Viability and Longevity of Human Miniaturized Living Myocardial Slices
Abstract
1. Introduction
2. Materials and Methods
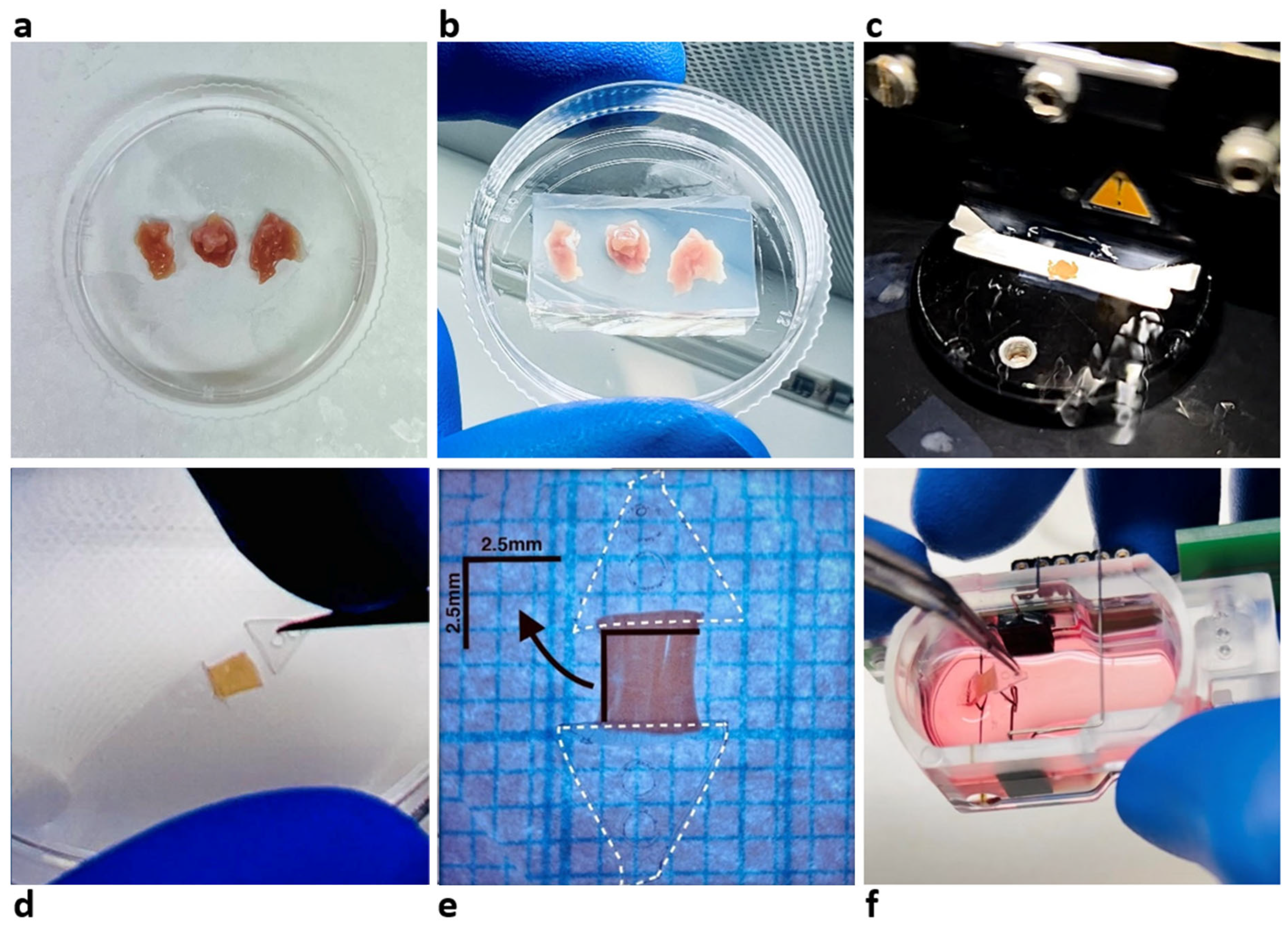
2.1. Tissue Acquisition
2.2. LMS Preparation and Culture
2.3. Biomechanical Profile Measurement
2.4. Mathematical Modeling and Statistics
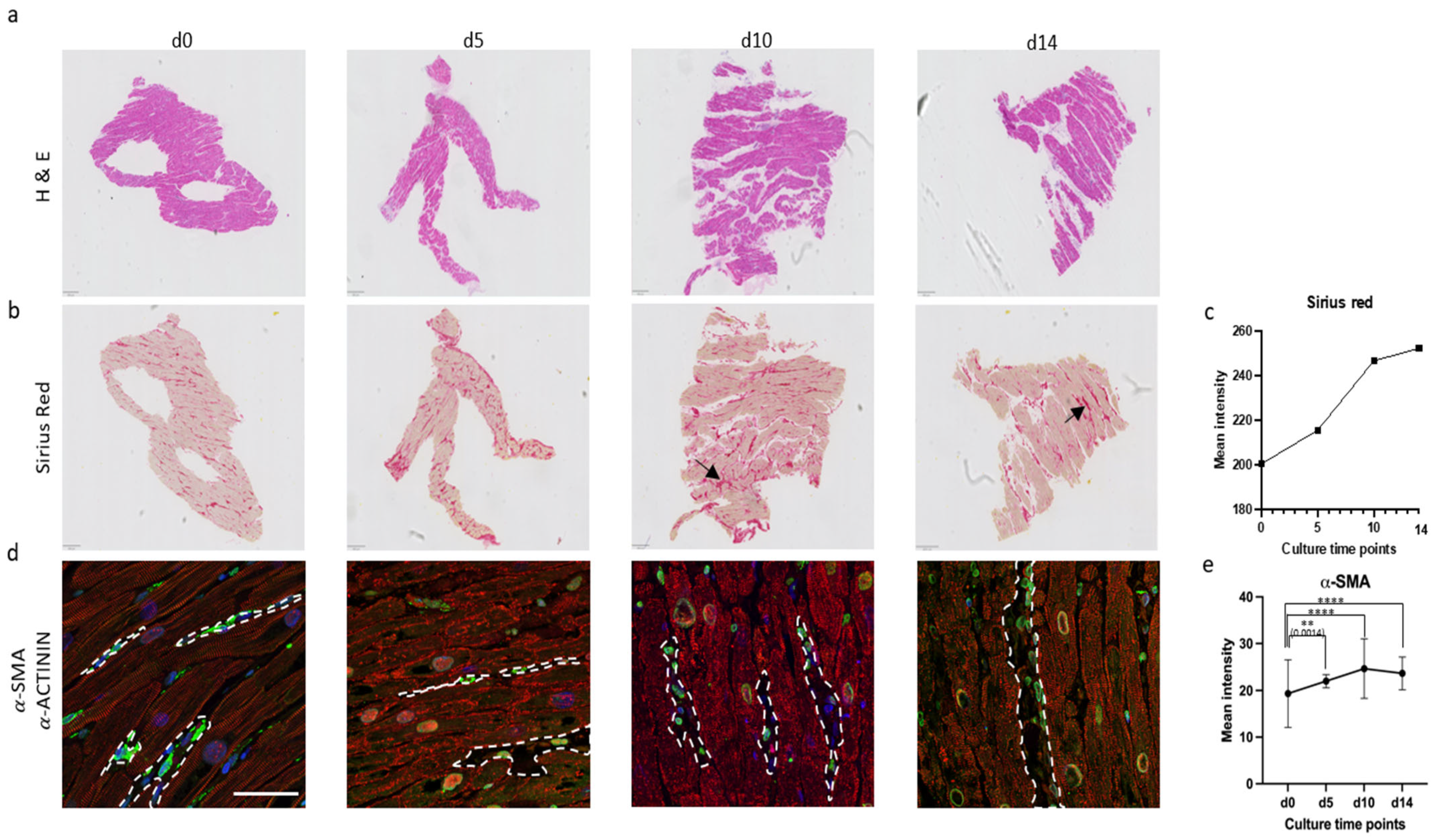
2.5. Histology of Prolonged Culture
2.6. Paraffin Embedding
2.7. Immunohistochemical Staining
3. Results
3.1. Statistical Model and Biomechanical Profiles Across Groups
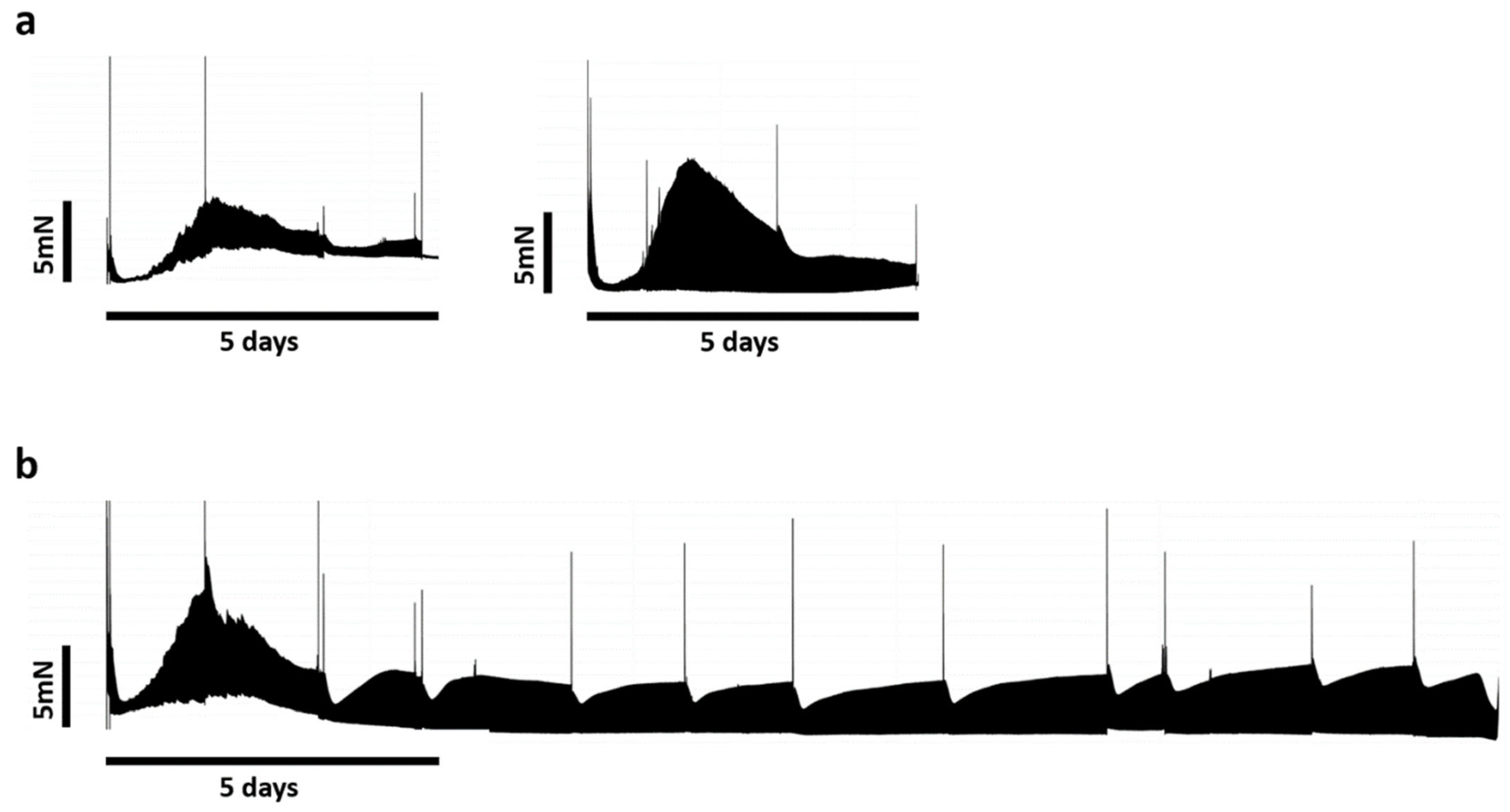
3.2. Biomimetic Culture and Viability of Mini-LMS
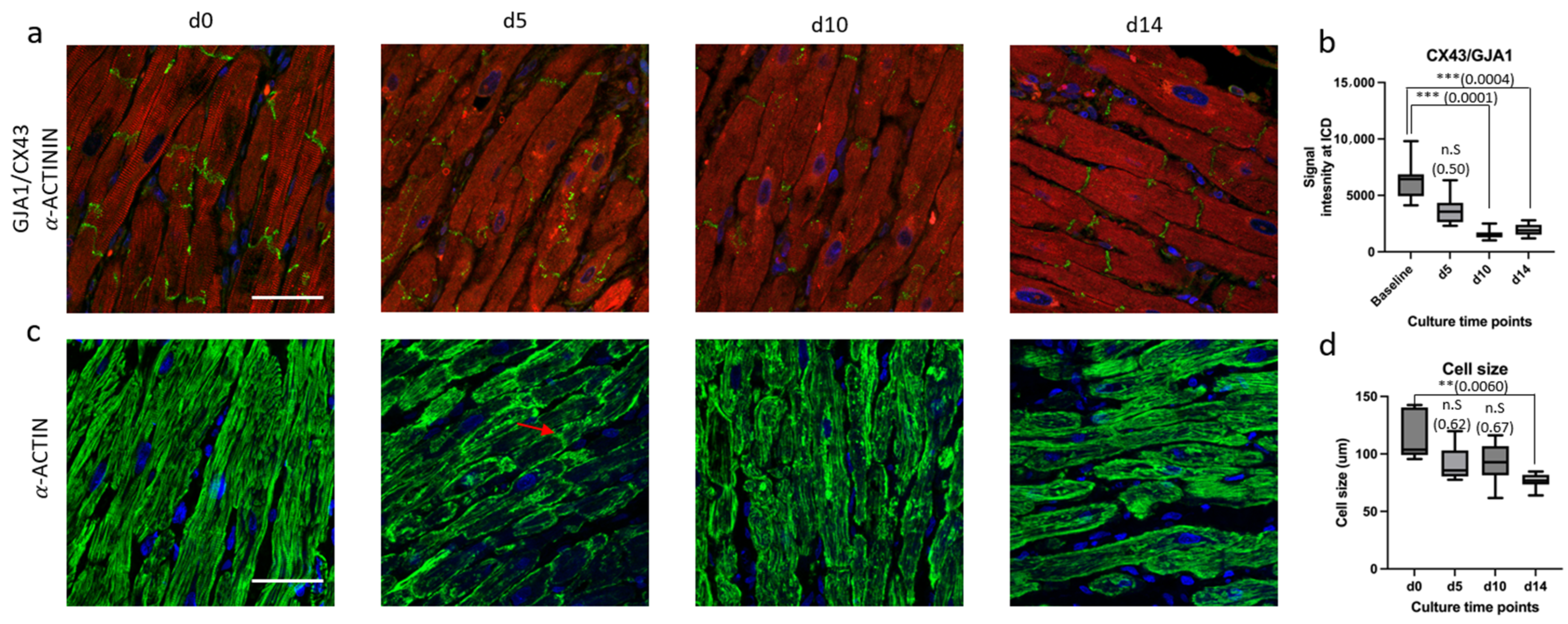
3.3. Micro-Architectural Organization and Cellular Morphology
4. Discussion
4.1. Statistical Modeling and Biomechanical Profile Across Groups
4.2. Biomimetic Culture of Mini-LMSs and Structural Changes
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LMSs | Living Myocardial Slices |
| mini-LMSs | Miniaturized LMSs |
| LV | Left Ventricle |
| TMDs | Triangular Mounting Devices |
| BMCCs | Biomimetic Cultivation Chambers |
| Fmax | Maximum Contraction Force |
| CD | Contraction Duration |
| AUC | Area under the Contraction Curve |
| TTP | Time-to-Peak |
| TTR | Time-to-Relaxation |
| AIC | Akaike Information Criterion |
| IQR | Median and Inter Quartile Range |
| PBS | Phosphate-Buffered Saline |
