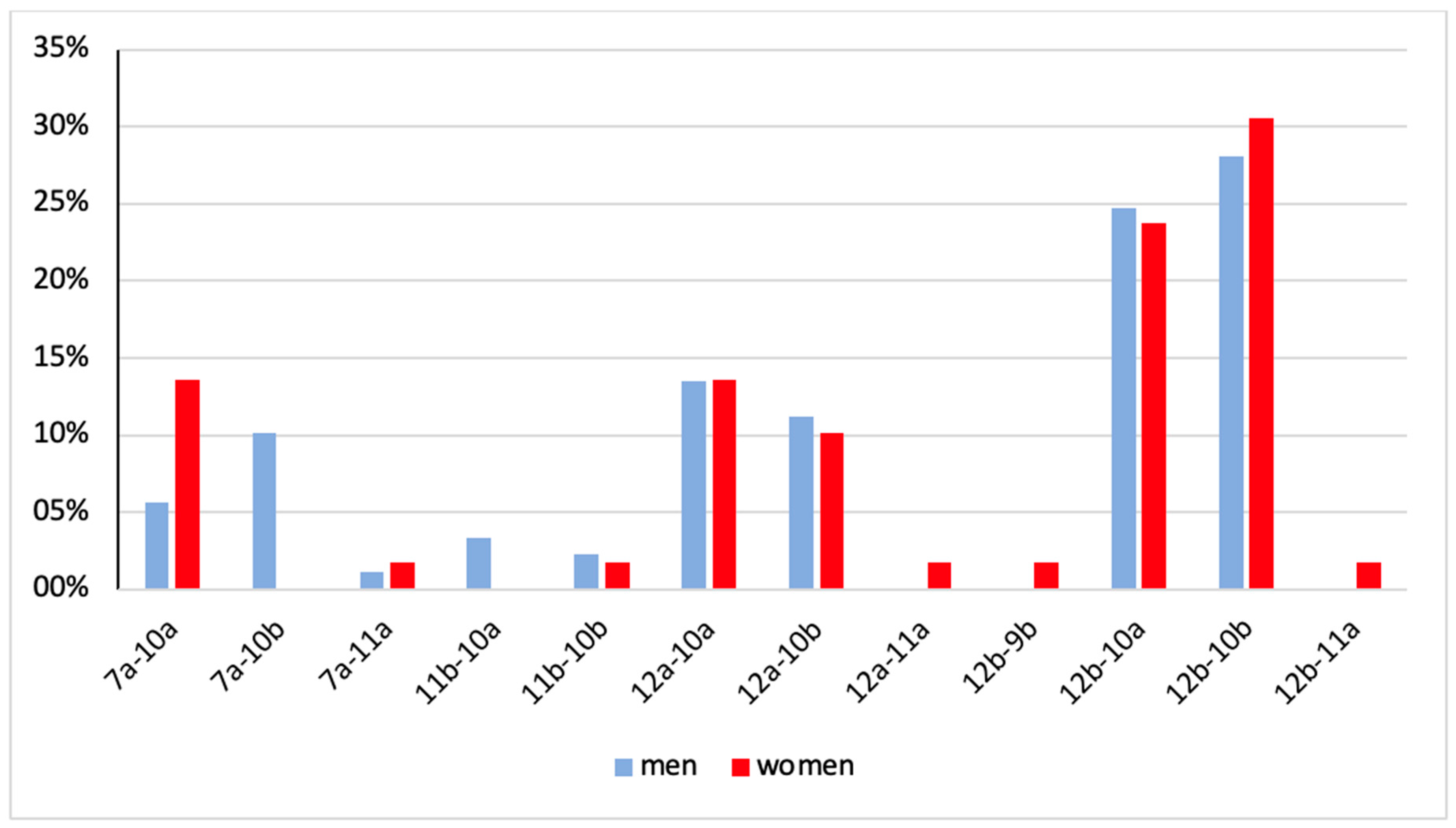
| 7a-10a | 7a-10b | 7a-11a | 11b-10a | 11b-10b | 12a-10a | 12a-10b | 12a-11a | 12b-9b | 12b-10a | 12b-10b | 12b-11a | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| men | 5 (5.6%) | 9 (10.1%) | 1 (1.1%) | 3 (3.4%) | 2 (2.3%) | 12 (13.5%) | 10 (11.2%) | 0 (0.0%) | 0 (0.0%) | 22 (24.7%) | 25 (28.1%) | 0 (0.0%) | 89 (100%) |
| women | 8 (13.6%) | 0 (0.0%) | 1 (1.7%) | 0 (0.0%) | 1 (1.7%) | 8 (13.6%) | 6 (10.7%) | 1 (1.7%) | 1 (1.7%) | 14 23.7%) | 18 (30.5%) | 1 (1.7%) | 59 (100%) |
| total | 13 (8.8%) | 9 (6.1%) | 2 (1.4%) | 3 (2.0%) | 3 (3.0%) | 20 (13.5%) | 16 (10.8%) | 1 (0.1%) | 1 (0.1%) | 36 (24.3%) | 43 (29.1%) | 1 (0.1%) | 148 (100%) |
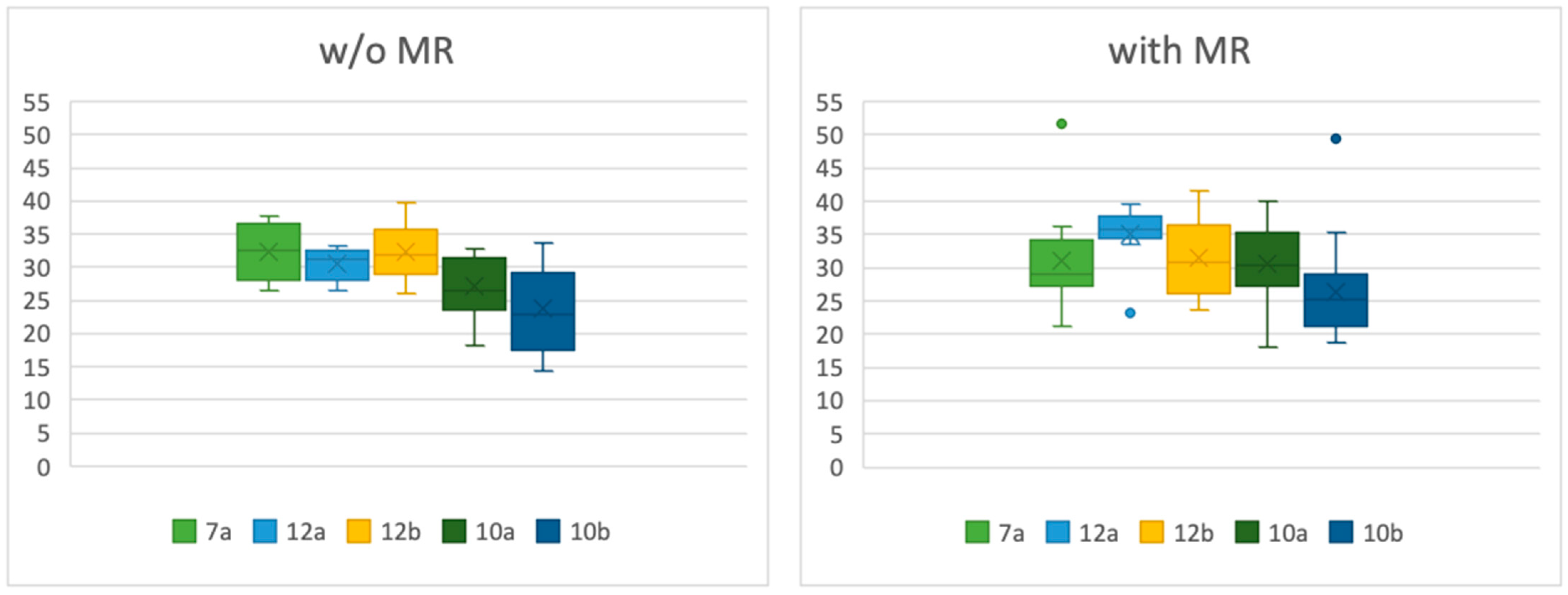
| w/o MI | with MI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | SD | Mean | Median | Min | Max | SD | Mean | Median | p-Value | |
| 7a | 26.6 | 37.8 | 4.58 | 32.38 | 32.55 | 26.6 | 51.7 | 7.24 | 32.04 | 29.3 | 0.5135 |
| 12a | 26.4 | 33.3 | 2.53 | 30.53 | 31.3 | 23.2 | 39.5 | 4.77 | 35.04 | 35.7 | 0.0095 |
