Navigating Cardiotoxicity in Immune Checkpoint Inhibitors: From Diagnosis to Long-Term Management
Abstract
1. Introduction
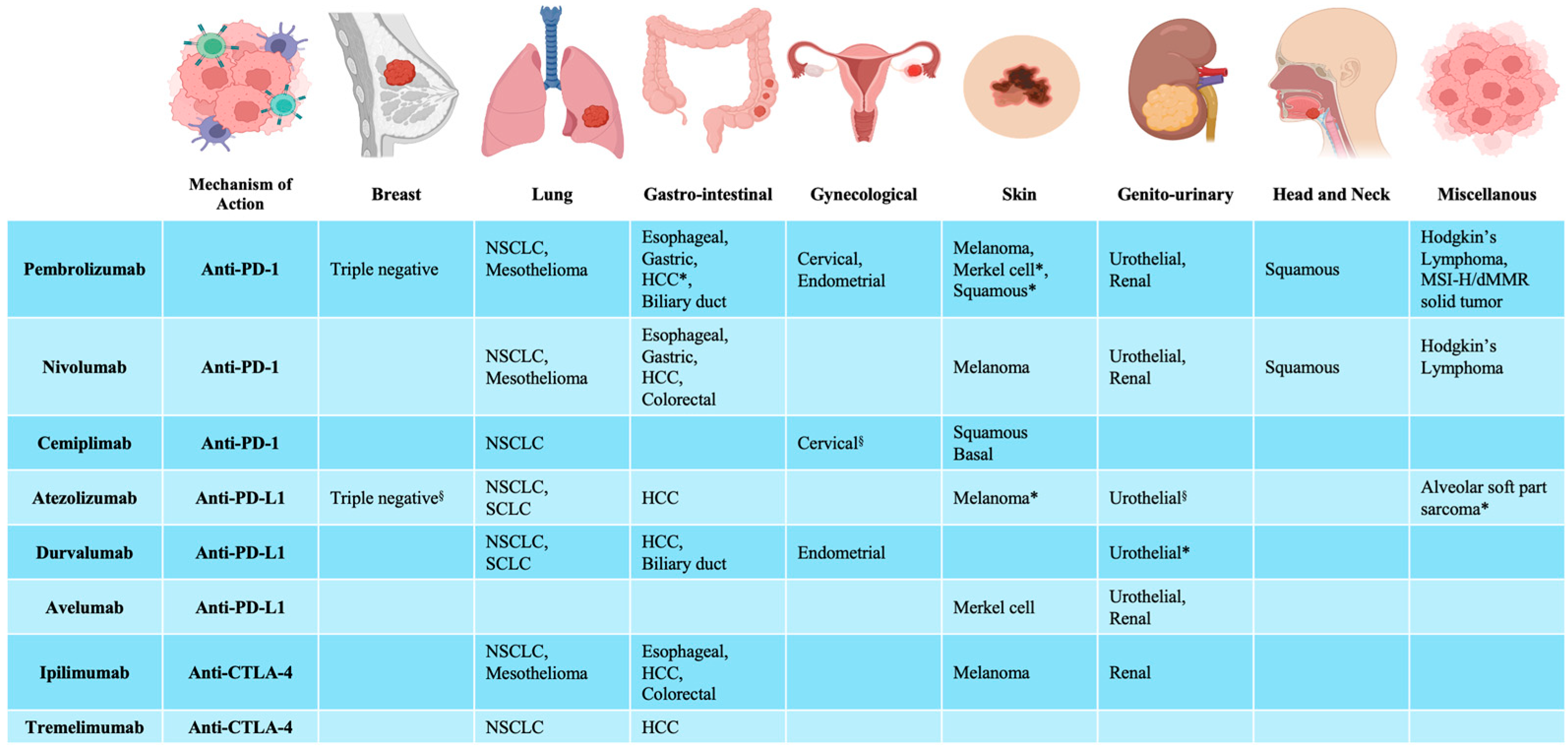
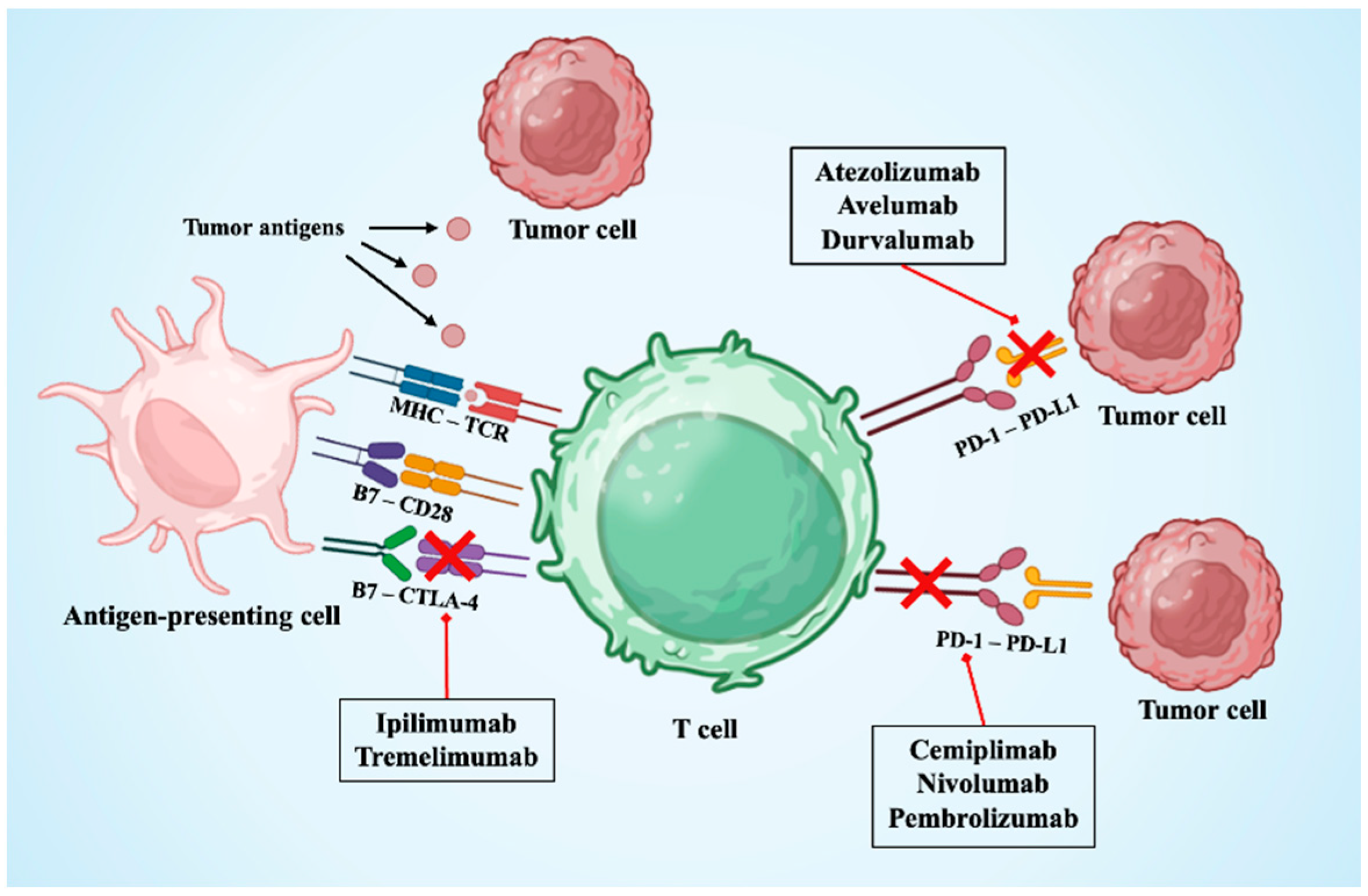
2. Immune Checkpoint Inhibitors
3. Cardiotoxicity Rationale and Onset
3.1. Pathophysiology
3.2. Preclinical and Animal Studies
3.3. Non-Immune ICI Toxicities
4. Risk Factors Related to Cardiotoxicity
4.1. Demographics
4.2. Pre-Existing Cardiovascular Conditions
4.3. Underlying Autoimmune Diseases
4.4. Tumor Type
4.5. Genetic Factors
5. Cardiovascular Complications
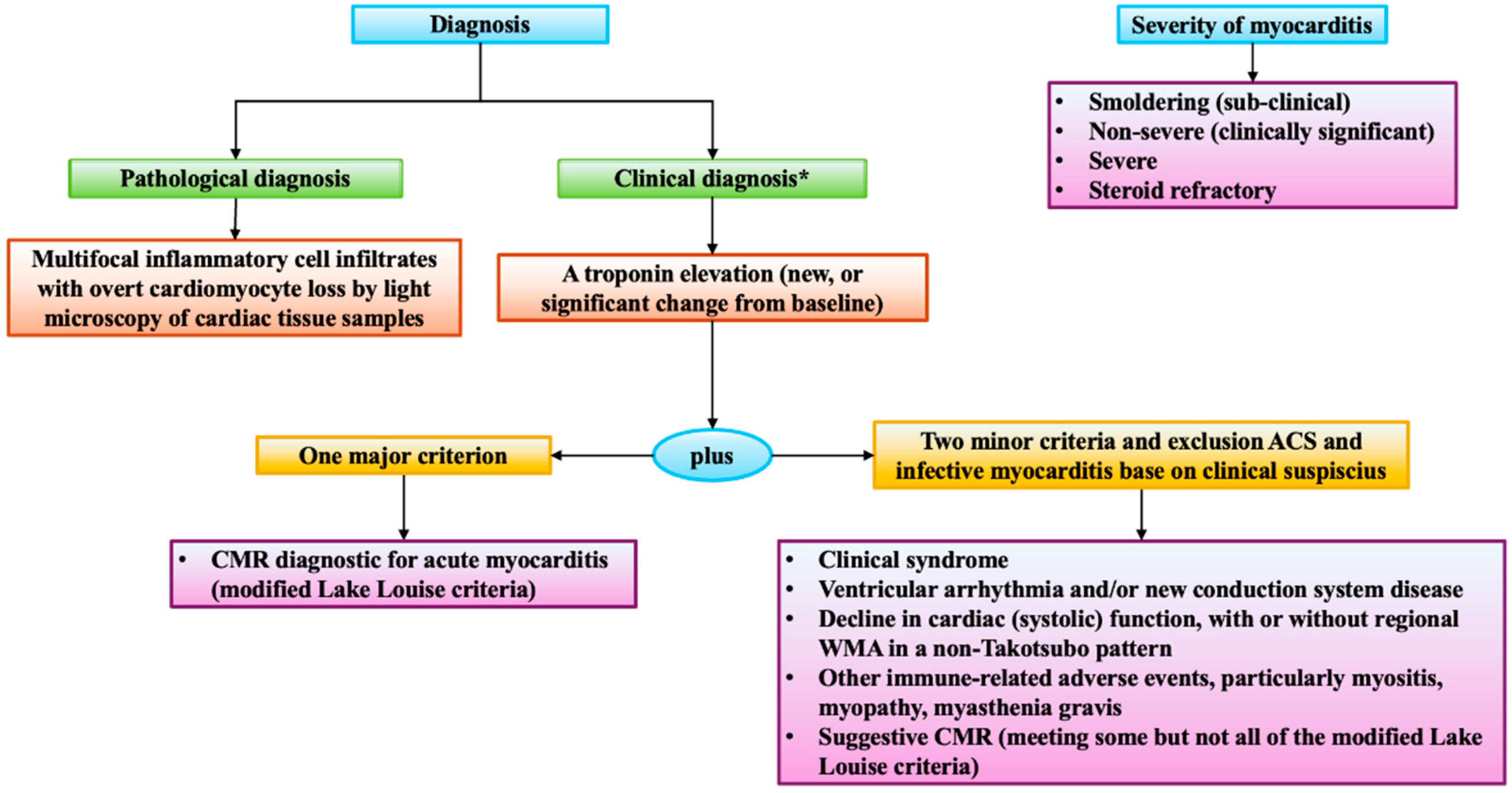
5.1. Myocarditis
5.2. Pericarditis
5.3. Arrhythmias
5.4. Heart Failure
5.5. Vasculitis
5.6. Atherosclerosis
6. Immunotherapies Rechallenge
7. Future Perspectives
- Surveillance during ICI induction. Considering that 40–80% of those events occurred within the first month of ICI therapy, and 50% of myocarditis resulted in fatal outcomes, improvement in the first month of therapy would be crucial. Beyond the routine follow-up in high-risk patients before each ICI cycle, immediate cardio-oncology evaluation should not be delayed upon new ECG or biomarker changes [67,112,113]. Gong et al. proposed a surveillance strategy for patients undergoing ICIs [114]. Additionally, new biomarkers such as single-nucleotide polymorphism in miR-146a and miR-34 seem to predict irAEs in patients treated with ICIs, including cardiac toxicity [115,116,117,118,119].