| PFA | Paraformaldehyde |
| HE | Hematoxylin and Eosin |
| ICD | Intercalated Discs |
| FFPE | Formalin-Fixed, Paraffin-Embedded |
References
- Perbellini, F.; Thum, T. Living myocardial slices: A novel multicellular model for cardiac translational research. Eur. Heart J. 2020, 41, 2405–2408. [Google Scholar] [CrossRef] [PubMed]
- Amesz, J.H.; Zhang, L.; Everts, B.R.; De Groot, N.M.S.; Taverne, Y. Living myocardial slices: Advancing arrhythmia research. Front. Physiol. 2023, 14, 1076261. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Terracciano, C.M.; Perbellini, F. Myocardial Slices: An Intermediate Complexity Platform for Translational Cardiovascular Research. Cardiovasc. Drugs Ther. 2019, 33, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Pitoulis, F.G.; Watson, S.A.; Perbellini, F.; Terracciano, C.M. Myocardial slices come to age: An intermediate complexity in vitro cardiac model for translational research. Cardiovasc. Res. 2020, 116, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Milting, H.; Fein, E.; Reiser, E.; Lu, K.; Seidel, T.; Schinner, C.; Schwarzmayr, T.; Schramm, R.; Tomasi, R.; et al. Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat. Commun. 2019, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Dendorfer, A.; Thum, T.; Perbellini, F. A practical guide for investigating cardiac physiology using living myocardial slices. Basic Res. Cardiol. 2020, 115, 61. [Google Scholar] [CrossRef] [PubMed]
