Long-Term Prognosis in Patients with ST-Elevation Myocardial Infarction Complicated by Heart Failure with Preserved Left Ventricular Ejection Fraction
Abstract
1. Introduction
2. Materials and Methods
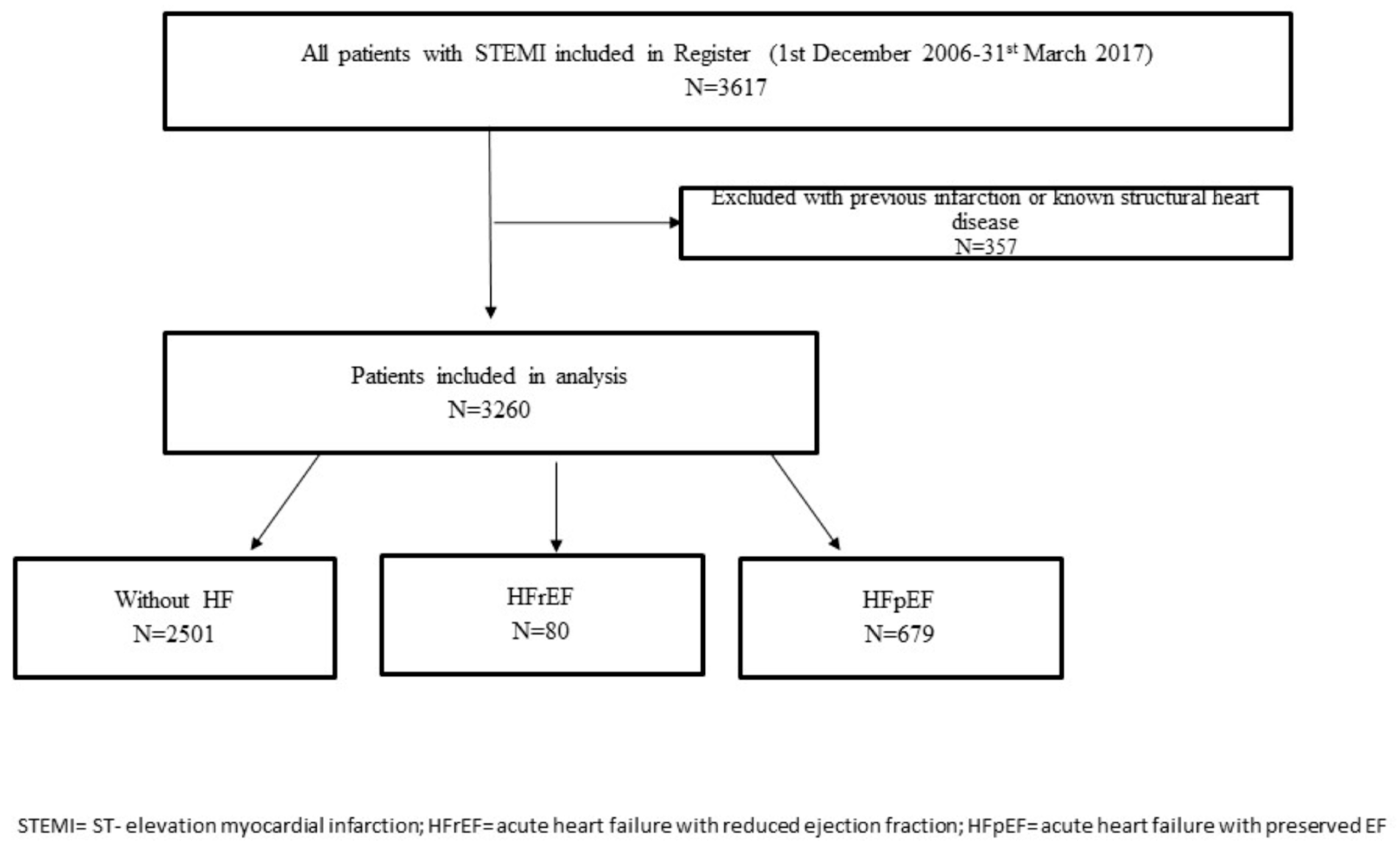
2.1. Study Population, Inclusion and Exclusion Criteria, Data Collection, and Definitions
2.2. Ethics
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Patient Baseline Characteristics and the Incidence of HF
4.2. Prognosis in STEMI Patients with HFpEF
4.3. Mechanisms of HF Development in STEMI Patients
4.4. Clinical Implications
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, J. Persistent mortality and heart failure burden of anterior ST-segment elevation myocardial infarction following primary percutaneous coronary intervention: Real-world evidence from the US Medicare Data Set. BMJ Open 2023, 13, e070210. [Google Scholar] [CrossRef]
- Emet, S.; Elitok, A.; Karaayvaz, E.B.; Engin, B.; Cevik, E.; Tuncozgur, A.; Aydogan, M.; Mercanoglu, F.; Ozcan, M.; Oncul, A. Predictors of left ventricle ejection fraction and early in-hospital mortality in patients with ST-segment elevation myocardial infarction: Single-center data from a tertiary referral university hospital in Istanbul. SAGE Open Med. 2019, 7, 2050312119871785. [Google Scholar] [CrossRef] [PubMed]
- Mavungu Mbuku, J.M.; Mukombola Kasongo, A.; Goube, P.; Miltoni, L.; Nkodila Natuhoyila, A.; M’Buyamba-Kabangu, J.R.; Longo-Mbenza, B.; Kianu Phanzu, B. Factors associated with complications in ST-elevation myocardial infarction: A single-center experience. BMC Cardiovasc. Disord. 2023, 23, 468. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, K.H.; Lee, N.; Park, H.; Cho, J.Y.; Yoon, H.J.; Ahn, Y.; Jeong, M.H.; Cho, J.G. Timing of heart failure development and clinical outcomes in patients with acute myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1193973. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.V.; Holmes, D.; Anderson, M.; Wang, T.Y.; Kontos, M.C.; Wiviott, S.D.; Scirica, B.M. Risk of heart failure complication during hospitalization for acute myocardial infarction in a contemporary population: Insights from the National Cardiovascular Data ACTION Registry. Circ. Heart Fail. 2012, 5, 693–702. [Google Scholar] [CrossRef]
