Oral Anticoagulants in Chronic Thromboembolic Pulmonary Hypertension: Tradition or Innovation?
Abstract
1. Introduction
2. Methods
3. Results and Discussion
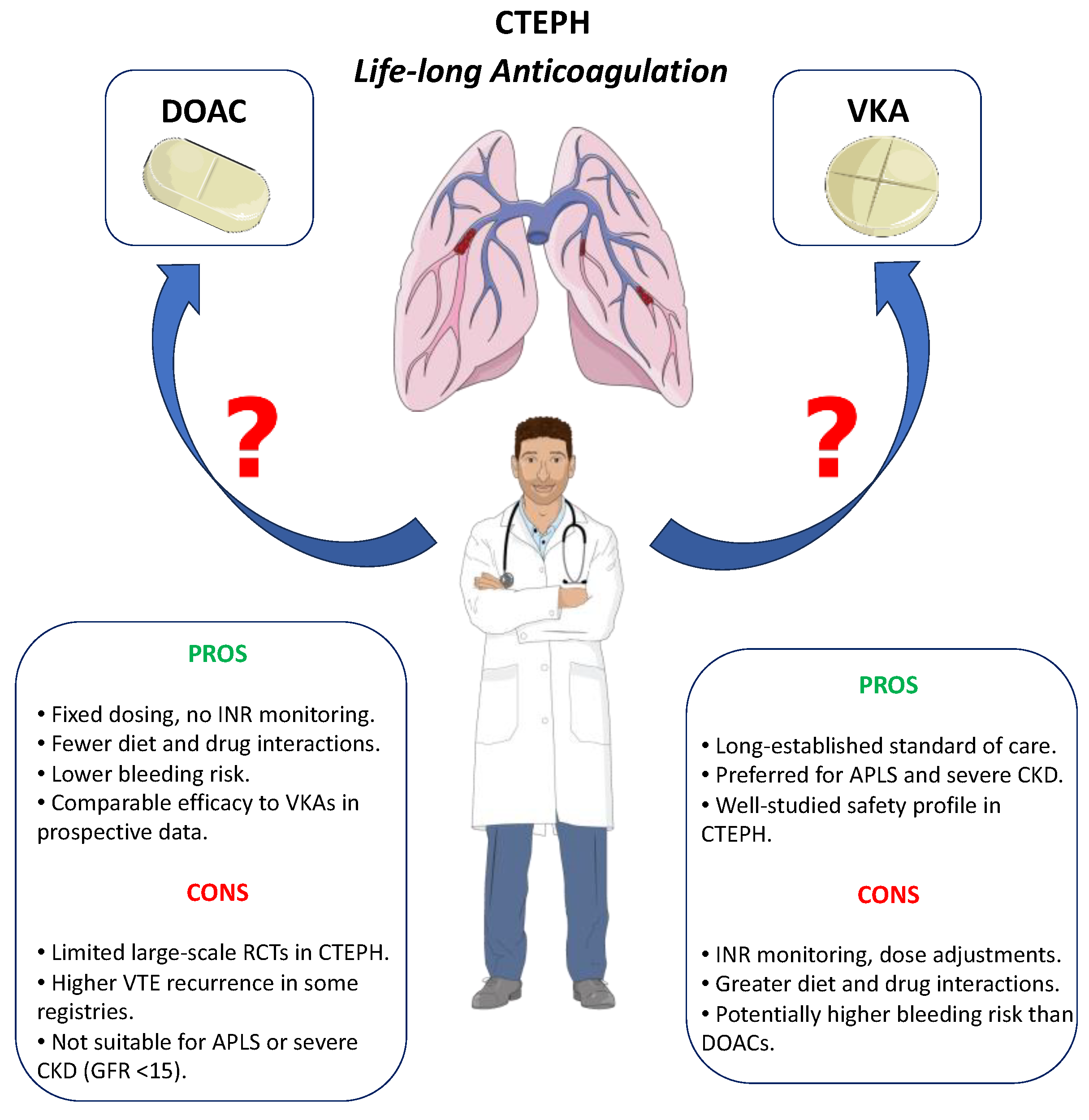
- -
- are DOACs as effective as VKAs in preventing further venous thromboembolic events?