- Amesz, J.H.; Langmuur, S.J.J.; Epskamp, N.; Bogers, A.; de Groot, N.M.S.; Manintveld, O.C.; Taverne, Y. Acute Biomechanical Effects of Empagliflozin on Living Isolated Human Heart Failure Myocardium. Cardiovasc. Drugs Ther. 2023, 38, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Amesz, J.H.; Langmuur, S.J.J.; Zhang, L.; Manintveld, O.C.; Schinkel, A.F.L.; de Jong, P.L.; de Groot, N.M.S.; Taverne, Y.J.H.J. Biomechanical response of ultrathin slices of hypertrophic cardiomyopathy tissue to myosin modulator mavacamten. Biomed. Pharmacother. 2024, 170, 116036. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Scigliano, M.; Bardi, I.; Ascione, R.; Terracciano, C.M.; Perbellini, F. Preparation of viable adult ventricular myocardial slices from large and small mammals. Nat. Protoc. 2017, 12, 2623–2639. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, E.C.H.; Amesz, J.H.; Sadeghi, A.H.; de Groot, N.M.S.; Manintveld, O.C.; Taverne, Y.J.H.J. Preclinical Models of Cardiac Disease: A Comprehensive Overview for Clinical Scientists. Cardiovasc. Eng. Technol. 2024, 15, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Amesz, J.H.; Langmuur, S.J.J.; van Schie, M.S.; Taverne, Y. Production of living myocardial slices from circulatory death hearts after ex vivo heart perfusion. JTCVS Tech. 2022, 13, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.M. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1092–H1099. [Google Scholar] [CrossRef] [PubMed]