- TCR profiling as predictive markers. Monitoring peripheral α-myosin-reactive T-cells may identify patients at increased myocarditis risk. Won et al., in a murine model, showed that PD-1 and CTLA-4 blockade led to the expansion and cardiac infiltration of α-myosin–specific CD8+ T cells, directly implicating them in the pathogenesis of myocardial injury [120]. These findings support the notion that loss of peripheral tolerance to cardiac-restricted antigens, such as α-myosin, is a central mechanism driving ICI-related cardiotoxicity and may offer a rationale for developing antigen-targeted therapeutic strategies.
- Neutrophil-to-lymphocyte ratio as an early myocarditis risk flag. A recent publication by Xue et al. evaluated the predictive value of the neutrophil-to-lymphocyte ratio in ICI-related myocarditis in a cohort of 146 patients affected by non-small cell lung cancer [121]. Among the 11.64% of patients who developed ICI-related myocarditis, an elevation in neutrophil-to-lymphocyte ratio ≥3.25 was reported as the most significant indicator of event occurrence (HR 11.094; 95% CI, 3.186–38.631; p < 0.001).
- Longitudinal cardio-oncology monitoring. The long-term consequences of ICI-related myocarditis remain unknown. The potential role of myocarditis as a “second hit” in genetically or otherwise predisposed individuals, potentially leading to cardiomyopathy (including, but not limited to, dilated cardiomyopathy), is receiving increasing attention [122,123]. However, no data are currently available regarding this mechanism in the context of ICI-related myocarditis. Likewise, there is no evidence on whether ICI-related myocarditis can act as a trigger for the development of cardiomyopathy, nor is there consensus on the optimal duration of follow-up for affected patients.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cha, J.-H.; Chan, L.-C.; Song, M.S.; Hung, M.-C. New Approaches on Cancer Immunotherapy. Cold Spring Harb. Perspect. Med. 2020, 10, a036863. [Google Scholar] [CrossRef] [PubMed]
- Rui, R.; Zhou, L.; He, S. Cancer immunotherapies: Advances and bottlenecks. Front. Immunol. 2023, 14, 1212476. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Lu, X.-J. Cancer immunotherapy: Current applications and challenges. Cancer Lett. 2020, 480, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, X.; Qie, R. Immunotherapy-associated cardiovascular toxicities: Insights from preclinical and clinical studies. Front. Oncol. 2024, 14, 1347140. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Alexander, G.; Chu, J.H.; Markopoulos, A.; Maloul, G.; Ayub, M.T.; Fidler, M.J.; Okwuosa, T.M. Immune Checkpoint Inhibitors and Cardiotoxicity: A Comparative Meta-Analysis of Observational Studies and Randomized Controlled Trials. J. Am. Heart Assoc. 2024, 13, e032620. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bhatti, S.A.; Ying, J. Immune Checkpoint Inhibitors—Associated Cardiotoxicity. Cancers 2022, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.; Lichtman, A.H.; Sharpe, A.H.; Galluzzi, L.; Kitsis, R.N. Immune checkpoint inhibitor–associated myocarditis: Manifestations and mechanisms. J. Clin. Investig. 2021, 131, e145186. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Lessomo, F.Y.N.; Mandizadza, O.O.; Mukuka, C.; Wang, Z.-Q. A comprehensive review on immune checkpoint inhibitors induced cardiotoxicity characteristics and associated factors. Eur. J. Med. Res. 2023, 28, 495. [Google Scholar] [CrossRef] [PubMed]
- Cautela, J.; Rouby, F.; Salem, J.-E.; Alexandre, J.; Scemama, U.; Dolladille, C.; Cohen, A.; Paganelli, F.; Ederhy, S.; Thuny, F. Acute Coronary Syndrome with Immune Checkpoint Inhibitors: A Proof-of-Concept Case and Pharmacovigilance Analysis of a Life-Threatening Adverse Event. Can. J. Cardiol. 2020, 36, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Puglisi, S.; Pirrone, C.; Martelli, V.; Catalano, F.; Nardin, S.; Seeber, A.; Puccini, A.; Sciallero, S. The role of immunotherapy in microsatellites stable metastatic colorectal cancer: State of the art and future perspectives. Front. Oncol. 2023, 13, 1161048. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, L.; Nardin, S.; Sacco, G.; Ferrante, M.; Rossi, G.; Barletta, G.; Bennicelli, E.; Dellepiane, C.; Tagliamento, M.; Pollone, B.R.; et al. Immune Checkpoint Inhibitors and Targeted Therapies in Early-Stage Non-Small-Cell Lung Cancer: State-of-the-Art and Future Perspectives. Cancers 2025, 17, 652. [Google Scholar] [CrossRef] [PubMed]