- Ng, V.G.; Lansky, A.J.; Meller, S.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Brodie, B.; Shah, R.; Mehran, R.; Stone, G.W. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: The HORIZONS-AMI trial. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 67–77. [Google Scholar] [CrossRef]
- Yilmaz, A.S.; Kahraman, F.; Ergül, E.; Çetin, M. Left Atrial Volume Index to Left Ventricular Ejection Fraction Ratio Predicted Major Adverse Cardiovascular Event in ST-Elevated Myocardial Infarction Patients during 8 Years of Follow-up. J. Cardiovasc. Echogr. 2021, 31, 227–233. [Google Scholar] [CrossRef]
- Lenell, J.; Lindahl, B.; Erlinge, D.; Jernberg, T.; Spaak, J.; Baron, T. Global longitudinal strain in long-term risk prediction after acute coronary syndrome: An investigation of added prognostic value to ejection fraction. Clin. Res. Cardiol. 2024, 114, 709–718. [Google Scholar] [CrossRef]
- Antonelli, L.; Katz, M.; Bacal, F.; Makdisse, M.R.P.; Correa, A.G.; Pereira, C.; Franken, M.; Fava, A.N.; Junior, C.V.S.; Pesaro, A.E.P. Heart failure with preserved left ventricular ejection fraction in patients with acute myocardial infarction. Arq. Bras. Cardiol. 2015, 105, 145–150. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Z. Predictors of in-hospital heart failure in patients with acute anterior wall ST-segment elevation myocardial infarction. Int. J. Cardiol. 2023, 375, 104–109. [Google Scholar] [CrossRef]
- Hamilton, E.; Desta, L.; Lundberg, A.; Alfredsson, J.; Christersson, C.; Erlinge, D.; Kellerth, T.; Lindmark, K.; Omerovic, E.; Reitan, C.; et al. Prevalence and prognostic impact of left ventricular systolic dysfunction or pulmonary congestion after acute myocardial infarction. ESC Heart Fail. 2023, 10, 1347–1357. [Google Scholar] [CrossRef]
- De Luca, L.; Cicala, S.D.; D’Errigo, P.; Cerza, F.; Mureddu, G.F.; Rosato, S.; Badoni, G.; Seccareccia, F.; Baglio, G. Impact of age, gender and heart failure on mortality trends after acute myocardial infarction in Italy. Int. J. Cardiol. 2022, 348, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Hoedemaker, N.P.; Roolvink, V.; de Winter, R.J.; van Royen, N.; Fuster, V.; García-Ruiz, J.M.; Er, F.; Gassanov, N.; Hanada, K.; Okumura, K.; et al. Early intravenous beta-blockers in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: A patient-pooled meta-analysis of randomized clinical trials. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Yndigegn, T.; Lindahl, B.; Mars, K.; Alfredsson, J.; Benatar, J.; Brandin, L.; Erlinge, D.; Hallen, O.; Held, C.; Hjalmarsson, P.; et al. Beta-Blockers after Myocardial Infarction and Preserved Ejection Fraction. N. Engl. J. Med. 2024, 390, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Teng, T.H.K.; Finn, J.; Knuiman, M.; Briffa, T.; Stewart, S.; Sanfilippo, F.M.; Ridout, S.; Hobbs, M. Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: A population-based study of 20,812 patients with first acute myocardial infarction in Western Australia. J. Am. Heart Assoc. 2013, 2, e000172. [Google Scholar] [CrossRef]
- Sulo, G.; Igland, J.; Nygård, O.; Vollset, S.E.; Ebbing, M.; Poulter, N.; Egeland, G.M.; Cerqueira, C.; Jørgensen, T.; Tell, G.S. Prognostic Impact of In-Hospital and Postdischarge Heart Failure in Patients with Acute Myocardial Infarction: A Nationwide Analysis Using Data From the Cardiovascular Disease in Norway (CVDNOR) Project. J. Am. Heart Assoc. 2017, 6, e005277. [Google Scholar] [CrossRef]
- Jiang, H.; Fang, T.; Cheng, Z. Mechanism of heart failure after myocardial infarction. J. Int. Med. Res. 2023, 51, 3000605231202573. [Google Scholar] [CrossRef]
- Xu, M.; Yan, L.; Xu, J.; Yang, X.; Jiang, T. Predictors and prognosis for incident in-hospital heart failure in patients with preserved ejection fraction after first acute myocardial infarction: An observational study. Medicine 2018, 97, e11093. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Zhu, Y.; Zeng, J.; Huang, H.; Yang, W.; Peng, K.; Wu, M. A diagnostic prediction model for the early detection of heart failure following primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. Am. J. Cardiovasc. Dis. 2024, 14, 208–219. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Vaduganathan, M.; Claggett, B.L.; John, J.E.; Desai, A.S.; Lewis, E.F.; Zile, M.R.; Carson, P.; Jhund, P.S.; Kober, L.; et al. Myocardial Infarction in Heart Failure with Preserved Ejection Fraction: Pooled Analysis of 3 Clinical Trials. JACC Heart Fail. 2020, 8, 618–626. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; García, C.; de Antonio, M.; Fernandez-Nofrerías, E.; Domingo, M.; Zamora, E.; Moliner, P.; Lupón, J. Impact of a ‘stent for life’ initiative on post-ST elevation myocardial infarction heart failure: A 15 year heart failure clinic experience. ESC Heart Fail. 2018, 5, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Tomoaia, R.; Beyer, R.S.; Simu, G.; Serban, A.M.; Pop, D. Understanding the role of echocardiography in remodeling after acute myocardial infarction and development of heart failure with preserved ejection fraction. Med. Ultrason. 2019, 21, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lenselink, C.; Ricken, K.W.; Groot, H.E.; de Bruijne, T.J.; Hendriks, T.; van der Harst, P.; Voors, A.A.; Lipsic, E. Incidence and predictors of heart failure with reduced and preserved ejection fraction after ST-elevation myocardial infarction in the contemporary era of early percutaneous coronary intervention. Eur. J. Heart Fail. 2024, 26, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef]
- Bennett, K.M.; Hernandez, A.F.; Chen, A.Y.; Mulgund, J.; Newby, L.K.; Rumsfeld, J.S.; Hochman, J.S.; Hoekstra, J.W.; Ohman, E.M.; Gibler, W.B.; et al. Heart failure with preserved left ventricular systolic function among patients with non-ST-segment elevation acute coronary syndromes. Am. J. Cardiol. 2007, 99, 1351–1356. [Google Scholar] [CrossRef]
- Mrdovic, I.; Savic, L.; Lasica, R.; Krljanac, G.; Asanin, M.; Brdar, N.; Djuricic, N.; Marinkovic, J.; Perunicic, J. Efficacy and safety of tirofiban-supported primary percutaneous coronary intervention in patients pretreated with 600 mg clopidogrel: Results of propensity analysis using the clinical center of Serbia STEMI register. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 56–66. [Google Scholar] [CrossRef]
- Kamon, D.; Sugawara, Y.; Soeda, T.; Okamura, A.; Nakada, Y.; Hashimoto, Y.; Ueda, T.; Nishida, T.; Onoue, K.; Okayama, S.; et al. Predominant subtype of heart failure after acute myocardial infarction is heart failure with non-reduced ejection fraction. ESC Heart Fail. 2021, 1, 317–325. [Google Scholar] [CrossRef]
- Akhtar, K.H.; Khan, M.S.; Baron, S.J.; Zieroth, S.; Estep, J.; Burkhoff, D.; Butler, J.; Fudim, M. The spectrum of post-myocardial infarction care: From acute ischemia to heart failure. Prog. Cardiovasc. Dis. 2024, 82, 15–25. [Google Scholar] [CrossRef]