- de Tombe, P.P.; Mateja, R.D.; Tachampa, K.; Ait Mou, Y.; Farman, G.P.; Irving, T.C. Myofilament length dependent activation. J. Mol. Cell. Cardiol. 2010, 48, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Monasky, M.M.; Biesiadecki, B.J.; Janssen, P.M. Increased phosphorylation of tropomyosin, troponin I, and myosin light chain-2 after stretch in rabbit ventricular myocardium under physiological conditions. J. Mol. Cell. Cardiol. 2010, 48, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Cao-Ehlker, X.; Fischer, C.; Lu, K.; Bruegmann, T.; Sasse, P.; Dendorfer, A.; Tomasi, R. Optimized Conditions for the Long-Term Maintenance of Precision-Cut Murine Myocardium in Biomimetic Tissue Culture. Bioengineering 2023, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Duff, J.; Bardi, I.; Zabielska, M.; Atanur, S.S.; Jabbour, R.J.; Simon, A.; Tomas, A.; Smolenski, R.T.; Harding, S.E.; et al. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat. Commun. 2019, 10, 2168. [Google Scholar] [CrossRef] [PubMed]
- Vahl, C.F.; Timek, T.; Bonz, A.; Kochsiek, N.; Fuchs, H.; Schäffer, L.; Rosenberg, M.; Dillmann, R.; Hagl, S. Myocardial length-force relationship in end stage dilated cardiomyopathy and normal human myocardium: Analysis of intact and skinned left ventricular trabeculae obtained during 11 heart transplantations. Basic Res. Cardiol. 1997, 92, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Perbellini, F.; Watson, S.A.; Scigliano, M.; Alayoubi, S.; Tkach, S.; Bardi, I.; Quaife, N.; Kane, C.; Dufton, N.P.; Simon, A.; et al. Investigation of cardiac fibroblasts using myocardial slices. Cardiovasc. Res. 2018, 114, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Chabanovska, O.; Lemcke, H.; Lang, H.; Vollmar, B.; Dohmen, P.M.; David, R.; Etz, C.; Neßelmann, C. Sarcomeric network analysis of ex vivo cultivated human atrial appendage tissue using super-resolution microscopy. Sci. Rep. 2023, 13, 13041. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Hartfield, E.M.; Crompton, L.A.; Badger, J.L.; Glover, C.P.; Kelly, C.M.; Rosser, A.E.; Uney, J.B.; Caldwell, M.A. Cross-regulation of Connexin43 and β-catenin influences differentiation of human neural progenitor cells. Cell Death Dis. 2014, 5, e1017. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.S.; van Veen, T.A.; de Bakker, J.M.; van Rijen, H.V. Functional consequences of abnormal Cx43 expression in the heart. Biochim. Biophys. Acta 2012, 1818, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lan, Y.; Zhao, Y.; Zhang, Q.; Lin, T.; Lin, K.; Guo, J.; Yan, Y. Expression of connexin 43 protein in cardiomyocytes of heart failure mouse model. Front. Cardiovasc. Med. 2022, 9, 1028558. [Google Scholar] [CrossRef] [PubMed]