| 12b | 26.0 | 39.7 | 4.36 | 32.33 | 31.8 | 23.6 | 41.5 | 5.61 | 31.45 | 30.8 | 0.6075 |
| 10a | 18.3 | 32.9 | 4.24 | 27.08 | 26.4 | 18.0 | 40.1 | 5.79 | 30.57 | 30.35 | 0.0459 |
| 10b | 14.3 | 33.6 | 5.91 | 23.72 | 22.9 | 18.8 | 49.5 | 6.77 | 26.41 | 25.2 | 0.6262 |
| w/o MI | with MI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | SD | Mean | Median | Min | Max | SD | Mean | Median | p-Value | |
| 7a | 35.5 | 35.5 | - | 35.5 | 35.5 | 28.4 | 37.2 | 3.10 | 31.89 | 31.3 | 0.2752 |
| 12a | 21.5 | 43.5 | 6.92 | 34.74 | 35.7 | 20.4 | 40.3 | 5.65 | 33.38 | 35.3 | 0.6014 |
| 12b | 24.5 | 41.5 | 4.43 | 33.97 | 35.1 | 23.2 | 45.4 | 5.67 | 33.86 | 32.7 | 0.8387 |
| 10a | 21.5 | 38.0 | 5.26 | 29.03 | 28.1 | 18.2 | 46.5 | 7.30 | 31.37 | 29.7 | 0.3400 |
| 10b | 18.5 | 35.8 | 7.07 | 36.07 | 24.9 | 17.3 | 40.3 | 7.1 | 27.41 | 25.0 | 0.5868 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Laethem, A.-J.; Figiel, J.; Mahnken, A.H.; Ramzan, R.; Irqsusi, M.; Vogt, S.; Rastan, A.J. Topography of the Papillary Muscles in the Mitral Valve Complex and Their Relevance for Mitral Valve Function. J. Cardiovasc. Dev. Dis. 2025, 12, 348. https://doi.org/10.3390/jcdd12090348
Van Laethem A-J, Figiel J, Mahnken AH, Ramzan R, Irqsusi M, Vogt S, Rastan AJ. Topography of the Papillary Muscles in the Mitral Valve Complex and Their Relevance for Mitral Valve Function. Journal of Cardiovascular Development and Disease. 2025; 12(9):348. https://doi.org/10.3390/jcdd12090348
Chicago/Turabian StyleVan Laethem, Alina-Jutta, Jens Figiel, Andreas H. Mahnken, Rabia Ramzan, Marc Irqsusi, Sebastian Vogt, and Ardawan J. Rastan. 2025. "Topography of the Papillary Muscles in the Mitral Valve Complex and Their Relevance for Mitral Valve Function" Journal of Cardiovascular Development and Disease 12, no. 9: 348. https://doi.org/10.3390/jcdd12090348
APA StyleVan Laethem, A.-J., Figiel, J., Mahnken, A. H., Ramzan, R., Irqsusi, M., Vogt, S., & Rastan, A. J. (2025). Topography of the Papillary Muscles in the Mitral Valve Complex and Their Relevance for Mitral Valve Function. Journal of Cardiovascular Development and Disease, 12(9), 348. https://doi.org/10.3390/jcdd12090348