- Trimarchi, G.; Pizzino, F.; Lilli, A.; De Caterina, A.R.; Esposito, A.; Dalmiani, S.; Mazzone, A.; Di Bella, G.; Berti, S.; Paradossi, U. Advanced Lung Cancer Inflammation Index as Predictor of All-Cause Mortality in ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 6059. [Google Scholar] [CrossRef]
- A McDonagh, T.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
| Characteristics | Without HF N = 2501 | HFpEF N = 80 | p-Value * | HFrEF N = 679 | p-Value ** |
|---|---|---|---|---|---|
| Age, years med (IQR) | 58 (45, 75) | 65 (45, 85) | <0.001 | 64 (46, 82) | 0.828 |
| Female, n (%) | 667 (26.6) | 22 (27.5) | 0.453 | 226 (33.2) | 0.064 |
| BMI, med (IQR) | 26.3 (22.1, 30.5) | 25.5 (20.1, 31.5) | 0.967 | 26.8 (21.8, 31.2) | 0.897 |
| Previous angina, n (%) | 174 (7) | 8 (10) | 0.173 | 57 (8.5) | 0.436 |
| Previous stroke, n (%) | 79 (3.2) | 4 (5) | 0.254 | 45 (6.6) | 0.234 |
| Diabetes, n (%) | 427 (17.1) | 19 (23.7) | 0.038 | 203 (29.8) | 0.535 |
| Hypertension, n (%) | 1652 (66.1) | 51 (63.8) | 0.730 | 488 (71.8) | 0.335 |
| HLP, n (%) | 1555 (62.2) | 32 (40) | 0.003 | 380 (55.9) | 0.064 |
| Smoking, n (%) | 1432 (57.3) | 30 (37.5) | 0.012 | 279 (41) | 0.792 |
| Family history, n (%) | 874 (34.5) | 24 (30) | 0.951 | 182 (26.8) | 0.238 |
| Pain duration, hours, med (IQR) | 2.5 (2, 3.5) | 2.5 (1.5, 3.5) | 0.678 | 3 (1.5, 4.5) | 0.444 |
| Systolic BP at admission, med (IQR) | 140 (110, 150) | 120 (110, 150) | 0.022 | 130 (110, 155) | 0.975 |
| Heart rate at admission med (IQR) | 76 (70, 85) | 100 (80, 116) | <0.001 | 90 (70, 98) | 0.063 |
| New-onset atrial fibrillation, n (%) | 104 (4.2) | 8 (9.7) | 0.021 | 114 (16.7) | 0.121 |
| Complete AV block, n (%) | 85 (3.4) | 7 (8.7) | 0.004 | 54 (7.9) | 0.808 |
| KIllip class II n (%) | N/A | 78 (97.5) | N/A | 509 (74.9) | <0.001 |
| Killip class III (%) | N/A | 2 (2.5) | N/A | 111 (16.3) | <0.001 |
| KIllip class IV (%) *** | N/A | 0 | N/A | 59 (8.6) | <0.001 |
| Multivessel disease, n (%) | 1323 (52.3) | 46 (56.9) | 0.480 | 471 (69.3) | 0.031 |
| LM stenosis, n (%) | 134 (5.4) | 5 (6.2) | 0.553 | 57 (8.4) | 0.502 |
| LAD as culprit vessel, n (%) | 819 (32.7) | 30 (37.5) | 0.234 | 466 (68.7) | <0.001 |