- Vilgelm, A.E.; Johnson, D.B.; Richmond, A. Combinatorial approach to cancer immunotherapy: Strength in numbers. J. Leukoc. Biol. 2016, 100, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; Parikh, R.; Gunturu, K.S.; Tariq, R.Z.; Dani, S.S.; Ganatra, S.; Nohria, A. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr. Oncol. Rep. 2021, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Medik, Y.B.; Patel, B.; Zamler, D.B.; Chen, S.; Chapman, T.; Schneider, S.; Park, E.M.; Babcock, R.L.; Chrisikos, T.T.; et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J. Exp. Med. 2023, 220, e20221333. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated with Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. npj Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-related adverse events of immune checkpoint inhibitors: A review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Liu, H.; Dai, J.; Zhao, J.; Zhu, S.; Zhang, X.; Liang, J.; Hu, X.; Zhao, J.; et al. The incidence of immune-related adverse events (irAEs) and their association with clinical outcomes in advanced renal cell carcinoma and urothelial carcinoma patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Cancer Treat. Rev. 2024, 129, 102787. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Champiat, S.; Lambotte, O.; Barreau, E.; Belkhir, R.; Berdelou, A.; Carbonnel, F.; Cauquil, C.; Chanson, P.; Collins, M.; Durrbach, A.; et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. 2016, 27, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bauersachs, J.; Berliner, D. Immune Checkpoint Inhibitor Associated Myocarditis and Cardiomyopathy: A Translational Review. Biology 2023, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, D.; Ma, Y.; Chen, X.; Dai, A.; Zhao, S.; Jin, X.; Gu, G. Cardiotoxicity associated with immune checkpoint inhibitors: Current status and future challenges. Front. Pharmacol. 2022, 13, 962596. [Google Scholar] [CrossRef] [PubMed]
- Baik, A.H.; Tsai, K.K.; Oh, D.Y.; Aras, M.A. Mechanisms and clinical manifestations of cardiovascular toxicities associated with immune checkpoint inhibitors. Clin. Sci. 2021, 135, 703–724. [Google Scholar] [CrossRef] [PubMed]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, Y.; Lu, J.; Zhang, Y.; Fan, G.; Tang, X.; Guo, W. Risk factors for cardiovascular adverse events from immune checkpoint inhibitors. Front. Oncol. 2023, 13, 1104888. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, M.L.; Meijers, W.C.; Screever, E.M.; Qin, J.; Carroll, M.G.; Sun, X.; Tannous, E.; Zhang, Y.; Sugiura, A.; Taylor, B.C.; et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature 2022, 611, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Salloum, F.N.; Tocchetti, C.G.; Ameri, P.; Ardehali, H.; Asnani, A.; de Boer, R.A.; Burridge, P.; Cabrera, J.-Á.; de Castro, J.; Córdoba, R.; et al. Priorities in Cardio-Oncology Basic and Translational Science: GCOS 2023 Symposium Proceedings: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncology 2023, 5, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Roy, M.D.; Golas, J.; Vitsky, A.; Ram, S.; Kumpf, S.W.; Martin, M.; Barletta, F.; Meier, W.A.; Hooper, A.T.; et al. Myocarditis in Cynomolgus Monkeys Following Treatment with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2019, 25, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Yousif, L.I.; Tanja, A.A.; de Boer, R.A.; Teske, A.J.; Meijers, W.C. The role of immune checkpoints in cardiovascular disease. Front. Pharmacol. 2022, 13, 989431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gan, Y.; Zhu, H.; Liu, Z.; Yao, X.; Cheng, C.; Liu, Z.; Su, C.; Zou, J. Role of mitochondrial metabolism in immune checkpoint inhibitors-related myocarditis. Front. Cardiovasc. Med. 2023, 10, 1112222. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008, 173, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Dinh, K.; Lombardo, F.; Kark, J. Doxorubicin cardiotoxicity in African Americans. J. Natl. Med. Assoc. 2004, 96, 196–199. [Google Scholar] [PubMed]
- Liu, J.-N.; Kong, X.-S.; Huang, T.; Wang, R.; Li, W.; Chen, Q.-F. Clinical Implications of Aberrant PD-1 and CTLA4 Expression for Cancer Immunity and Prognosis: A Pan-Cancer Study. Front. Immunol. 2020, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Yousif, L.I.; Screever, E.M.; Versluis, D.; Aboumsallem, J.P.; Nierkens, S.; Manintveld, O.C.; de Boer, R.A.; Meijers, W.C. Risk Factors for Immune Checkpoint Inhibitor–Mediated Cardiovascular Toxicities. Curr. Oncol. Rep. 2023, 25, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Algowhary, M.; Uguz, B.; Oliveira, D.C.; Ganyukov, V.; Zimbakov, Z.; Cercek, M.; Jensen, L.O.; Loh, P.H.; Calmac, L.; et al. Impact of Smoking Status on Mortality in STEMI Patients Undergoing Mechanical Reperfusion for STEMI: Insights from the ISACS–STEMI COVID-19 Registry. J. Clin. Med. 2022, 11, 6722. [Google Scholar] [CrossRef] [PubMed]