- Sheydina, A.; Riordon, D.R.; Boheler, K.R. Molecular mechanisms of cardiomyocyte aging. Clin. Sci. 2011, 121, 315–329. [Google Scholar] [CrossRef] [PubMed]
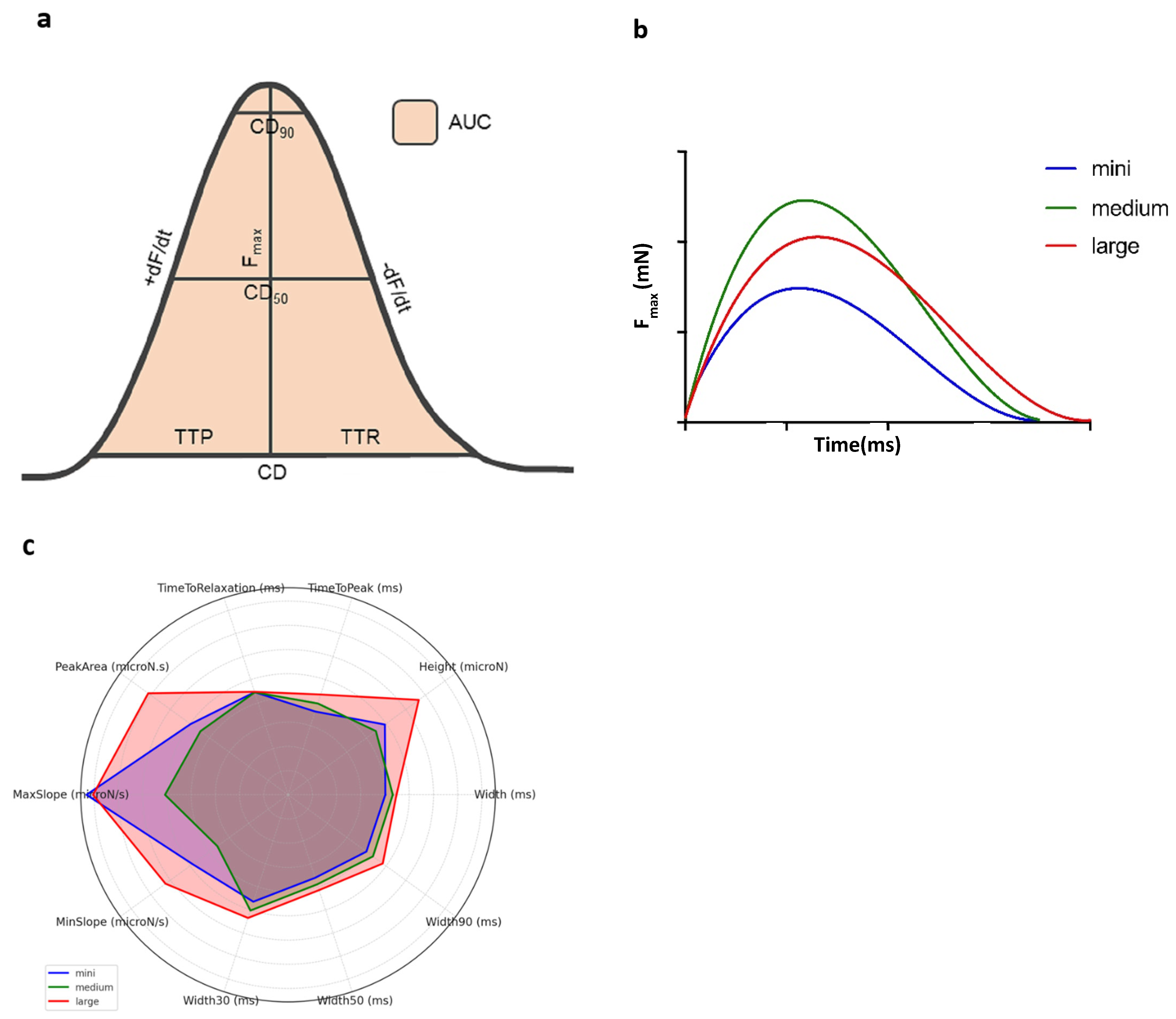
| Mini-LMSs (n = 34) | Medium LMSs (n = 25) | Large LMSs (n = 30) | H | p-Value | |
|---|---|---|---|---|---|
| CD (ms) | 501.5 (458.83–560.48) | 540 (481.47–611.02) | 559.16 (477.98–588.33) | 3.379 | 0.185 |
| Fmax (μN) | 1234.14 (583.63–2472.43) | 1119.4 (480.81–5004.09) | 1666.54 (1273.61–2141.51) | 1.953 | 0.377 |
| TTP (ms) | 180.85 (160.67–212.8) | 198.28 (175.07–216.28) | 217.77 (197.58–232.35) | 11.38 | 0.003 |
| TTR (ms) | 334.31 (269.75–402.33) | 333.81 (301.71–397.5) | 335.39 (287.08–371.57) | 1.061 | 0.589 |
| AUC (μN.s) | 372.82 (171.94–879.33) | 335.11 (156.39–1576.86) | 534.93 (386.14–779.6) | 3.843 | 0.146 |
| +dF/dt (μN/s) | 10,397.5 (4501.23–18553.02) | 6347.33 (3320.83–26712.99) | 10,056.81 (6366.42–15,181.58) | 0.543 | 0.762 |
| -dF/dt (μN/s) | −6088.98 (−11,129.17–3036.83) | −4510 (−20,011.67–2532.17) | −7811.38 (−11,055.67–5497.26) | 1.793 | 0.408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; van Steenis, Y.P.; Henry, S.; van