| RCA as culprit vessel, n (%) | 1230 (49.1) | 32 (40) | 0.123 | 128 (18.9) | <0.001 |
| Cx as culprit vessel, n (%) | 318 (12.7) | 9 (11.2) | 0.545 | 58 (8.6) | <0.001 |
| Stent implanted, n (%) | 2384 (95.3) | 71 (88.9) | 0.013 | 603 (88.9) | 0.989 |
| Postprocedural flow TIMI <3, n (%) | 55 (2.2) | 5 (6.2) | 0.008 | 86 (12.7) | <0.001 |
| Acute stent thrombosis, n (%) | 26 (1.1) | 1 (1.2) | 0.336 | 11 (1.6) | 0.883 |
| Peak CK MB U/L med (IQR) | 1673 (1430, 5435) | 2819 (1645, 4578) | 0.096 | 2883 (1435, 6789) | 0.009 |
| Peak Troponin I ng/mL, med (IQR) | 29 (14.3, 105) | 40 (30, 130,1) | 0.456 | 52.8 (34.6, 159) | 0.051 |
| WBC at admission × 109, med (IQR) | 11.1 (6.8–15.2) | 12.1 (7.7–16.4) | 0.081 | 12.3 (7–16.2) | 0.549 |
| Hemoglobin at admission g/L, med (IQR) | 143 (123, 163) | 142 (126, 156) | 0.061 | 139 (117, 152) | 0.678 |
| Baseline CKD, n (%) | 1711 (68.4) | 52 (65.2) | 0.108 | 517 (76.1) | 0.074 |
| EF, med (IQR) | 50 (40, 61) | 55 (50, 60) | 0.070 | 40 (30, 45) | <0.001 |
| LVEDD, med (IQR) | 5.5 (5.1, 5.8) | 5.5 (5, 5.7) | 0.130 | 5.7 (5.3, 6.7) | 0.018 |
| E/A ratio | 0.85 ± 0.39 | 0.79 ± 0.28 | 0.153 | 0.96 ± 0.65 | 0.014 |
| Therapy at discharge **** | |||||
| Beta blockers, n (%) | 2222 (88.9) | 65 (81.2) | 0.181 | 481 (70.8) | 0.178 |
| ACE inhibitors, n (%) | 2056 (82.2) | 62 (77.5) | 0.204 | 462 (68) | 0.417 |
| Statin, n (%) | 2206 (88.2) | 66 (82.5) | 0.559 | 481 (70.9) | 0.447 |
| Diuretic, n (%) | 259 (10.3) | 4 (5) | <0.001 | 236 (34.8) | <0.001 |
| Calcium antagonist, n (%) | 88 (3.5) | 4 (5) | 0.404 | 15 (2.2) | 0.184 |
| Amiodarone, n (%) | 64 (2.6) | 1 (1.2) | 0.074 | 25 (3.7) | 0.065 |
| Length of hospital stay, days, med (IQR) | 7 (5, 9) | 7 (6, 9) | 0.830 | 9 (7, 13) | <0.001 |
| In-hospital mortality, n (%) | 12 (0.5) | 1 (1.2) | 0.282 | 119 (17.5) | <0.001 |
| Without HF N = 2501 | HFpEF N = 80 | p-Value * | HFrEF N = 679 | p-Value ** | |
|---|---|---|---|---|---|
| 1 month mortality | 19 (0.7) | 1 (1.1) | 0.282 | 124 (18.2) | <0.001 |
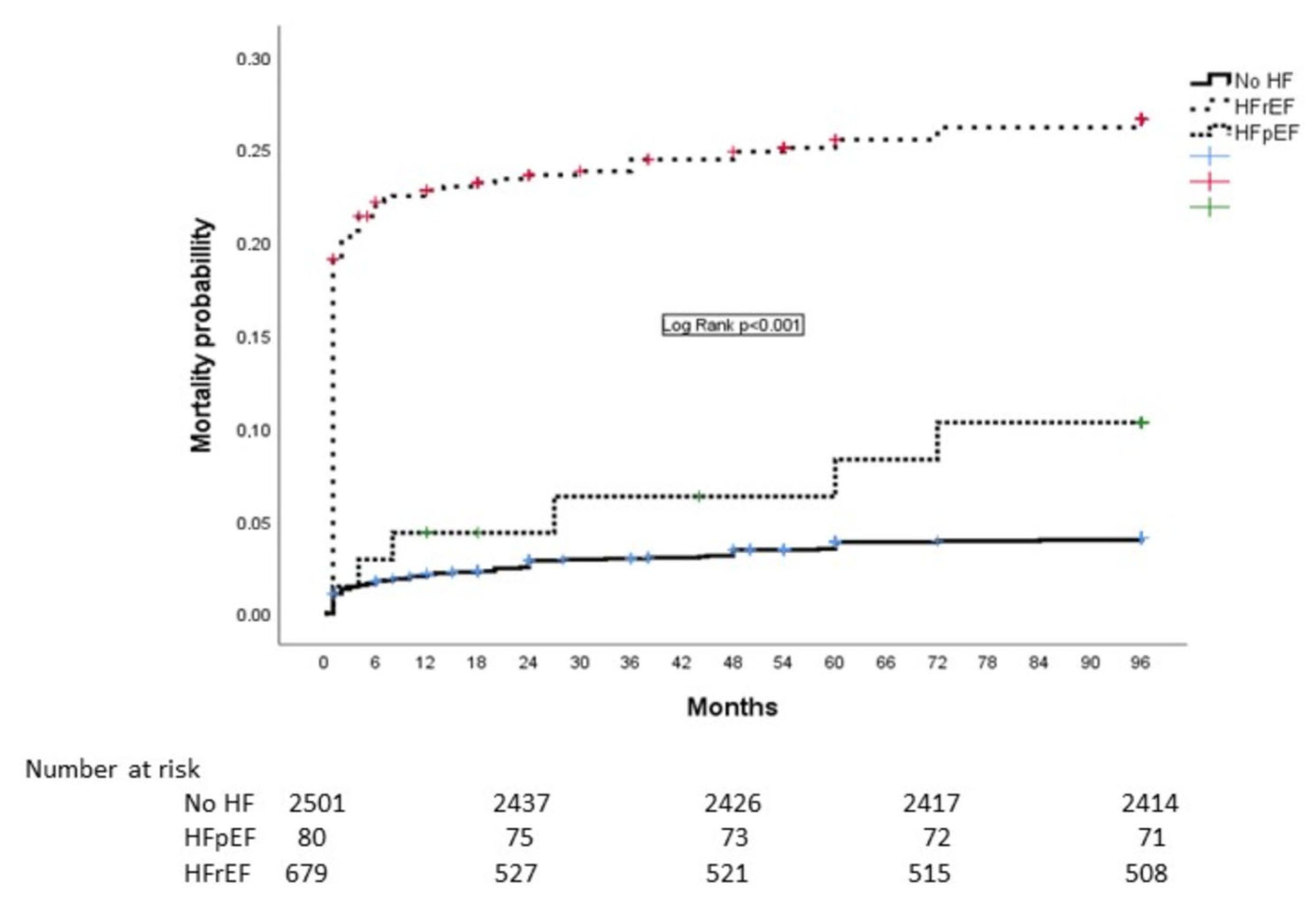
| 1-year mortality | 53 (2.1) | 4 (5) | 0.016 | 150 (22.1) | <0.001 |
| 8-year mortality | 87 (3.5) | 9 (11.2) | <0.001 | 171 (25.1) | <0.001 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Age, years | 1.07 (1.06–1.08) | <0.001 | 1.05 (1.04–1.06) | <0.001 |