- -
- do DOACs have the same safety profile as VKAs?
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTEPH | Chronic thromboembolic pulmonary hypertension |
| DOAC | Direct oral anticoagulants |
| ESC | European Society of Cardiology |
| INR | International Normalized Ratio |
| PH | Pulmonary hypertension |
| RCT | randomized controlled trials |
| VKA | Vitamin K antagonists |
| VTE | venous thromboembolism |
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Gavilanes-Oleas, F.A.; Alves, J.L.; Fernandes, C.J.C.; Prada, L.F.L.; Salibe, W.; Terra, M.; Morinaga, L.; Hoette, S.; Jardim, C.; Souza, R.; et al. Use of direct oral anticoagulants for chronic thromboembolic pulmonary hypertension. Clinics 2018, 73, e216. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Abe, K.; Funakoshi, K.; Tamura, Y.; Nakashima, N.; Todaka, K.; Taniguchi, Y.; Inami, T.; Adachi, S.; Tsujino, I.; et al. Long-term outcome of chronic thromboembolic pulmonary hypertension using direct oral anticoagulants and warfarin: A Japanese prospective cohort study. J. Thromb. Haemost. 2023, 21, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Bunclark, K.; Newnham, M.; Chiu, Y.; Ruggiero, A.; Villar, S.S.; Cannon, J.E.; Coghlan, G.; Corris, P.A.; Howard, L.; Jenkins, D.; et al. A multicenter study of anticoagulation in operable chronic thromboembolic pulmonary hypertension. J. Thromb. Haemost. 2020, 18, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Simonneau, G.; Pittrow, D.; Delcroix, M.; Pepke-Zaba, J.; Langleben, D.; Mielniczuk, L.M.; Subias, P.E.; Snijder, R.J.; Barberà, J.A.; et al. Oral anticoagulants (NOAC and VKA) in chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2022, 41, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.; Alotaibi, M.; Fernandes, T.M.; Kim, S.; Kerr, K.M.; Yang, J.; Pretorius, V.; Madani, M.; Kim, N.H. Direct oral anticoagulants in patients with chronic thromboembolic pulmonary hypertension and the presence of recent thrombus during pulmonary endarterectomy. Pulm. Circ. 2022, 12, e12110. [Google Scholar] [CrossRef] [PubMed]
- Barati, S.; Amini, H.; Ahmadi, Z.H.; Dastan, A.; Kashani, B.S.; Eskandari, R.; Dastan, F. Evaluating the efficacy and safety of rivaroxaban as a warfarin alternative in chronic thromboembolic pulmonary hypertension patients undergoing pulmonary endarterectomy: A randomized clinical trial. Rev. Port. Cardiol. 2023, 42, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Watanabe, H.; Taniguchi, Y.; Ikeda, N.; Inami, T.; Yasuda, S.; Murohara, T.; Hatano, M.; Tamura, Y.; Yamashita, J.; et al. A Multicenter, Single-Blind, Randomized, Warfarin-Controlled Trial of Edoxaban in Patients with Chronic Thromboembolic Pulmonary Hypertension: KABUKI Trial. Circulation 2024, 149, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.M.; Panama, G.; Kim, A.G.; Rayamajhi, S.; Abela, G.S. Clinical outcomes between direct oral anticoagulants versus vitamin K antagonists in chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, L.; Liang, S.; Liu, H. Direct Oral Anticoagulants in Chronic Thromboembolic Pulmonary Hypertension: First Meta-Analysis of Prospective Studies. Clin. Appl. Thromb. Hemost. 2024, 30, 10760296241257931. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, N.; Shirakawa, R.; Fukumoto, Y.; Sugimura, K.; Miyata, S.; Miura, Y.; Nochioka, K.; Miura, M.; Tatebe, S.; Aoki, T.; et al. Platelets are highly activated in patients of chronic thromboembolic pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Remková, A.; Šimková, I.; Valkovičová, T. Platelet abnormalities in chronic thromboembolic pulmonary hypertension. Int. J. Clin. Exp. Med. 2015, 8, 9700–9707. [Google Scholar] [PubMed] [PubMed Central]