- Nardin, M.; Verdoia, M.; Negro, F.; Rolla, R.; Tonon, F.; De Luca, G. Impact of active smoking on the immature platelet fraction and its relationship with the extent of coronary artery disease. Eur. J. Clin. Investig. 2020, 50, e13181. [Google Scholar] [CrossRef] [PubMed]
- Pivato, C.A.; Jones, D.; Cao, D.; Sartori, S.; Chiarito, M.; Nicolas, J.; Zhang, Z.; Beerkens, F.; Nardin, M.; Qiu, H.; et al. Prognostic Value of Baseline Inflammation in Diabetic and Nondiabetic Patients Undergoing Percutaneous Coronary Intervention. Can. J. Cardiol. 2022, 38, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Barbieri, L.; Schaffer, A.; Cassetti, E.; Nardin, M.; Bellomo, G.; Aimaretti, G.; Marino, P.; Sinigaglia, F.; De Luca, G. Impact of diabetes on uric acid and its relationship with the extent of coronary artery disease and platelet aggregation: A single-centre cohort study. Metabolism 2014, 63, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Mollace, R.; Longo, S.; Nardin, M.; Tavernese, A.; Musolino, V.; Cardamone, A.; Federici, M. Role of MASLD in CVD: A review of emerging treatment options. Diabetes Res. Clin. Pract. 2024, 217, 111891. [Google Scholar] [CrossRef] [PubMed]
- Nardin, M.; Verdoia, M.; Sartori, C.; Pergolini, P.; Rolla, R.; Barbieri, L.; Schaffer, A.; Marino, P.; Bellomo, G.; Suryapranata, H.; et al. Body Mass Index and Platelet Reactivity During Dual Antiplatelet Therapy with Clopidogrel or Ticagrelor. J. Cardiovasc. Pharmacol. 2015, 66, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Nardin, M.; Verdoia, M.; Barbieri, L.; De Luca, G. Impact of metabolic syndrome on mean platelet volume and its relationship with coronary artery disease. Platelets 2019, 30, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Prado, Y.; Ben Shimol, J.; Samson, O. Body mass index and immune-related adverse events in patients on immune checkpoint inhibitor therapies: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2021, 70, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, F.; Poto, R.; Aran, L.; Cuomo, A.; Galdiero, M.R.; Spadaro, G.; Abete, P.; Bonaduce, D.; Marone, G.; Tocchetti, C.G.; et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: Clinical Risk Factors. Curr. Oncol. Rep. 2021, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Escudier, M.; Cautela, J.; Malissen, N.; Ancedy, Y.; Orabona, M.; Pinto, J.; Monestier, S.; Grob, J.-J.; Scemama, U.; Jacquier, A.; et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor–Related Cardiotoxicity. Circulation 2017, 136, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.-E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for Severe Immune Checkpoint Inhibitor–Associated Myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wei, Y.; Li, L.; Ge, H.; Wang, Y.; Zeng, C.; Ma, F. Genetic factors in the pathogenesis of cardio-oncology. J. Transl. Med. 2024, 22, 739. [Google Scholar] [CrossRef] [PubMed]
- Chin, I.S.; Khan, A.; Olsson-Brown, A.; Papa, S.; Middleton, G.; Palles, C. Germline genetic variation and predicting immune checkpoint inhibitor induced toxicity. npj Genom. Med. 2022, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Reynolds, K.L.; Lyon, A.R.; Palaskas, N.; Neilan, T.G. The Evolving Immunotherapy Landscape and the Epidemiology, Diagnosis, and Management of Cardiotoxicity: JACC: CardioOncology Primer. JACC CardioOncol. 2021, 3, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Naidoo, J.; Neilan, T.G. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur. Heart J. 2022, 43, 4458–4468. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Rassaf, T.; Totzeck, M. Cardiotoxicity from immune checkpoint inhibitors. IJC Heart Vasc. 2019, 25, 100420. [Google Scholar] [CrossRef] [PubMed]
- Al-Kindi, S.G.; Oliveira, G.H. Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 392, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Olenchock, B.A.; Salem, J.-E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, G.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the Setting of Cancer Therapeutics. Circulation 2019, 140, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- D’sOuza, M.; Nielsen, D.; Svane, I.M.; Iversen, K.; Rasmussen, P.V.; Madelaire, C.; Fosbøl, E.; Køber, L.; Gustafsson, F.; Andersson, C.; et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: A nationwide Danish study. Eur. Heart J. 2021, 42, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 * and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
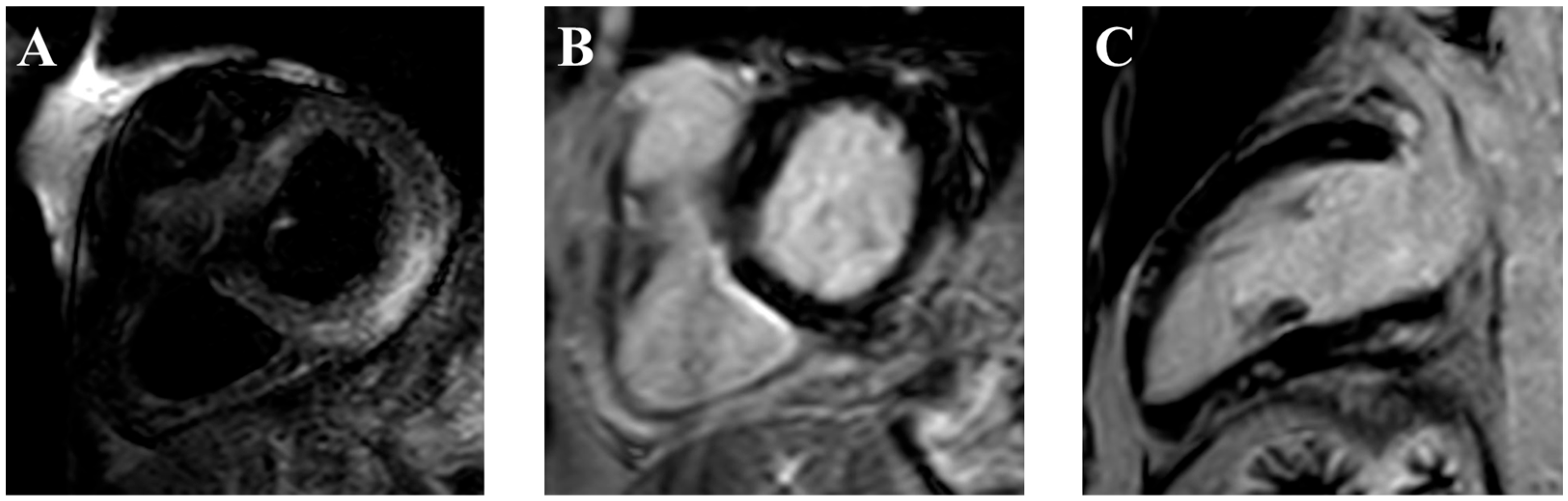