Doorn, E.C.H.; Amesz, J.H.; van de Woestijne, P.C.; de Groot, N.M.S.; Manintveld, O.C.; Bartelds, B.; Taverne, Y.J.H.J. Viability and Longevity of Human Miniaturized Living Myocardial Slices. J. Cardiovasc. Dev. Dis. 2025, 12, 269. https://doi.org/10.3390/jcdd12070269
Zhou Z, van Steenis YP, Henry S, van Doorn ECH, Amesz JH, van de Woestijne PC, de Groot NMS, Manintveld OC, Bartelds B, Taverne YJHJ. Viability and Longevity of Human Miniaturized Living Myocardial Slices. Journal of Cardiovascular Development and Disease. 2025; 12(7):269. https://doi.org/10.3390/jcdd12070269
Chicago/Turabian StyleZhou, Ziyu, Yvar P. van Steenis, Surya Henry, Elisa C. H. van Doorn, Jorik H. Amesz, Pieter C. van de Woestijne, Natasja M. S. de Groot, Olivier C. Manintveld, Beatrijs Bartelds, and Yannick J. H. J. Taverne. 2025. "Viability and Longevity of Human Miniaturized Living Myocardial Slices" Journal of Cardiovascular Development and Disease 12, no. 7: 269. https://doi.org/10.3390/jcdd12070269
APA StyleZhou, Z., van Steenis, Y. P., Henry, S., van Doorn, E. C. H., Amesz, J. H., van de Woestijne, P. C., de Groot, N. M. S., Manintveld, O. C., Bartelds, B., & Taverne, Y. J. H. J. (2025). Viability and Longevity of Human Miniaturized Living Myocardial Slices. Journal of Cardiovascular Development and Disease, 12(7), 269. https://doi.org/10.3390/jcdd12070269