| Author | Year of Publication | Study Design | Patients’ Characteristics | Type of Anticoagulation (Number of Patients) | Effectiveness in Prevention of VTE Recurrence * | Safety Profile * |
|---|---|---|---|---|---|---|
| Gavilanes-Oleas et al. [2] | 2018 | Retrospective | Non-operated | Rivaroxaban (16), Dabigatran (3), Apixaban (1) | No VTE recurrence | 1 major bleeding after traumatic fall |
| Hosokawa et al. [3] | 2023 | Prospective | Mixed | DOACs (481), VKA (446) | Similar VTE recurrence | Fewer major bleedings with DOACs |
| Bunclark et al. [4] | 2020 | Retrospective | Operated | DOAC (204), VKA (808) | Higher VTE recurrence with DOACs | |
| Humbert et al. [5] | 2022 | Prospective registry | Non-operated | DOAC (198), VKA (683) | Higher embolic events with DOACs | Similar bleeding |
| Jeong et al. [6] | 2022 | Retrospective | Operated | Not specified | More residual thrombus in DOAC group during PEA | |
| Barati et al. [7] | 2023 | Prospective | Operated | Rivaroxaban (35), Warfarin (61) | No significant differences in outcomes | |
| KABUKI Trial [8] | 2024 | RCT | Operated | Edoxaban (37), Warfarin (37) | Similar VTE | Similar bleeding rates |
| Salazar et al. [9] | 2024 | Meta-analysis of data from 10 studies | Mixed | Mixed DOACs vs. VKA | Similar VTE | Similar bleeding rates |
| Zhang et al. [10] | 2024 | Meta-analysis | Mixed | DOAC (751), VKA (1287) | Similar effectiveness | Trend to lower bleeding with DOACs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laviola, D.; Manzi, G.; Recchioni, T.; Luise, M.C.; Mercurio, V.; Mihai, A.; Badagliacca, R.; Papa, S.; Vizza, C.D. Oral Anticoagulants in Chronic Thromboembolic Pulmonary Hypertension: Tradition or Innovation? J. Cardiovasc. Dev. Dis. 2025, 12, 271. https://doi.org/10.3390/jcdd12070271
Laviola D, Manzi G, Recchioni T, Luise MC, Mercurio V, Mihai A, Badagliacca R, Papa S, Vizza CD. Oral Anticoagulants in Chronic Thromboembolic Pulmonary Hypertension: Tradition or Innovation? Journal of Cardiovascular Development and Disease. 2025; 12(7):271. https://doi.org/10.3390/jcdd12070271
Chicago/Turabian StyleLaviola, Domenico, Giovanna Manzi, Tommaso Recchioni, Maria Cristina Luise, Valentina Mercurio, Alexandra Mihai, Roberto Badagliacca, Silvia Papa, and Carmine Dario Vizza. 2025. "Oral Anticoagulants in Chronic Thromboembolic Pulmonary Hypertension: Tradition or Innovation?" Journal of Cardiovascular Development and Disease 12, no. 7: 271. https://doi.org/10.3390/jcdd12070271
APA StyleLaviola, D., Manzi, G., Recchioni, T., Luise, M. C., Mercurio, V., Mihai, A., Badagliacca, R., Papa, S., & Vizza, C. D. (2025). Oral Anticoagulants in Chronic Thromboembolic Pulmonary Hypertension: Tradition or Innovation? Journal of Cardiovascular Development and Disease, 12(7), 271. https://doi.org/10.3390/jcdd12070271