- Cadour, F.; Cautela, J.; Rapacchi, S.; Varoquaux, A.; Habert, P.; Arnaud, F.; Jacquier, A.; Meilhac, A.; Paganelli, F.; Lalevée, N.; et al. Cardiac MRI Features and Prognostic Value in Immune Checkpoint Inhibitor–induced Myocarditis. Radiology 2022, 303, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.Y.; Arbune, A.; Soufer, A.; Ragheb, E.; Kwan, J.M.; Lamy, J.; Henry, M.; Cuomo, J.R.; Charifa, A.; Gallegos, C.; et al. Left ventricular myocardial strain and tissue characterization by cardiac magnetic resonance imaging in immune checkpoint inhibitor associated cardiotoxicity. PLoS ONE 2021, 16, e0246764. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Zhang, L.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Cabral, M.; Awadalla, M.; Nohria, A.; Zlotoff, D.A.; Thuny, F.; et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients with Immune Checkpoint Inhibitor-Associated Myocarditis. J. Am. Coll. Cardiol. 2021, 77, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Man, X.; Wang, H.; Chen, C.; Cong, X.; Sun, L.; Sun, X.; Zhang, J.; Yang, L. Efficacy of high-dose steroids versus low-dose steroids in the treatment of immune checkpoint inhibitor-associated myocarditis: A case series and systematic review. Front. Immunol. 2025, 16, 1455347. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Buhlaiga, N.; Thébault, P.; Lapointe, R.; Johnson, N.A.; Miller, W.H. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N. Engl. J. Med. 2019, 380, 2375–2376. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.Y.; Blackley, E.; McLean, C.; Moore, M.; Bergin, P.; Gill, S.; Haydon, A. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br. J. Cancer 2017, 117, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Tocchetti, C.G.; Farmakis, D.; Koop, Y.; Andres, M.S.; Couch, L.S.; Formisano, L.; Ciardiello, F.; Pane, F.; Au, L.; Emmerich, M.; et al. Cardiovascular toxicities of immune therapies for cancer—a scientific statement of the Heart Failure Association (HFA) of the ESC and the ESC Council of Cardio-Oncology. Eur. J. Heart Fail. 2024, 26, 2055–2076. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Power, J.R.; Dolladille, C.; Ozbay, B.; Procureur, A.; Ederhy, S.; Palaskas, N.L.; Lehmann, L.H.; Cautela, J.; Courand, P.-Y.; Hayek, S.S.; et al. Immune checkpoint inhibitor-associated myocarditis: A novel risk score. Eur. Heart J. 2025, ehaf315. [Google Scholar] [CrossRef] [PubMed]
- Mudra, S.E.; Rayes, D.L.; Agrawal, A.; Kumar, A.K.; Li, J.Z.; Njus, M.; McGowan, K.; Kalam, K.A.; Charalampous, C.; Schleicher, M.; et al. Immune checkpoint inhibitors and pericardial disease: A systematic review. Cardio-Oncology 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Drobni, Z.D.; Zafar, A.; Quinaglia, T.; Hartmann, S.; Gilman, H.K.; Raghu, V.K.; Gongora, C.; Sise, M.E.; Alvi, R.M.; et al. Pericardial disease in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e002771. [Google Scholar] [CrossRef] [PubMed]
- Giancaterino, S.; Abushamat, F.; Duran, J.; Lupercio, F.; DeMaria, A.; Hsu, J.C. Complete heart block and subsequent sudden cardiac death from immune checkpoint inhibitor–associated myocarditis. Heart Case Rep. 2020, 6, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Andres, M.S.; Ramalingam, S.; Rosen, S.D.; Baksi, J.; Khattar, R.; Kirichenko, Y.; Young, K.; Yousaf, N.; Okines, A.; Huddart, R.; et al. The spectrum of cardiovascular complications related to immune-checkpoint inhibitor treatment. Cardio-Oncology 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Cottu, A.; Delaval, L.; Forestier, A.; Tomelleri, A.; Campochiaro, C.; Bond, M.; Dion, J.; Gury, A.; Savary, X.; Dhote, R.; et al. Immune checkpoint inhibitors-induced large vessel vasculitis: Clinical characteristics and management from a European multicentre study. Rheumatology 2025, keaf172. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.C.; Mullany, S.; Quinlivan, A.; Craig, A.; New-Tolley, J.; Slattery, J.; Sukumaran, S.; Klebe, S.; Craig, J.E.; Siggs, O.M.; et al. Eosinophilic Vasculitis and Arteritic Anterior Ischemic Optic Neuropathy Associated with Anti-PD-L1 Therapy. J. Immunother. 2022, 45, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Laera, N.; Malerba, P.; Vacanti, G.; Nardin, S.; Pagnesi, M.; Nardin, M. Impact of Immunity on Coronary Artery Disease: An Updated Pathogenic Interplay and Potential Therapeutic Strategies. Life 2023, 13, 2128. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Nardin, M.; Gioscia, R.; Negro, F.; Marcolongo, M.; Suryapranata, H.; Kedhi, E.; De Luca, G. Novara Atherosclerosis Study Group (NAS) Higher neutrophil-to-lymphocyte ratio (NLR) increases the risk of suboptimal platelet inhibition and major cardiovascular ischemic events among ACS patients receiving dual antiplatelet therapy with ticagrelor. Vasc. Pharmacol. 