| In-hospital HF | 8.03 (5.78–11.95) | <0.001 | 4.78 (3.21–7.12) | <0.001 |
| HFrEF | 7.85 (6.05–10.18) | <0.001 | 4.89 (3.19–6.42) | <0.001 |
| HFpEF | 2.43 (1.06–5.57) | 0.013 | 1.85 (1.26–4.25) | 0.012 |
| Post-procedural flow TIMI < 3 | 6.47 (4.79–8.75) | <0.001 | 2.84 (2.06–3.92) | <0.001 |
| New-onset AF | 4.39 (3.28–5.87) | <0.001 | 1.47 (1.07–2.01) | 0.017 |
| Multivessel disease | 2.58 (2.02–3.95) | <0.001 | 1.39 (1.07–1.80) | 0.014 |
| Anaemia at admission | 2.17 (1.95–3.74) | <0.001 | ||
| Diabetes | 2.11 (1.62–2.73) | <0.001 | ||
| Baseline CKD | 1.46 (1.11–1.67) | 0.051 | ||
| LAD culprit vessel | 1.46 (1.04–1.68) | 0.050 | ||
| Male gender | 1.42 (1.08–1.57) | 0.053 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savic, L.; Simic, D.; Lasica, R.; Krljanac, G.; Matic, D.; Asanin, M.; Stankovic, S.; Antonijevic, N.; Mrdovic, I. Long-Term Prognosis in Patients with ST-Elevation Myocardial Infarction Complicated by Heart Failure with Preserved Left Ventricular Ejection Fraction. J. Cardiovasc. Dev. Dis. 2025, 12, 272. https://doi.org/10.3390/jcdd12070272
Savic L, Simic D, Lasica R, Krljanac G, Matic D, Asanin M, Stankovic S, Antonijevic N, Mrdovic I. Long-Term Prognosis in Patients with ST-Elevation Myocardial Infarction Complicated by Heart Failure with Preserved Left Ventricular Ejection Fraction. Journal of Cardiovascular Development and Disease. 2025; 12(7):272. https://doi.org/10.3390/jcdd12070272
Chicago/Turabian StyleSavic, Lidija, Damjan Simic, Ratko Lasica, Gordana Krljanac, Dragan Matic, Milika Asanin, Sanja Stankovic, Nebojsa Antonijevic, and Igor Mrdovic. 2025. "Long-Term Prognosis in Patients with ST-Elevation Myocardial Infarction Complicated by Heart Failure with Preserved Left Ventricular Ejection Fraction" Journal of Cardiovascular Development and Disease 12, no. 7: 272. https://doi.org/10.3390/jcdd12070272
APA StyleSavic, L., Simic, D., Lasica, R., Krljanac, G., Matic, D., Asanin, M., Stankovic, S., Antonijevic, N., & Mrdovic, I. (2025). Long-Term Prognosis in Patients with ST-Elevation Myocardial Infarction Complicated by Heart Failure with Preserved Left Ventricular Ejection Fraction. Journal of Cardiovascular Development and Disease, 12(7), 272. https://doi.org/10.3390/jcdd12070272