2020, 132, 106765. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sasaki, N.; Yamashita, T.; Emoto, T.; Kasahara, K.; Mizoguchi, T.; Hayashi, T.; Yodoi, K.; Kitano, N.; Saito, T.; et al. Overexpression of Cytotoxic T-Lymphocyte—Associated Antigen-4 Prevents Atherosclerosis in Mice. Arter. Thromb. Vasc. Biol. 2016, 36, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Lv, S.; Liu, B.; Liu, Z.; Luo, Y.; Kong, W.; Xu, Q.; Feng, J.; Wang, X. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE−/− mice. Cardiovasc. Res. 2013, 97, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Drobni, Z.D.; Gongora, C.; Taron, J.; Suero-Abreu, G.A.; Karady, J.; Gilman, H.K.; Supraja, S.; Nikolaidou, S.; Leeper, N.; Merkely, B.; et al. Impact of immune checkpoint inhibitors on atherosclerosis progression in patients with lung cancer. J. Immunother. Cancer 2023, 11, e007307. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, R.; Hoeller, C.; Pichler, V.; Mitterhauser, M.; Karanikas, G.; Haug, A.; Li, X.; Hacker, M. Immune Checkpoint Inhibitor Therapy Induces Inflammatory Activity in Large Arteries. Circulation 2020, 142, 2396–2398. [Google Scholar] [CrossRef] [PubMed]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Dolladille, C.; Akroun, J.; Morice, P.-M.; Dompmartin, A.; Ezine, E.; Sassier, M.; Da-Silva, A.; Plane, A.-F.; Legallois, D.; L’oRphelin, J.-M.; et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: A safety meta-analysis. Eur. Heart J. 2021, 42, 4964–4977. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.; Manisty, C.; Cheng, R.; Dastidar, A.; Mamas, M.; Ghosh, A. Immune checkpoint inhibitors: Unravelling atherosclerotic cardiovascular risk. Atherosclerosis 2025, 403, 119147. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Zaid, M.A.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bermas, B.; Braaten, T.; Budde, L.E.; et al. NCCN Guidelines ® Insights: Management of Immunotherapy-Related Toxicities, Version 2.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019, 5, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Gougis, P.; Jochum, F.; Abbar, B.; Dumas, E.; Bihan, K.; Lebrun-Vignes, B.; Moslehi, J.; Spano, J.-P.; Laas, E.; Hotton, J.; et al. Clinical spectrum and evolution of immune-checkpoint inhibitors toxicities over a decade—A worldwide perspective. eClinicalMedicine 2024, 70, 102536. [Google Scholar] [CrossRef] [PubMed]
- Menachery, S.M.; Hang, Y.; Pritchard, L.; Poklepovic, A.; Bottinor, W. Immune Checkpoint Inhibitor Rechallenge in a Patient with Previous Fulminant Myocarditis. Am. J. Cardiol. 2023, 199, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Armanious, M.; Huang, J.; Jeong, D.; Druta, M.; Fradley, M.G. Case of pembrolizumab-induced myocarditis presenting as torsades de pointes with safe re-challenge. J. Oncol. Pharm. Pract. 2020, 26, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Bailly, G.; Robert-Halabi, M.; Vion, P.-A.; Allenbach, Y.; Abbar, B.; Bretagne, M.; Salem, J.-E. Rechallenge After Severe Immune Checkpoint Inhibitor Myocarditis. JACC CardioOncology 2025, 7, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Chye, A.M.; Nordman, I.I.C.; Sverdlov, A.L. Successful immune checkpoint inhibitor rechallenge after immune-related pericarditis: Clinical case series. Front. Cardiovasc. Med. 2022, 9, 964324. [Google Scholar] [CrossRef] [PubMed]
- Allouchery, M.; Lombard, T.; Martin, M.; Rouby, F.; Sassier, M.; Bertin, C.; Atzenhoffer, M.; Miremont-Salame, G.; Perault-Pochat, M.-C.; Puyade, M. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J. Immunother. Cancer 2020, 8, e001622. [Google Scholar] [CrossRef] [PubMed]
- Eldani, C.; Kostine, M.; Faure, M.; Lazaro, E.; Rigothier, C.; Hiriart, J.B.; Teulières, B.; Poullenot, F.; Haissaguerre, M.; Zysman, M.; et al. Safety of immune checkpoint inhibitor rechallenge after severe immune-related adverse events: A retrospective analysis. Front. Oncol. 2024, 14, 1403658. [Google Scholar] [CrossRef] [PubMed]
- Frascaro, F.; Bianchi, N.; Sanguettoli, F.; Marchini, F.; Meossi, S.; Zanarelli, L.; Tonet, E.; Serenelli, M.; Guardigli, G.; Campo, G.; et al. Immune Checkpoint Inhibitors-Associated Myocarditis: Diagnosis, Treatment and Current Status on Rechallenge. J. Clin. Med. 2023, 12, 7737. [Google Scholar] [CrossRef] [PubMed]
- Bylsma, S.; Yun, K.; Patel, S.; Dennis, M.J. Immune Checkpoint Inhibitor Rechallenge After Prior Immune Toxicity. Curr. Treat. Options Oncol. 2022, 23, 1153–1168. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.S.; Cooper, L.T.; Kerneis, M.; Funck-Brentano, C.; Silvain, J.; Brechot, N.; Hekimian, G.; Ammirati, E.; Ben M’barek, B.; Redheuil, A.; et al. Systematic analysis of drug-associated myocarditis reported in the World Health Organization pharmacovigilance database. Nat. Commun. 2022, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Anquetil, C.; Salem, J.-E.; Lebrun-Vignes, B.; Johnson, D.B.; Mammen, A.L.; Stenzel, W.; Léonard-Louis, S.; Benveniste, O.; Moslehi, J.J.; Allenbach, Y. Immune Checkpoint Inhibitor–Associated Myositis. Circulation 2018, 138, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.H.; Cautela, J.; Palaskas, N.; Baik, A.H.; Meijers, W.C.; Allenbach, Y.; Alexandre, J.; Rassaf, T.; Müller, O.J.; Aras, M.; et al. Clinical Strategy for the Diagnosis and Treatment of Immune Checkpoint Inhibitor–Associated Myocarditis. JAMA Cardiol. 2021, 6, 1329. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.F.; Cascino, G.J.; Murtagh, G.; Akhter, N. Circulating Biomarkers for Cardiotoxicity Risk Prediction. Curr. Treat. Options Oncol. 2021, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Marschner, D.; Falk, M.; Javorniczky, N.R.; Hanke-Müller, K.; Rawluk, J.; Schmitt-Graeff, A.; Simonetta, F.; Haring, E.; Dicks, S.; Ku, M.; et al. MicroRNA-146a regulates immune-related adverse events caused by immune checkpoint inhibitors. JCI Insight 2020, 5, e132334. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chen, H.; Chen, D.; Ye, Y.; Xie, C.; Hou, M. PD-1 inhibitor inducing exosomal miR-34a-5p expression mediates the cross talk between cardiomyocyte and macrophage in immune checkpoint inhibitor–related cardiac dysfunction. J. Immunother. Cancer 2020, 8, e001293. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.-C.; Liu, X.-M.; Liang, L.-R.; Wang, L.-F.; Zhong, J.-C. Targeting the microRNA-34a as a Novel Therapeutic Strategy for Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 8, 784044. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.; Walch-Rückheim, B.; Friedmann, K.S.; Rheinheimer, S.; Tänzer, T.; Glombitza, B.; Sester, M.; Lenhof, H.-P.; Hoth, M.; Schwarz, E.C.; et al. miR-34a: A new player in the regulation of T cell function by modulation of NF-κB signaling. Cell Death Dis. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zou, C.; Chen, H.; Xie, C.; Hou, M. Immune checkpoint inhibitor induces cardiac injury through polarizing macrophages via modulating microRNA-34a/Kruppel-like factor 4 signaling. Cell Death Dis. 2020, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Won, T.; Kalinoski, H.M.; Wood, M.K.; Hughes, D.M.; Jaime, C.M.; Delgado, P.; Talor, M.V.; Lasrado, N.; Reddy, J.; Čiháková, D. Cardiac myosin-specific autoimmune T cells contribute to immune-checkpoint-inhibitor-associated myocarditis. Cell Rep. 2022, 41, 111611. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, C.; Shao, J.; Wang, L.; Han, Y.; Wang, J.; Wang, J. Predictive Value of Neutrophil-to-Lymphocyte Ratio for Immune Checkpoint Inhibitor-Related Myocarditis Among Patients Treated for Non-Small-Cell Lung Cancer. Cancer Innov. 2025, 4, e163. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, J.A.J.; Heymans, S.R.B. Dilated cardiomyopathy: Second hits knock-down the heart. Eur. Heart J. 2024, 45, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardin, S.; Ruffilli, B.; Costantini, P.; Mollace, R.; Taglialatela, I.; Pagnesi, M.; Chiarito, M.; Soldato, D.; Cao, D.; Conte, B.; et al. Navigating Cardiotoxicity in Immune Checkpoint Inhibitors: From Diagnosis to Long-Term Management. J. Cardiovasc. Dev. Dis. 2025, 12, 270. https://doi.org/10.3390/jcdd12070270
Nardin S, Ruffilli B, Costantini P, Mollace R, Taglialatela I, Pagnesi M, Chiarito M, Soldato D, Cao D, Conte B, et al. Navigating Cardiotoxicity in Immune Checkpoint Inhibitors: From Diagnosis to Long-Term Management. Journal of Cardiovascular Development and Disease. 2025; 12(7):270. https://doi.org/10.3390/jcdd12070270
Chicago/Turabian StyleNardin, Simone, Beatrice Ruffilli, Pietro Costantini, Rocco Mollace, Ida Taglialatela, Matteo Pagnesi, Mauro Chiarito, Davide Soldato, Davide Cao, Benedetta Conte, and et al. 2025. "Navigating Cardiotoxicity in Immune Checkpoint Inhibitors: From Diagnosis to Long-Term Management" Journal of Cardiovascular Development and Disease 12, no. 7: 270. https://doi.org/10.3390/jcdd12070270
APA StyleNardin, S., Ruffilli, B., Costantini, P., Mollace, R., Taglialatela, I., Pagnesi, M., Chiarito, M., Soldato, D., Cao, D., Conte, B., Verdoia, M., Gennari, A., & Nardin, M. (2025). Navigating Cardiotoxicity in Immune Checkpoint Inhibitors: From Diagnosis to Long-Term Management. Journal of Cardiovascular Development and Disease, 12(7), 270. https://doi.org/10.3390/jcdd12070270








